Abstract
TBX1 encodes a DNA binding transcription factor that is commonly deleted in human DiGeorge syndrome and plays an important role in heart development. Mechanisms of Tbx1 function, such as Tbx1 interacting regulatory proteins and transcriptional target specificity, are largely unknown. Ash2l is the mammalian homolog of Drosophila Ash2 (absent small homeotic 2) and is a core component of a multimeric histone methyltransferase complex that epigenetically regulates transcription via methylation of histone lysine residues. We undertook an unbiased yeast two-hybrid screen to look for functionally relevant Tbx1-interacting proteins and report a physical and functional interaction between Tbx1 and Ash2l. Tbx1 interacts with Ash2l in both yeast and mammalian cells and Ash2l acts as a transcriptional co-activator in luciferase reporter assays. Expression analysis shows that Tbx1 and Ash2l have overlapping mRNA and protein expression patterns during development. By generating an Ash2l knockout mouse utilizing gene-trap technology, we show that although Ash2l heterozygous mice are normal, Ash2l-null embryos die early during gestation. Thus, Ash2l is required for the earliest stages of embryogenesis. Furthermore, our finding of a physical interaction between Tbx1 and Ash2l suggest that at least some functions of Tbx1 may be mediated by direct interactions with a histone methyltransferase complex.
Keywords: DiGeorge syndrome, epigenetics, chromatin, development, transcription
Introduction
DiGeorge syndrome is a common disorder with a high incidence of congenital heart defects and accumulating data suggest that the Tbx1 transcription factor plays a critical developmental role in its pathogenesis. Animal studies using mouse models have implicated Tbx1 as a critical gene within the commonly deleted region of chromosome 22 and three different TBX1 mutations have been described in families with the classical features of DiGeorge syndrome but without evidence of a chromosomal deletion at 22q11.1 One of these mutations occurred in the highly conserved T-box DNA binding domain. This domain is not only important for the DNA binding functions of the transcription factor, but is also known to mediate the interaction with transcriptional co-regulators. For example, the homeodomain transcription factor, Nkx2.5, and the zinc-finger transcription factor, Gata4, interact with the T-box domain of Tbx202 and Tbx183 to modulate transcriptional pathways important for cardiac development. The Tbx5 T-box also interacts with Nkx2.5.4 In addition, the Tbx18 T-box interacts with the paired-box transcription factor, Pax3, to regulate mesodermal differentiation during somitic development.5
Mechanisms regulating the specificity of T-box protein-dependent transcriptional activation are not entirely clear. In part, this may be dependent on specific DNA sequence in regulatory regions of direct downstream targets. The consensus in vitro DNA binding site is known for some T-box proteins, including Tbx15, Tbx18 and Brachyury (T).3,6– 9 Brachyury binds optimally as a dimer to a palindromic DNA sequence containing 24 basepairs,7,8 but is also able to bind as a monomer to half of the palindromic DNA sequence, the T/2 site. Many T-box family members bind to the full T site and the T/2 site with varying affinities,3,7 although this binding affinity does not correlate with the ability to mediate transcription. Therefore, DNA binding alone is not sufficient to explain the T-box protein specificity. T-box proteins physically interact with other transcription factors,2 – 5,10– 15 mediated by domains outside the T-box, which provides another layer of specificity in their transcriptional regulation. Another exciting observation is that further specificity of T-box protein transcriptional regulation may be conferred through association with epigenetic modification complexes that result in chromatin changes and permissive transcriptional states.16 –18
We undertook a search for functionally relevant Tbx1-interacting proteins to explore mechanisms of transcriptional regulation relevant to the pathogenesis of DiGeorge syndrome and the biology of heart development. In this study, we report a physical and functional interaction between Tbx1 and Ash2l. Ash2l is the mammalian homolog of the Drosophila protein Ash2 (absent small homeotic 2). Ash2l is a core component of a multimeric histone methyltransferase (HMT) protein complex19,20 and may also function as an oncoprotein.20 HMT complexes modulate transcription by catalyzing the methylation of arginine or lysine residues of N-terminal tails of core histones. In general, lysine methylation functions to modulate transcription and DNA repair, whereas arginine methylation is only known to alter transcription. For example, in the case of dimethylation or trimethylation of core histone 3 lysine 4 (H3K4) residues, these histone modifications functionally result in chromatin unfolding, or euchromatin, and facilitate transcription activation.21 These epigenetic modifications play a critical role in myriad biological events from development to oncogenesis.
Materials and methods
Yeast two-hybrid assay
Mouse Tbx1 was cloned into the pGBKT7 vector (Clontech, Mountain View, CA, USA) and was used as bait to screen a pretransformed mouse cDNA E11 library (Clontech). The AH109 yeast strain was transformed with the pGBKT7-Tbx1 plasmid and mated with the pretransformed Y187 yeast strain. Transformants were screened under high stringency using SD/-Trp/-Leu/-His/-Ade growth conditions. To verify the yeast interactions, individual candidate clones and pGBKT7-Tbx1 were co-transformed into AH109 yeast and grown under the same high stringency conditions with the addition of X-α-gal.
