Abstract
Many species of fish display morphological divergence between individuals feeding on macroinvertebrates associated with littoral habitats (benthic morphotypes) and individuals feeding on zooplankton in the limnetic zone (limnetic morphotypes). Threespine stickleback (Gasterosteus aculeatus L.) have diverged along the benthic-limnetic axis into allopatric morphotypes in thousands of populations and into sympatric species pairs in several lakes. However, only a few well known populations have been studied because identifying additional populations as either benthic or limnetic requires detailed dietary or observational studies. Here we develop a Fisher’s linear discriminant function based on the skull morphology of known benthic and limnetic stickleback populations from the Cook Inlet Basin of Alaska and test the feasibility of using this function to identify other morphologically divergent populations. Benthic and limnetic morphotypes were separable using this technique and of 45 populations classified, three were identified as morphologically extreme (two benthic and one limnetic), nine as moderately divergent (three benthic and six limnetic) and the remaining 33 populations as morphologically intermediate. Classification scores were found to correlate with eye size, the depth profile of lakes, and the presence of invasive northern pike (Esox lucius). This type of classification function provides a means of integrating the complex morphological differences between morphotypes into a single score that reflects the position of a population along the benthic-limnetic axis and can be used to relate that position to other aspects of stickleback biology.
Keywords: benthic morphotypes, Cook Inlet Basin, discriminant analysis, Esox lucius, Gasterosteus aculeatus, geometric morphometrics, limnetic morphotypes, northern pike, trophic morphotypes
INTRODUCTION
Many species display local adaptations that allow for the efficient use of available food resources. In some groups, these adaptations result in two or more specialized morphotypes with distinct differences in body morphology, cranial-facial morphology, feeding behavior, or niche use that are recognizable across many independent populations. In freshwater fish, trophic polymorphisms are exceptionally common; they have been described for 97 species comprising 52 genera and 17 families (Robinson & Wilson, 1994). Of the accounts that deal with lacustrine species, the majority (37 out of 48) include divergence between morphotypes feeding predominantly on macroinvertebrates associated with the substrates of shallow littoral habitats (benthic morphotypes) and morphotypes feeding primarily on zooplankton suspended in the limnetic zones of lakes (limnetic morphotypes; McPhail, 1984; Robinson & Wilson, 1994; Uchii et al., 2007). Differentiation along this benthic-limnetic axis is generally continuous but sometimes results in populations that feed almost exclusively on one prey type or another (McPhail, 1984).
The differences between these two morphotypes consist of numerous traits that influence the foraging efficiency of fish feeding in different habitats and on different types of prey (Schluter & McPhail, 1992; McPhail, 1994; Robinson & Wilson, 1994; Wimberger, 1994). Benthic fish typically have deeper heads and bodies, fewer gill rakers, more robust pharyngeal teeth, and smaller eyes. Benthic fish generally live in shallow areas of lakes where structurally complex habitats dominate and macroinvertebrates associated with the substrate or submerged vegetation are the most abundant prey type (Scheffer, 1998). In this environment, the deep body of the benthic morphotype provides a mechanical advantage that minimizes turning radius and increases maneuverability (Walker, 1997; Gerstner, 1999; Blake, 2004), thus increasing foraging efficiency (Werner, 1977; Ehlinger & Wilson, 1988; Walker, 1997; Svanbäck & Eklöv, 2003). The deep head of the benthic morphotype confers increased suction feeding performance, which is advantageous when feeding on large prey that are often embedded in or clinging to the substrate (Webb, 1984; Norton & Brainerd, 1993; Ferry-Graham, Bolnick & Wainwright, 2002; Carroll et al., 2004). Fewer and more widely spaced gill rakers may improve benthic foraging efficiency by allowing debris taken in during suction to pass out of the buccal cavity through the gills (Werner, 1977; Gross & Anderson, 1984; Amundsen, Bohn & Vaga, 2004). In many taxa, benthic morphotypes display robust pharyngeal plates and molarform teeth which increase foraging efficiency when feeding on hard bodied prey such as snails and Sphaeriid clams that require crushing (Vermeij & Covich, 1978; Meyer, 1989; Wainwright, 2006). Smaller eyes in benthic morphotypes have not been directly linked to increased foraging performance in littoral habitats; however, Hulsey, Mims, & Streelman (2007) provide some evidence that reduced eye size may allow for more developed facial musculature.