Cell culture and immunoprecipitation
JEG3 cells (HTB-36; ATCC, Manassas, VA, USA) were grown in Roswell Park Memorial Institute-1640 media (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum. Just prior to transfection, RPMI-1640 media were replaced with OptiMEM (Invitrogen) media. Cells at 60–80% confluence were transfected with 2 μg of plasmid DNA per 10 cm dish using the FuGENE HD (Roche, Indianapolis, IN, USA) transfection reagent. Transfection complex was prepared at 4:1 reagent:DNA ratio in 500 μLOptiMEM per dish. Growth media were replaced with RPMI-1640 18–24 h after transfection and cells were harvested 48 h after transfection. Whole-cell lysates were extracted with lysis buffer (50 mmol/ L Tris-HCl [pH 8.0], 150 mmol/L NaCl, 20 mmol/L tetrasodium pyrophosphate, 10 mmol/L EDTA and 1% Triton X-100) containing protease inhibitors (Roche, Indianapolis, IN, USA). Cell lysates were incubated with 5 μg rabbit anti-V5 (Sigma-Aldrich, St Louis, MO, USA) or rabbit IgG antibodies. Immunoprecipitated protein was analyzed by Western blot with mouse anti-myc antibody (1:5000; Invitrogen).
Vector construction and gene targeting
Vector construction and mouse AK7.1 embryonic stem (ES) cell targeting was performed as part of the Soriano Laboratory Gene-Trap Project (http://www.drbsinai.org/soriano/db5.html).22,23 The Ash2l-targeted AK7.1 ES cells correspond to Clone ID# S3-11A1 and utilized the ROSAFARY gene-trap vector.22 ES cell retroviral infection, electroporation, 3′ rapid amplification of cDNA ends (RACE) and 5′ genomic sequencing have been described previously.23 The targeted ES cells were injected into C57BL/6J blastocysts and resulting chimeric mice were bred to produce germline offspring that were used for these studies. These mice were maintained on a mixed C57BL/6J;129 genetic background. The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all animal protocols.
Southern blot
Southern blot hybridization was performed on genomic DNA from ES cells digested with HindIII, PstI and EcoRI. The 5′ flanking probe used detects a 10.8-, 6.4- or 10.8-kb wild-type band and a 1.5-, 4.5-, or 3.9-kb targeted band, respectively, for each of the three restriction enzymes.
Genotyping
Genotyping of Ash2l gene-trap mice was performed using standard polymerase chain reaction (PCR) techniques using an annealing temperature of 60°C for the wild-type allele and 62°C for the mutant allele. The following genotyping primers were used: Ash2l-F5 – 5′-CGTCTTTTCCACCCACA ACTACC-3′ and Ash2l-R9 – 5′-ACCAGGACCCGCTCCA GC-3′ for the wild-type allele; and Ash2l-F5 and Ash2l-U5R2 – 5′-CTGTTCCTTGGGAGGGTCTC-3′ for mutant allele. The resulting wild-type and mutant PCR products were 572 and 252 basepairs, respectively. For blastocyst genotyping, genomic DNA was amplified by isothermic multiple displacement amplification with the GenomiPhi V2 DNA Amplification Kit (GE HealthCare, Piscataway, NJ, USA).
Reverse transcription PCR
Total RNA was extracted and purified from ES cell colonies with Trizol (Invitrogen) and the RNeasy mini kit (Qiagen, Valencia, CA, USA). First-strand cDNA was synthesized using random hexamers with the SuperScript III system (Invitrogen). Ash2l and βgeo* reverse transcription (RT)-PCR primer sequences are indicated in supplemental Tables S1 and S2.
Immunohistochemistry, radioactive in situ hybridization
Horseradish peroxidase immunostaining was performed as described previously24 using anti-Ash2 A300-112A (1:100; Bethyl Laboratories, Montgomery, TX, USA). In situ hybridization was performed as described previously.25,26 Protocols are available at http://www.med.upenn.edu/mcrc.
Luciferase assays
JEG3 cells were grown in six-well dishes to 50–80% confluency. The TBX1 and Ash2l expression constructs were co-transfected with the brachyury (T) half-site luciferase reporter (2xT-tk-GL2)27 and phRL-TK reporter vectors (Promega, Madison, WI, USA) using Lipofectamine transfection reagent (Invitrogen). Firefly and Renilla luciferase activity were measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s recommended protocol. All results are normalized for transfection efficiency and represent the average of nine experiments.
Results
Tbx1 interacts with Ash2l in a yeast two-hybrid assay
In order to identify novel Tbx1-interacting proteins, we performed a yeast two-hybrid assay using full-length mouse Tbx1 protein as bait and a mouse embryonic (E) day 11 cDNA library as prey. The mouse Tbx1 coding region was subcloned into the pGBKT7 vector and transformed into AH109 yeast. Yeast mating was carried out between these transformed AH109 yeast cells and the pretransformed cDNA library. Mated yeast cells were grown under high stringency conditions. The interaction screen was performed in triplicate and over 900 colonies grew. Plasmid DNA was isolated from 86 colonies. After sequencing and translation, protein sequence comparison was made between the GAL4 fusion protein of the sequenced clone and public protein databases. The translated proteins aligned to 25 entries in the online databases. Thus, these 25 proteins were subjected to further verification in yeast.