Limnetic morphotypes have traits that are well suited for feeding on planktonic prey. Zooplankton often display patchy abundance and distributions within lakes (Omori & Hamner, 1982; del Giorgio & Gasol, 1995) and planktivorous fish often travel relatively large distances to find productive patches (Webb, 1984; Walker, 1997); this selects for the more fusiform body shape of limnetic morphotypes. The numerous, fine gill rakers of limnetic morphotypes likely increase the efficiency of feeding on small planktonic organisms by acting as a sieve to filter them out as water is forced from the buccal cavity (Magnuson & Heitz, 1971; Gibson, 1988; Gerking, 1994; Langeland & Nøst, 1995; but see Wright, O’Brien & Luecke, 1983; Drenner et al., 1987; Gerking, 1994). The relatively large eye size associated with limnetic morphotypes is assumed to be an adaptation to feeding on small prey since visual acuity increases with eye size in fishes (Protasov, 1970; Land & Nilsson, 2002).
Many structures associated with feeding in teleosts are located on the skull (Liem, 1993; Gerking, 1994; Caldecutt & Adams, 1998). Jaw structure and arrangement, shape and location of the opercular bones, orbit morphology, arrangement of the suspensorial bones, and many other osteological features influence the effectiveness of feeding (Norton, 1991; Wainwright, 1996; Huckins, 1997) and vary depending on the feeding mechanism being employed (Gosline, 1973; Liem, 1993; Westneat, 2004). Since fish feeding on benthic prey typically utilize a suction feeding mechanism while fish feeding on planktonic prey typically rely more on ram feeding (Gerking, 1994), many of these features should differ between benthic and limnetic morphotypes. These features are widespread over the skull (Liem, 1993), and thus it is difficult to measure differences in their morphology as a whole. Geometric morphometrics, a landmark based technique of measuring and analyzing shape, allows for the examination of the integrated effects of many features on the skull morphology of fish (Zelditch et al., 2004).
The morphological differences found between benthic and limnetic morphotypes represent local adaptation to ecologically contrasting habitats. Trophic traits are genetically controlled (McKaye et al., 1984; Lavin & McPhail, 1985; Hindar, Ryman & Stahl, 1986; McPhail, 1994; Bernatchez et al., 1996; Robinson & Wilson, 1996; Bernatchez, Chouinard & Lu, 1999; Gíslason et al., 1999; Lu & Bernatchez, 1999; Peichel et al., 2001; Proulx & Magnan, 2004), and often evolve in parallel in populations that are independently derived (Bell & Foster, 1994b; Robinson & Wilson, 1994). Additionally, similar variation is found across many unrelated taxa (Robinson & Wilson, 1994; Taylor, 1999; Robinson & Schluter, 2000). However, some plasticity in these traits exists (Day & McPhail, 1996; Robinson & Wilson, 1996; Peres-Neto & Magnan, 2004; Proulx & Magnan, 2004).
Differentiation along the benthic-limnetic axis is evident both within and between populations and between species (Bell & Andrews, 1997). Because differentiation along the benthic-limnetic axis occurs at levels on both sides of the species “boundary”, it has been implicated as a potential means of speciation in several groups of freshwater fishes such as Arctic char (Malmquist et al., 1992; Savvaitova, 1995; Adams et al., 1998; Jonsson & Skúlason, 2000; Jonsson & Jonsson, 2001; Guiguer et al., 2002; McCarthy et al., 2004), sunfish (Mittelbach, Osenberg & Wainwright, 1992; Robinson & Wilson, 1996; Jastrebski & Robinson, 2004), and numerous other groups (Schluter & McPhail, 1992; Robinson & Wilson, 1994; Bernatchez et al., 1996; Schluter, 1996; Logan et al., 2000; Grey, 2001; Ostbye et al., 2006).
The threespine stickleback (Gasterosteus aculeatus L.; hereafter referred to as ‘stickleback’) is a small fish, widely distributed throughout the boreal and temperate zones of the northern hemisphere. The species inhabits coastal marine, brackish, and an astounding array of freshwater habitats ranging from tiny ephemeral streams in arid desert regions to large Arctic lakes. Among these habitats, populations can be wholly marine, anadromous, or strict residents of fresh water (Bell & Foster, 1994b). A broad geographical and ecological distribution, together with the fragmentation of the gene pool into many thousands of isolated populations in freshwater habitats, has generated immense phenotypic diversity within the species complex. This diversity, coupled with the high incidence of repeated parallel evolution between populations, have made the species complex a model system for the study of ecological speciation (Bell & Foster, 1994b; Hendry et al., 2009). Benthic and limnetic morphotypes are common in threespine stickleback and have evolved repeatedly in parallel both allopatrically in thousands of lakes around the northern hemisphere, and sympatrically in several lakes in coastal British Columbia (McPhail, 1984; Schluter & McPhail, 1992; Baker et al., 2005).
Many aspects of stickleback behavior, morphology, and life-history are thought to be linked to foraging (Bell & Foster, 1994b), and have been intensively studied in the sympatric species pairs of British Columbia (Bentzen & McPhail, 1984; Nagel & Schluter, 1998; Hatfield & Schluter, 1999; Odling-Smee, Boughman & Braithwaite, 2008) and in a few well known allopatric populations (Walker, 1997; Caldecutt & Adams, 1998; Baker et al., 2005; Purnell et al., 2006; Travis, 2007; Snowberg & Bolnick, 2008). A relatively small number of populations have been used extensively in studies of stickleback trophic morphology because identifying additional populations as either benthic or limnetic requires detailed dietary or observational studies for all populations being considered. In this study we develop a classification function based on the skull morphology of previously confirmed benthic and limnetic stickleback populations from the Cook Inlet Basin of Alaska and test the feasibility of using this function to identify other morphologically divergent populations. This type of classification function provides a means of integrating the many complex morphological differences between morphotypes into a single score that reflects the relative position of a population along the benthic-limnetic axis. We also demonstrate how the “benthic-limnetic” scores produced by such a classification function can be used to test hypotheses regarding the relationship between trophic morphotype and lake habitat.
METHODS
Fish Collection and Preservation
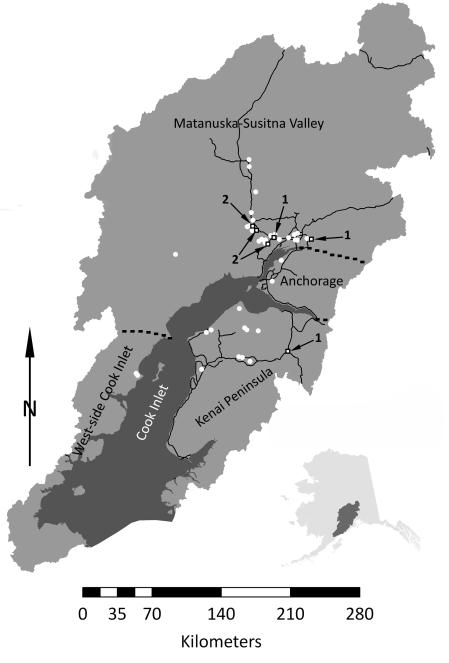
Threespine stickleback were collected from 45 freshwater lakes throughout the Cook Inlet Basin (Fig. 1; Table 1) between 2002 and 2009 using unbaited 1/8″ or 1/4″ wire mesh minnow traps set from shore. Trapped stickleback were sacrificed with an overdose of MS-222 anesthetic, rinsed in lake water, and fixed in 10% formalin buffered with dolomite. After 2 to 8 weeks fish were removed from formalin, rinsed in a running water bath, and preserved in 70% ethanol. All fish were then bleached in a 0.05% hydrogen peroxide solution to remove pigmentation and stained with 1% alizarin red S to facilitate the analysis of bony structures.