The pGBKT7-Tbx1 vector and the plasmid of candidate clones were co-transformed into AH109 yeast cells and grown on SD/-Trp/-Leu/-His/-Ade plates containing X-α-gal. The pGBKT7 vector alone was used as negative control. Only those plasmid clones that allowed yeast survival when co-transformed with the pGBKT7-Tbx1 plasmid but not with control pGBKT7 vector were considered to interact with Tbx1 (Figure 1a). As a result, 20 proteins were identified to have an interaction with Tbx1 in yeast. Among them, eight colonies with high galactosidase activity were chosen for further analysis. The clones included Ash2l, Mnat1, Rhox5, Psap, Sox11, Tmem55a, Wdr68 and Tsen54. Ash2l was particularly intriguing, as two non-overlapping interacting domains were independently found to interact with Tbx1 in our yeast two-hybrid screen. Ash2l is the vertebrate homolog of Drosophila Ash2 and has been implicated in histone methylation and resulting transcriptional regulation as part of an HMT complex.
Figure 1.
Tbx1 physically and functionally interacts with Ash2l in yeast and mammalian cells. (a) Tbx1 fused to the GAL4 DNA-BD interacts with Ash2l fused to the GAL4-AD allowing for yeast survival. (b) Full-length Tbx1-V5 co-immunoprecipitates with full-length myc-Ash2l and the myc-Ash2l mutants Δ100–150 and Δ310–413, but not Δ6–290. Protein was extracted from transiently transfected JEG3 whole-cell lysates. Immunoprecipitations were performed with anti-V5 or control IgG and complexes were analyzed by Western blot using HRP-conjugated anti-myc antibody. (c) Full-length Tbx1-V5 co-immunoprecipitates with full-length myc-Ash2l and the myc-Ash2l mutants 1–310, 1–405 and SPRY del but not 1–145 or 413–516. Methods were identical to those described in (b). (d) Full-length myc-Ash2l co-immunoprecipitates with full-length Tbx1-V5 and the Tbx1-V5 mutants F137Y, G299S and Trunc (Tbx1delC; Stoller and Epstein33). Methods were identical to those described in (b). (e) Schematic representation of full-length and mutant Ash2l constructs used to map the Ash2l interaction domain. The ability (+) or inability (−) of full-length Tbx1 to immunoprecipitate the Ash2l proteins is indicated. (f) Transcriptional co-activation of a luciferase reporter by TBX1 and Ash2l. Transient transfection of JEG3 cells with Ash2l augments TBX1-dependent transcriptional activation in a dose-dependent manner. Results are normalized for transfection efficiency and are expressed as mean + SD of nine experiments. The statistical significance of differences between groups was analyzed by the paired Student’s t-test. BD, binding domain; AD, activation domain; HRP, horse radish peroxidase; SD, standard deviation
Tbx1 physically interacts with Ash2l in mammalian cells
To further show that Ash2l may be an important co-factor for Tbx1, we confirmed the interaction in mammalian cells. V5-tagged full-length Tbx1 and myc-tagged Ash2l mammalian expression constructs were generated. V5-tagged Tbx1 was transiently co-transfected with either myc-tagged Ash2l or with control plasmids into JEG3 chor-iocarcinoma cells. These cells have previously been shown to allow for robust activation in functional Tbx1 assays.27 This is presumably due to the presence of transcriptional co-factors important for Tbx1-dependent activation. After precipitating with either rabbit anti-V5 antibody or rabbit IgG from whole-cell extracts, samples were analyzed by Western blotting with mouse anti-myc antibody. Full-length Ash2l specifically interacts with full-length Tbx1, while there was no non-specific binding in control experiments (Figures 1b–d). The known functional domains of Ash2l include a conserved plant homeodomain (PHD) finger, a SPla/RYanodine receptor (SPRY) motif28 and a bipartite nuclear localization signal.29 PHD domains bind to histone-methylated lysine residues (e.g. H3K4) resulting in conformational changes in chromatin structure and changes in transcriptional activity.30 The SPRY domain is an evolutionarily ancient motif present in many divergent protein families. It may be involved in protein–protein interactions, although its function is largely unknown.31 We further characterized the Tbx1–Ash2l interaction by mapping the minimal interacting domain of Ash2l by co-immunoprecipitation (summarized in Figure 1e). We transiently expressed full-length Tbx1 with various truncations of Ash2l in JEG3 cells and performed co-immunoprecipitations with whole-cell extracts. Deletion of the PHD domain (Δ100–150) has no effect on the Ash2l–Tbx1 physical interaction, but a larger deletion (Δ6–290) abolishesthis interaction, suggesting that Ash2l amino acids 150–289 are required for the interaction with Tbx1 (Figure 1b). The 150–289 interaction domain is sufficient to mediate the interaction with Tbx1 (1–310, 1–405), and when deleted (1–145), the interaction is abolished (Figure 1c). This region does not include the SPRY domain. This observation was confirmed by experiments with SPRY deletion constructs (SPRY del, 1–405) to confirm that this domain is not required for the interaction with Tbx1 (Figure 1c).