Figure 1.
Map of the Cook Inlet Basin showing major geographic regions (separated by dashed boarders) and the locations of 45 stickleback populations. Black rimmed squares and arrows indicate populations used in the training sets with 1 signifying populations used in the benthic training group and 2 signifying populations used in the limnetic training group. White circles are populations of uncertain morphotype. North arrow indicates true north.
Table 1.
Locations, putative morphotypes based on previous dietary analysis, and pike/stocking statuses of sampled lakes. Blank morphotype values indicate populations with no published accounts of diet and a question mark following morphotype indicates conflicting or limited evidence. P indicates pike presence and S indicates stocked lakes (data obtained from Alaska Department of Fish and Game lake files).
| Lake Name | Region | Latitude | Longitude | Morphotype | Pike/Stocked |
|---|---|---|---|---|---|
| AK062 (unnamed) | Kenai Peninsula | 60.794 | −150.330 | ||
| Bear | West-side Cook Inlet | 60.420 | −152.378 | ||
| Bear Paw | Mat-Su Valley | 61.614 | −149.756 S | S | |
| Beaver House | Mat-Su Valley | 61.574 | −149.863 | Limnetic? e, h, i, j |
S |
| Bird | Mat-Su Valley | 60.972 | −150.409 | ||
| Blanket | Mat-Su Valley | 61.591 | −149.874 | ||
| Boot | Mat-Su Valley | 61.717 | −150.117 | S | |
| Caswell | Mat-Su Valley | 62.017 | −149.967 | P, S | |
| Cheney | Anchorage | 61.203 | −149.758 | P, S | |
| Christiansen | Mat-Su Valley | 62.315 | −150.063 | S | |
| Coal Creek | Mat-Su Valley | 61.489 | −151.568 | ||
| Corcoran | Mat-Su Valley | 61.574 | −149.688 | Benthic f, g, h, i, j |
|
| Crane | Kenai Peninsula | 60.792 | −150.955 | ||
| Crystal | Mat-Su Valley | 61.710 | −150.100 | P, S | |
| Echo | Kenai Peninsula | 60.438 | −151.161 | S | |
| Falk | Mat-Su Valley | 61.566 | −149.049 | ||
| Finger | Mat-Su Valley | 61.606 | −149.279 | P,S | |
| Fish | Mat-Su Valley | 62.251 | −150.065 | P | |
| Grouse | Kenai Peninsula | 60.776 | −150.287 | ||
| Hidden | Kenai Peninsula | 60.486 | −150.263 | ||
| Horseshoe | Mat-Su Valley | 61.572 | −149.928 | P | |
| Kashwitna | Mat-Su Valley | 61.833 | −150.076 | Limnetic? e, f, g, h, j |
P |
| Loberg | Mat-Su Valley | 61.560 | −149.258 | Limnetic? h | S |
| Long | Mat-Su Valley | 61.578 | −149.764 | Limnetic e, f, g, h, i, j |
P, S |
| Lynda | Mat-Su Valley | 61.571 | −149.836 | Limnetic? e, f, g, h, i, j |
|
| Lynne | Mat-Su Valley | 61.712 | −150.039 | Limnetic b, i | S |
| Matanuska | Mat-Su Valley | 61.556 | −149.229 | S | |
| Milo | Mat-Su Valley | 61.670 | −150.092 | P | |
| Mud | Mat-Su Valley | 61.563 | −148.949 | Benthic a, c, e, f, g, h, i |
|
| Peterson | Kenai Peninsula | 60.525 | −150.396 | P | |
| Psalm | Anchorage | 61.383 | −149.563 | ||
| Rocky | Mat-Su Valley | 61.556 | −149.825 | P, S | |
| South Rolly | Mat-Su Valley | 61.669 | −150.126 | Limnetic d, e | P, S |
| Stormy | Kenai Peninsula | 60.771 | −151.047 | P | |
| Tern | Kenai Peninsula | 60.533 | −149.550 | Benthic c, d, h | |