Ash2l functionally interacts with Tbx1
To determine the contribution of Ash2l in Tbx1-mediated transcriptional activity, we transiently co-transfected JEG3 cells with Tbx1 and Ash2l in luciferase reporter assays. Transient transfection of Tbx1 in JEG3 cells results in a 10-fold activation of the 2xT-tk-GL227 over control levels. The Ash2l-containing HMT complex has previously been associated with transcriptional activation,20,28,32 and consistent with the hypothesis that Ash2l is a Tbx1 co-factor, we show that co-transfection of Ash2l augments this activation in a dose-dependent manner (Figure 1f). This provides evidence that not only do Ash2l and Tbx1 physically interact, but in addition they functionally interact as transcriptional activators.
Known human missense mutations do not affect the physical interaction between Tbx1 and Ash2l
Prior studies in our lab showed that a human frame-shift mutation in TBX1 results in the absence of a critical nuclear localization signal.33 The mechanism by which two other human TBX1 missense mutations lead to a functional defect was not fully elucidated, although other investigators subsequently reported that these mutations might be gain-of-function mutations.27 To determine if these missense mutations result in an altered interaction with Ash2l, we performed further immunoprecipitation analysis. We transiently expressed myc epitope-tagged Ash2l with either full-length V5-tagged Tbx1 or Tbx1 mutants in JEG3 cells, immunoprecipitated with anti-V5 antibody or IgG, and then detected precipitated Ash2l with anti-myc antibody. As shown in Figure 1d, these mutants retain the ability to interact physically with Ash2l. Although the mutations are associated with human DiGeorge syndrome, the mechanism is not due to an alteration in the interaction of Tbx1 with an Ash2l HMT complex.
Ash2l expression overlaps with areas of Tbx1 expression in the developing mouse embryo
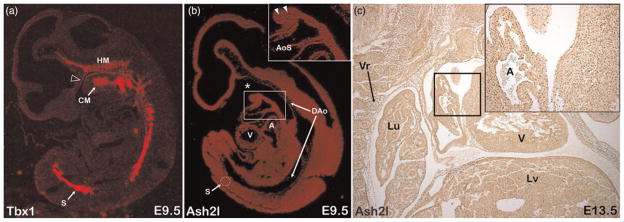
If Tbx1 and Ash2l functionally interact in vivo, then they should be co-expressed. Hence, we performed Ash2l expression analysis for comparison with the well-documented expression patterns of Tbx1. Tbx1 is expressed in early embryonic pharyngeal endoderm, the somite, head mesenchyme and in the cardiac outflow tract myocardium (Figure 2).34– 36 We analyzed wild-type mid-gestation mouse embryos by in situ hybridization and by immunohistochemistry to look at overall Ash2l expression patterns. Immunostaining and in situ hybridization analysis revealed that Ash2l, both at the mRNA and the protein level, is broadly expressed, including in Tbx1 expression domains (Figure 2). These immunohistochemistry experiments also reveal that Ash2l is a nuclear protein with little or no cytoplasmic expression. Immunostaining experiments utilizing an antigen-specific blocking peptide as negative control supported the specificity of this staining pattern (data not shown). These Ash2l expression data are consistent with prior reports.28,29
Figure 2.
Ash2l is broadly expressed in the mid-gestation mouse embryo. (a) Parasagittal section of E9.5 embryo showing Tbx1 expression by in situ hybridization. (b) Parasagittal section of E9.5 embryo showing Ash2l expression by in situ hybridization. (c) Parasagittal section of E13.5 embryo showing Ash2l protein expression by immunohistochemistry. Head mesenchyme (HM), pharyngeal arch core mesoderm (CM), pharyngeal endoderm (open arrowhead), ventricle (V), atria (A), somite (S), aortic sac (AoS), dorsal aorta (DAo), pharyngeal region of foregut diverticulum (asterisk), origin of first and second aortic arch arteries (closed arrowheads), vertebra (Vr), lung (Lu) and liver (Lv) are indicated for reference. E, embryonic age
Ash2l is critical for the earliest stages of embryonic development
To determine the effect of the loss of Ash2l in the mouse, we generated Ash2l knockout mice utilizing gene-trap targeted ES cells. The Ash2l gene-trap ES cell line harbors a promoter trap whereby a promoter-less cassette (βgeo*) comprised of a LacZ-neomycin fusion downstream of an adenoviral splice acceptor site integrates under the control of an endogenous promoter and functions as an artificial 3′ terminal exon.22 Analysis of 5′ genomic sequence and 3′ RACE sequence revealed that the retroviral gene-trap cassette inserted between the alternative exons 1a and 1b of Ash2l (Figure 3a). Blastocyst injections with Ash2l gene-trap ES cells resulted in a chimeric mouse that was capable of germ-line transmission. The resulting progeny were genotyped by PCR. To confirm the predicted splicing event between exon 1a and the βgeo* cassette, we extracted mRNA from targeted and wild type ES cells derived from healthy heterozygous adult gene-trap mice (see below) and analyzed them by RT-PCR. The predicted exon 1a-βgeo* species was present only in targeted cells, but not in wild-type cells (Figure 3b), as predicted. In addition, there was no exon 1b-βgeo* species, showing that this gene-trap only directly targeted one isoform of Ash2l. Comparison of the relative levels of isoforms 1a and 1b mRNA in ES cells showed that the 1a isoform is predominant during early development (Figure 3c). In Ash2l targeted ES cells, there was equal mRNA expression of the two isoforms, suggesting a compensatory upregulation of the 1b isoform upon disruption of the transcription of the 1a isoform. We further confirmed the targeting by analyzing Ash2l gene-trap ES cells by Southern blot of genomic DNA digested with three different restriction enzymes. While this Southern analysis of wild-type ES cells revealed a single genomic fragment, there was an additional predicted fragment for each of the restriction enzymes in heterozygous ES cells, thus confirming the targeting at the Ash2l locus (Figure 3e).