| Trapper Joe | Kenai Peninsula | 60.760 | −150.079 | ||
| Vera | Mat-Su Valley | 61.713 | −150.138 | S | |
| Visnaw | Mat-Su Valley | 61.619 | −149.679 | Limnetic? a, h, j |
S |
| Wadell | West-side Cook Inlet | 60.408 | −152.356 | ||
| Walby | Mat-Su Valley | 61.619 | −149.211 | S | |
| Wallace | Mat-Su Valley | 61.574 | −149.572 | P | |
| Wasilla | Mat-Su Valley | 61.586 | −149.396 | P | |
| Watson | Kenai Peninsula | 60.539 | −150.465 | ||
| West Beaver | Mat-Su Valley | 61.586 | −149.844 | S | |
| Willow | Mat-Su Valley | 61.744 | −150.057 | Benthic? h, i, j | S |
M. Travis, unpublished data,
P. Park, unpublished data,
Morphological Analysis
Morphological analyses were completed using approximately 40 (minimum of 36) fish from each population. Only fish measuring > 32 mm standard length (SL; anterior tip of premaxilla to posterior border of hyperal plate) were included because most stickleback have developed adult body shape by this size (Walker, 1993). Photographs of the left lateral aspect of the head of each fish were taken using a digital camera mounted on a fluorescent dissecting scope (Leica MZFLIII microscope with a Leica DFC340FX camera). Photographs were adjusted for brightness and contrast. In order to cover all features of the head, up to four photographs were taken; multiple photographs of a specimen were merged into a single composite image using a reposition only function in Adobe Photoshop (version 10.0.1).
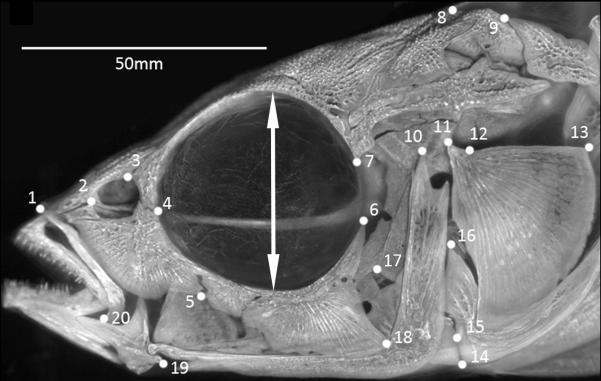
Twenty biologically homologous anatomical landmarks that cover the form of the stickleback skull (Fig. 2; Cresko & Lum, unpublished) were digitized using the tpsDIG2 program (version 2.12; Rohlf, 2008a). One landmark was not included in analyses due to inconsistent visibility and repeatability. The remaining 19 landmarks were aligned using the generalized Procrustes procedure and the resulting configurations used to obtain partial warps in the tpsRelw software package (version 1.46; Rohlf, 2008b). In addition to geometric morphometric data, vertical orbit diameter was also measured from the digital images using the measuring tool in tpsDig2.
Figure 2.
Locations of anatomical landmarks used to study skull morphology in threespine stickleback. Note landmark 17 has been excluded. Double sided arrow indicates vertical orbit diameter measurement.
Development of Classification
Partial warp scores were used in the R statistical package (version 2.9.1; www.r-project.org) to develop a Fisher’s linear discriminant function (FLDA) to classify specimens along the axis that best separates benthic and limnetic stickleback. A FLDA was used rather than a standard linear discriminant analysis (LDA) because it does not assume normally distributed classes or equal class covariances as LDA does. When the assumptions of LDA are met the two methods are equivalent (Hair et al., 2009). When both FLDA and LDA were compared with these data the results were qualitatively the same (Willacker, 2009).