Figure 3.
Generation of Ash2l gene-trap knockout mice. (a) Targeting strategy of Ash2l gene-trap. The ROSAFARY retroviral cassette inserted between Ash2l exons 1a and 1b. The expected sizes of the HindIII (H), PstI (P) and EcoRI (E) digests recognized by the indicated probe (**) are shown. (b) Confirmation of predicted Ash2l exon 1a-βgeo* splicing. RT-PCR shows the exon 1a-βgeo* species in heterozygous, but not wild–type (WT) Ash2l ES cells. An exon 1b-βgeo* species is not present in either heterozygous or WT cells. (c) Ash2l isoform 1a is predominant in ES cells. RT-PCR shows that the Ash2l 1a isoform is more highly expressed in WT cells compared with the 1b isoform. In heterozygous Ash2l ES cells, there is equal expression of the two isoforms. (d) Schematic representation of alternative splicing. Ash2l exons 1a and 1b splice with exon 2. In Ash2l gene-trap mice, exon 1a splices with βgeo*. Exon 1b-βgeo* splicing is shown for illustration. Primers used for RT-PCR are indicated. (e) Genomic Southern blot of WT (+/+) and targeted Ash2l gene-trap ES cells (+/−). WT (10.8, 6.4 or 10.8 kb) and mutant (1.5, 4.5 or 3.9 kb) bands are shown, respectively, for the HindIII, PstI and EcoRI restriction enzyme digests. RT-PCR, reverse transcription-polymerase chain reaction; ES, embryonic stem
Mice heterozygous for Ash2l were born without phenotypic abnormalities in expected Mendelian ratios and survived well past one year of age with normal fertility (data not shown). To determine the phenotype of the homozygous knockout state, we performed multiple crosses of Ash2l heterozygous mice. Of 80 total live born pups 30/80 (37%) were wild type, 50/80 (63%) were heterozygous and 0/80 (0%) were homozygous for the Ash2l gene-trap (Table 1). To establish at what stage of development loss of Ash2l results in embryonic lethality, we crossed heterozygous mice and harvested embryos at progressively earlier stages of development. We were unable to identify null embryos at any postimplantation stage tested (Table 1). We then isolated individual blastocysts, amplified genomic DNA by multiple displacement amplification and performed Ash2l genotyping by PCR. At this early stage of development, we were able to identify null blastocysts at close to Mendelian ratios (Table 1). We then attempted to generate blastocyst-derived Ash2l null ES cells for functional analysis. Although we were able to derive multiple wild-type and heterozygous ES cell lines (data not shown), we were unable to isolate any null ES cell lines. This underscores the notion that Ash2l is essential for the earliest stages of embryonic development.
Table 1.