The FLDA was developed using the variance-covariance matrix of partial warp scores from three known “extreme” benthic populations (Corcoran, Mud, and Tern Lakes) and three known “extreme” limnetic populations (Long, Lynne, and South Rolly Lakes) as a training set for establishing the separation of benthic and limnetic morphotypes. The populations used to develop this discriminant function were chosen based on studies of stickleback diet and feeding behavior that have shown them to be the most extreme of each morphotype known in the Cook Inlet Basin (Walker, 1997; Caldecutt & Adams, 1998; Foster, Scott & Cresko, 1998; Purnell et al., 2006; Purnell et al., 2007; Travis, 2007; Karve, von Hippel & Bell, 2008; von Hippel, 2008; M. Travis, unpublished data; P. Park, unpublished data). Following FLDA, a Hotelling’s T2 test was performed in R to confirm that the differentiation between the groups was significant. Cross validation using a leave-one-out procedure that drew repeated (10 000 iterations) random samples (n = 100) from each training group was used to predict the group to which each individual in the training set belonged and assess the accuracy of classifications within the training set. A leave-one-out approach was used to cross-validate the training set because the division of the dataset into separate development and validation samples would have resulted in a loss of statistical power. This method does not compromise the validity of the cross-validation (Hair et al., 2009). Following cross-validation, the FLDA classification function was used to calculate discriminant scores for each individual from the populations of uncertain morphotype. These individual discriminant scores were then used to calculate population mean discriminant scores (PMDS). The distributions of individual discriminant scores across populations were normally distributed, as were the PMDS (Willacker, 2009). The PMDS were adjusted so that the inter-population mean was zero. The divergences between PMDS and the inter-population mean were then used to classify populations as extreme benthic/limnetic adapted (PMDS >2 SD from inter-population mean), moderately benthic/limnetic adapted (PMDS 1-2 SD from inter-population mean), or intermediate (PMDS <1 SD from inter-population mean).
Statistical Analysis
Data on 13 lake habitat variables (Table 2), stocking history and the presence of invasive northern pike (Esox lucius L.; Table 1) were included in habitat analyses. Statistical analyses were carried out using the R statistical package (version 2.9.1). The PMDS was used in all statistical tests utilizing discriminant scores.
Table 2.
Habitat variables tested and their associated sample size, mean, and standard deviation. Water chemistry data were obtained from a dataset compiled by stickleback researchers beginning in 1989 (Bell & Ortí, 1994; Bell et al., 1993; Jones et al., 2003; Walker, 1997) and from the Alaska Lakes Assessment conducted by the U.S. Environmental Protection Agency in 2008. Physical habitat data were obtained from Alaska Department of Fish and Game lake files. See Willacker (2009) for details on the compilation of the dataset.
| Parameter | n | SD | |
|---|---|---|---|
| Alkalinity | 32 | 43.61 | 33.96 |
| Ammonia (NH3) | 28 | 7.46 | 12.64 |
| Calcium | 34 | 11.77 | 9.20 |
| Chlorophyll A | 33 | 1.93 | 4.42 |
| Color | 34 | 14.53 | 8.75 |
| Lake area | 45 | 154.35 | 255.29 |
| Maximum depth | 31 | 34.83 | 29.15 |
| Mean depth | 23 | 13.12 | 8.28 |
| Percent of lake <1.52m | 17 | 38.53 | 17.61 |
| Percent of lake <3.05m | 23 | 55.50 | 23.41 |
| Total nitrogen (TN) | 30 | 271.97 | 91.99 |
| Total phosphorus (TP) | 34 | 8.34 | 3.64 |
| Turbidity | 35 | 0.97 | 0.45 |
Linearly measured morphometric traits are impacted by the size of the fish. Therefore, the mean orbit diameter of each population was regressed against its mean centroid size and the residuals were used in subsequent analyses. The association between PMDS and the residuals of orbit diameter was tested using Pearson’s product-moment correlation.
Principle components analysis was used to reduce the dimensionality of the lake habitat variables and identify groups of variables that best characterized lake habitat differences. The significance of loadings on each principle component (PC) was assessed using the power-level method outlined in Hair et al. (2009). Using this method, only loadings of ± 0.80 or greater were considered significant. The relationships between principle components with significant loadings and PMDS were assessed using Pearson’s product-moment correlation. Complete habitat data were only available for 17 of the 45 lakes and therefore the sample size in these correlations was small. Because of this, the correlations were also performed using the non-parametric Spearman rank correlation. Results were qualitatively the same and only the parametric correlations are reported.
Two t-tests were conducted to test for differences in PMDS due to the stocking of salmonids and the invasion of northern pike.
RESULTS
Classification Function
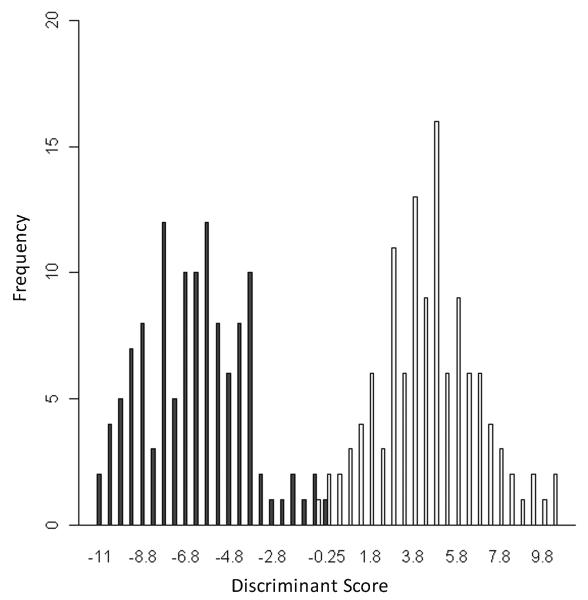
The discriminant scores for individuals in the training set showed a bimodal distribution (Fig. 3). Individual scores for fish from the benthic training group (n = 120 fish from three populations) ranged from −10.83 to −0.09 with a mode of −5.98, while individual scores for the limnetic training group (n = 120 fish from three populations) ranged from −0.69 to 10.28 with a mode of 4.23. Less than 2% of individuals fell into the range of overlap.
Figure 3.
Histogram of discriminant scores for benthic (dark bars) and limnetic (light bars) training groups of threespine stickleback from the Cook Inlet Basin, Alaska.
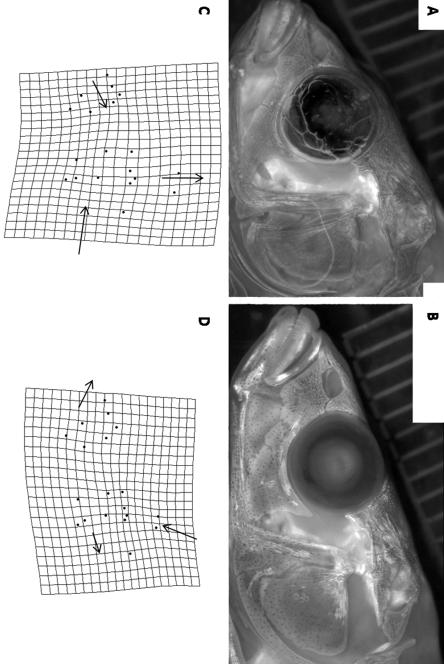
Morphological differences between the two training groups were readily apparent in images of the fish (Fig. 4 A and B). Relative warps analysis and thin-plate spline deformations illustrate that fish with lower discriminant scores (more benthic) typically have shorter, deeper skulls and a shorter snout while fish with higher discriminant scores have shallow, elongate skulls with longer snouts (Fig. 4 C and D). The results of a Hotelling’s T2 test confirmed that the apparent differences in the two training groups were significant (T2 = 32.30; F34, 203 = 1.49; p <0.0001). Cross-validation of the training set showed that 97.1% of all individuals were classified into the correct morphotype when repeated random samples were drawn.
Figure 4.