Summary of genotypes in Ash2l heterozygote intercrosses
| Genotype | E3.5 | E8.5–E11.5 | P0 |
|---|---|---|---|
| Ash2l−/− | 7 | 0 | 0 |
| Ash2l+/− | 28 | 41 | 50 |
| Ash2l+/+ | 5 | 21 | 30 |
| χ2 | P = 0.042 | P < 0.001 | P < 0.001 |
E, embryonic age
Discussion
Epigenetic mechanisms of gene regulation are important during embryogenesis. Some of these epigenetic mechanisms include DNA methylation and histone modifications such as histone acetylation, phosphorylation and methylation. The trithorax group (TrxG) was originally defined in Drosophila as a group of proteins required for the positive regulation of Hox genes and which functionally oppose the Polycomb group of transcriptional repressors.37 The TrxG proteins are a heterogeneous group of chromatin modifying complexes and include the MLL (mixed-lineage leukemia), NURF, TAC1, Ash1 and SWI/SNF complexes.38,39 The specific chromatin modification can vary, but some TrxG complexes function as HMTs and mediate the methylation of H3K4. The SET (Su(var)3–9, enhancer of zeste, Trx) domain catalyzes the methylation of lysine residues and is present in some TrxG proteins, including Drosophila Trx. Many of the TrxG proteins are conserved across species as divergent as yeast and human. The yeast SET domain-containing protein, Set1, was the first protein shown to mediate the methylation of H3K4. Although Set1 is the only H3K4 HMT in yeast, there are many human Set1 homologs including Set1a, Set1b and MLL1–4.40 Of the SET domain-containing MLL proteins, MLL1, the human homolog of Drosophila Trx, is the best characterized due to its important role in hematopoietic cancers.38 The MLL1 multimeric protein complex includes WD40 repeat domain 5 (Wdr5), retinoblastoma binding protein 5 (Rbbp5) and Ash2l. The triad of Wdr5, Rbbp5 and Ash2l form a core complex that is capable of forming a larger complex through interactions with other SET domain proteins such as Set1, MLL2 and MLL3.40 H3K4 methylation, mediated by Ash2l-containing HMT complexes, results in the promotion of cellular differentiation by positively regulating the expression of specific Hox genes during development, including Hoxc819,28 and HoxA9.41 Interestingly, animal models in which Hox genes, such as Hoxa3, have been targeted phenocopy many features of mutant Tbx1 mouse models and human DiGeorge syndrome.42
In this study we show that the T-box transcription factor, Tbx1, interacts both physically and functionally with Ash2l. The multimeric Ash2l HMT complex19,20 catalyzes the methylation of histone lysine residues. H3K4 can undergo mono-, di- or trimethylation.21 The transcriptional start sites of and genomic regions in close proximity to actively transcribed genes are highly associated with trimethyl-H3K4 and dimethyl-H3K4.43,44 Methylation of H3K4 may be a conserved function of some, if not all, T-box family members. Miller et al.17 have shown that multiple T-box proteins, including Tbx1, are able to induce a dimethyl-H3K4 state, although this is not sufficient for transcriptional activation across family members. The hematopoietic T-box transcription factor, T-bet, is both necessary and sufficient to establish the dimethyl-H3K4 state, but not the trimethyl-H3K4 state at the CXCR3 and IFN-γ promoters. The T-box DNA binding domain directly mediates the T-bet HMT complex interaction.17,18 Other T-box proteins, Eomes, Tbx6 and Brachyury, are also able to recruit methyltransferase activity to the CXCR3 promoter, but do not uniformly result in transcriptional activation. This suggests that events other than lysine methylation are required for factor-specific activation of target genes.18 Interestingly, the T-bet physically interacts with Rbbp5,17 which is also a conserved homolog in the Ash2l HMT complex.28,45 Other T-box transcription factors have a role in histone biology and chromatin remodeling. Tbx5 directly interacts with BAF (brahma-related gene 1-associated factor) chromatin remodeling complexes16 and the T-box transcription factor, Tbr-1, directly interacts with CASK (calmodulin-associated serine/threonine kinase) that, by association with the nuclear protein, CINAP (CASK-interacting nucleosome assembly protein), binds histones and facilitates nucleosome assembly.46,47
Ash2l, as part of a structural platform with Wdr5 and Rbbp5,40 in concert with SET domain proteins catalyzes lysine methylation in a context-dependent manner. Knockdown of Ash2l as part of an MLL2 HMT complex results in decreased H3K4 trimethylation of the β-globin gene.32 Ash2l, as part of a different MLL2 HMT complex interacting with the paired-box transcription factor, Pax7, regulates Myf5 by trimethylating H3K4 during myogen-esis.45 In vitro studies of a reconstituted MLL1 complex reveals that the absence of Ash2l results in no change in monomethyl-H3K4, but results in a complete absence of trimethyl-H3K4 and a marked decrease in dimethyl-H3K4. The differential effects on dimethylation and trimethylation may be directly attributable to the PHD domain of Ash2l as the PHD domain has a lower affinity for dimethyl-K4 peptides compared with trimethyl-K4 peptides.30 In vivo, chromatin immunoprecipitation experiments in 293 cells reveal that Ash2l knockdown primarily results in decreased trimethyl-H3K4 (and possibly dimethyl-H3K4) at the HOXC8 locus, but not the HOXA9 locus.48 The absence of an effect on H3K4 methylation at the HOXA9 locus is consistent with prior reports that Ash2l knockdown has no effect on HOXA9 transcription in HeLa cells.41 This in vivo finding has been duplicated in HeLa cells in which Ash2l knockdown resulted in both a global reduction in trimethyl-H3K4 as well as a reduction in trimethyl-H3K4 at the GAPDH, E2F2, and HOXA11 loci.49 Thus, this work supports our findings and the idea that Ash2l-containing HMT complexes are involved in Tbx1 transcription factor-dependent transcriptional regulation.
In addition, we show that, consistent with prior studies, Ash2l28 and Tbx1 expression patterns overlap,34 –36 supporting a co-operative role during embryogenesis. Our analysis of targeted Ash2l mutant mice reveals that Ash2l expression is absolutely essential during early development. We show that when the Ash2l locus is disrupted, no survivors can be identified during mid- or late gestation. Ash2l-null embryos reach blastocyst stage but, ex vivo, these cells are unable to form ES cells. Ash2l is expressed in the developing blastocyst,50 and we speculate that maternally derived Ash2l protein is sufficient for epigenetic regulatory function up to the blastocyst stage, but shortly after this stage, the contribution of embryonic Ash2l is required for further development and required for ES cell maintenance and/or proliferation. This requirement underscores the myriad developmental genes that are likely regulated by the Ash2l-containing HMT complexes.
Supplementary Material
Acknowledgments
We thank the Soriano Laboratory for the gene-trap ES cells. This research was supported by NHLBI K08-HL086633 and the Pediatric Scientist Development Program (NICHD K12-HD00850) to JZS and by NIH 1P01HL075215 to JAE.
Footnotes
Author contributions: All authors participated in the design, interpretation of the studies, analysis of the data and review of the manuscript. LH, FH, DDZ, JY and JZS conducted the experiments; CCT and BDG supplied critical reagents (Ash2l expression constructs); and JAE and JZS wrote the manuscript.
References
- 1.Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–73. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- 2.Stennard FA, Costa MW, Elliott DA, Rankin S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau BG, Zorn AM, Harvey RP. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev Biol. 2003;262:206–24. doi: 10.1016/s0012-1606(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 3.Farin HF, Bussen M, Schmidt MK, Singh MK, Schuster-Gossler K, Kispert A. Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on Groucho corepressors. J Biol Chem. 2007;282:25748–59. doi: 10.1074/jbc.M703724200. [DOI] [PubMed] [Google Scholar]
- 4.Hiroi Y, Kudoh S, Monzen K, Ikeda Y, Yazaki Y, Nagai R, Komuro I. Tbx5 associates with Nkx2–5 and synergistically promotes cardiomyocyte differentiation. Nat Genet. 2001;28:276–80. doi: 10.1038/90123. [DOI] [PubMed] [Google Scholar]
- 5.Farin HF, Mansouri A, Petry M, Kispert A. T-box protein Tbx18 interacts with the paired box protein Pax3 in the development of the paraxial mesoderm. J Biol Chem. 2008;283:25372–80. doi: 10.1074/jbc.M802723200. [DOI] [PubMed] [Google Scholar]
- 6.Conlon FL, Fairclough L, Price BM, Casey ES, Smith JC. Determinants of T box protein specificity. Development. 2001;128:3749–58. doi: 10.1242/dev.128.19.3749. [DOI] [PubMed] [Google Scholar]
- 7.Tada M, Smith JC. T-targets: clues to understanding the functions of T-box proteins. Dev Growth Differ. 2001;43:1–11. doi: 10.1046/j.1440-169x.2001.00556.x. [DOI] [PubMed] [Google Scholar]
- 8.Kispert A, Herrmann BG. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 1993;12:3211–20. doi: 10.1002/j.1460-2075.1993.tb05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kispert A, Hermann BG. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 1993;12:4898–9. doi: 10.1002/j.1460-2075.1993.tb05990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 2002;16:1234–46. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowotschin S, Liao J, Gage PJ, Epstein JA, Campione M, Morrow BE. Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field. Development. 2006;133:1565–73. doi: 10.1242/dev.02309. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi JK, Mileikovskaia M, Koshiba-Takeuchi K, Heidt AB, Mori AD, Arruda EP, Gertsenstein M, Georges R, Davidson L, Mo R, Hui CC, Henkelman RM, Nemer M, Black BL, Nagy A, Bruneau BG. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development. 2005;132:2463–74. doi: 10.1242/dev.01827. [DOI] [PubMed] [Google Scholar]
- 13.Leconte L, Lecoin L, Martin P, Saule S. Pax6 interacts with cVax and Tbx5 to establish the dorsoventral boundary of the developing eye. J Biol Chem. 2004;279:47272–7. doi: 10.1074/jbc.M406624200. [DOI] [PubMed] [Google Scholar]
- 14.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–7. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 15.Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt–Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–21. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 16.Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, Henkelman RM, Wrana JL, Rossant J, Bruneau BG. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–12. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Huang AC, Miazgowicz MM, Brassil MM, Weinmann AS. Coordinated but physically separable interaction with H3K27-demethylase and H3K4-methyltransferase activities are required for T-box protein-mediated activation of developmental gene expression. Genes Dev. 2008;22:2980–93. doi: 10.1101/gad.1689708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis MD, Miller SA, Miazgowicz MM, Beima KM, Weinmann AS. T-bet’s ability to regulate individual target genes requires the conserved T-box domain to recruit histone methyltransferase activity and a separate family member-specific transactivation domain. Mol Cell Biol. 2007;27:8510–21. doi: 10.1128/MCB.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, Kay GF, Hayward NK, Hess JL, Meyerson M. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–97. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 20.Luscher-Firzlaff J, Gawlista I, Vervoorts J, Kapelle K, Braunschweig T, Walsemann G, Rodgarkia-Schamberger C, Schuchlautz H, Dreschers S, Kremmer E, Lilischkis R, Cerni C, Wellmann A, Luscher B. The human trithorax protein hASH2 functions as an oncoprotein. Cancer Res. 2008;68:749–58. doi: 10.1158/0008-5472.CAN-07-3158. [DOI] [PubMed] [Google Scholar]
- 21.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Chen WV, Delrow J, Corrin PD, Frazier JP, Soriano P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat Genet. 2004;36:304–12. doi: 10.1038/ng1306. [DOI] [PubMed] [Google Scholar]
- 23.Chen WV, Soriano P. Gene trap mutagenesis in embryonic stem cells. Methods Enzymol. 2003;365:367–86. [PubMed] [Google Scholar]
- 24.Stoller JZ, Degenhardt KR, Huang L, Zhou DD, Lu MM, Epstein JA. Cre reporter mouse expressing a nuclear localized fusion of GFP and beta-galactosidase reveals new derivatives of Pax3-expressing precursors. Genesis. 2008;46:200–4. doi: 10.1002/dvg.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wawersik S, Epstein JA. Gene expression analysis by in situ hybridization. Radioactive probes. Methods Mol Biol. 2000;137:87–96. doi: 10.1385/1-59259-066-7:87. [DOI] [PubMed] [Google Scholar]
- 26.Gitler AD, Lu MM, Jiang YQ, Epstein JA, Gruber PJ. Molecular markers of cardiac endocardial cushion development. Dev Dyn. 2003;228:643–50. doi: 10.1002/dvdy.10418. [DOI] [PubMed] [Google Scholar]
- 27.Zweier C, Sticht H, Aydin-Yaylagul I, Campbell CE, Rauch A. Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet. 2007;80:510–7. doi: 10.1086/511993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan CC, Sindhu KV, Li S, Nishio H, Stoller JZ, Oishi K, Puttreddy S, Lee TJ, Epstein JA, Walsh MJ, Gelb BD. Transcription factor Ap2delta associates with Ash2l and ALR, a trithorax family histone methyltransferase, to activate Hoxc8 transcription. Proc Natl Acad Sci USA. 2008;105:7472–7. doi: 10.1073/pnas.0711896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikegawa S, Isomura M, Koshizuka Y, Nakamura Y. Cloning and characterization of ASH2L and Ash2l, human and mouse homologs of the Drosophila ash2 gene. Cytogenet Cell Genet. 1999;84:167–72. doi: 10.1159/000015248. [DOI] [PubMed] [Google Scholar]
- 30.Mellor J. It takes a PHD to read the histone code. Cell. 2006;126:22–4. doi: 10.1016/j.cell.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes DA, de Bono B, Trowsdale J. Relationship between SPRY and B30.2 protein domains. Evolution of a component of immune defence? Immunology. 2005;116:411–7. doi: 10.1111/j.1365-2567.2005.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demers C, Chaturvedi CP, Ranish JA, Juban G, Lai P, Morle F, Aebersold R, Dilworth FJ, Groudine M, Brand M. Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus. Mol Cell. 2007;27:573–84. doi: 10.1016/j.molcel.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoller JZ, Epstein JA. Identification of a novel nuclear localization signal in Tbx1 that is deleted in DiGeorge syndrome patients harboring the 1223delC mutation. Hum Mol Genet. 2005;14:885–92. doi: 10.1093/hmg/ddi081. [DOI] [PubMed] [Google Scholar]
- 34.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1–Tbx5, during early mouse development. Dev Dyn. 1996;206:379–90. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 35.Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol. 2001;235:62–73. doi: 10.1006/dbio.2001.0283. [DOI] [PubMed] [Google Scholar]
- 36.Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum Mol Genet. 2002;11:915–22. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- 37.Kennison JA. The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu Rev Genet. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- 38.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–33. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 39.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Crawford BD, Hess JL. MLL core components give the green light to histone methylation. ACS Chem Biol. 2006;1:495–8. doi: 10.1021/cb600367v. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–49. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–9. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- 43.Sims RJ, 3rd, Reinberg D. Histone H3 Lys 4 methylation: caught in a bind? Genes Dev. 2006;20:2779–86. doi: 10.1101/gad.1468206. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, III, Gingeras TR, Schreiber SL, Lander ES. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 45.McKinnell IW, Ishibashi J, Le Grand F, Punch VG, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsueh YP, Wang TF, Yang FC, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- 47.Wang GS, Hong CJ, Yen TY, Huang HY, Ou Y, Huang TN, Jung WG, Kuo TY, Sheng M, Wang TF, Hsueh YP. Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron. 2004;42:113–28. doi: 10.1016/s0896-6273(04)00139-4. [DOI] [PubMed] [Google Scholar]
- 48.Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–9. doi: 10.1038/nsmb1128. [DOI] [PubMed] [Google Scholar]
- 49.Steward MM, Lee JS, O’Donovan A, Wyatt M, Bernstein BE, Shilatifard A. Molecular regulation of H3K4 trimethylation by ASH2L, a shared subunit of MLL complexes. Nat Struct Mol Biol. 2006;13:852–4. doi: 10.1038/nsmb1131. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa T, Piao Y, Zhong J, Matoba R, Carter MG, Wang Y, Goldberg I, Ko MS. High-throughput screen for genes predominantly expressed in the ICM of mouse blastocysts by whole mount in situ hybridization. Gene Expr Patterns. 2006;6:213–24. doi: 10.1016/j.modgep.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.