Images of fish that most closely correspond to the A) benthic and B) limnetic mean skull morphologies and the distortion of the thin-plate spline that accompanies each configuration (C and D). Note scale bars (millimeters) embedded in images.
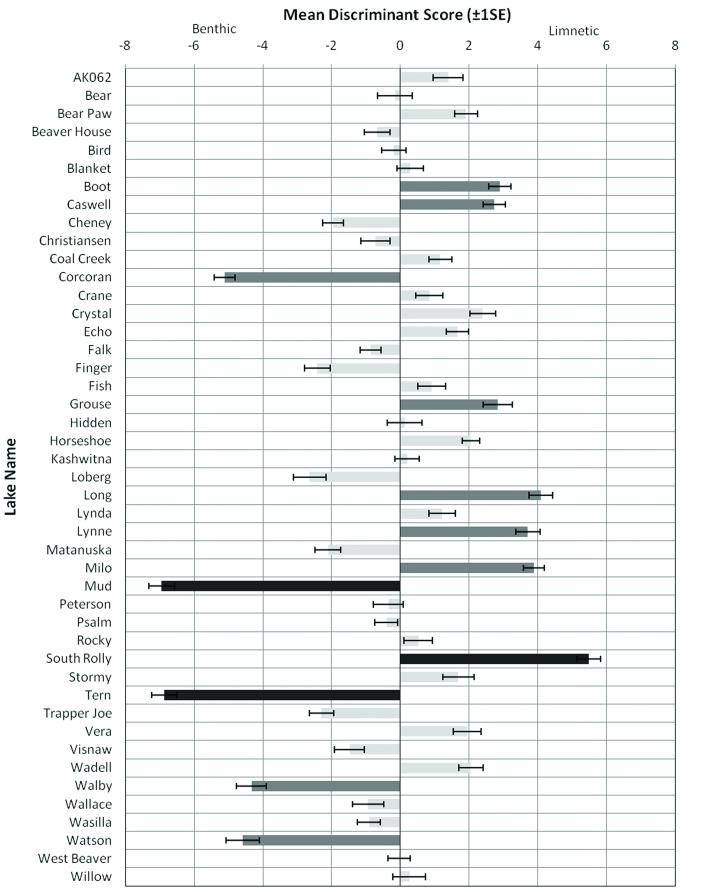
When the classification function was applied to individuals from populations of uncertain morphotypes, individual scores ranged from −12.28 to 11.35 (mode = 0.62). Extreme populations had mean discriminant scores greater than ± 5.45 (2+ SD from the inter-population mean), moderately adapted populations had scores between ± 2.73 and ± 5.45 (1-2 SD), and intermediate populations were less than ± 2.73 (<1 SD; Willacker, 2009). Of the 45 populations examined, three were identified as morphologically extreme (Fig. 5): Mud and Tern Lakes were classified as extreme benthic populations (PMDS = −6.95 and −6.87 respectively), while South Rolly Lake was classified as an extreme limnetic (PMDS = 5.48). Additionally, Corcoran, Walby and Watson lakes were also classified as moderately adapted to benthic habitat (PMDS = −5.11, −4.33, and −4.59 respectively), while Boot, Caswell, Grouse, Long, Lynne, and Milo lakes were classified as moderately adapted to limnetic conditions (PMDS = 2.89, 2.73, 2.84, 4.08, 3.71, and 3.88 respectively). The remaining 33 populations were classified as morphologically intermediate. All six populations used in the training set (Mud, Tern, Corcoran, Long, Lynne, South Rolly) had PMDS >1SD from the inter-population mean (Fig. 5).
Figure 5.
Population mean discriminant scores for 45 threespine stickleback populations from the Cook Inlet Basin, Alaska. Error bars indicate standard error within each population. The color of the bar indicates the divergence of each population mean discriminant score from the mean discriminant score across all populations; dark grey = >2SD, medium grey = 1SD-2SD, and light grey =<1SD.
Orbit Diameter
Population mean discriminant score was significantly correlated with residual orbit diameter; fish from populations with a more benthic PMDS had a significantly larger orbit diameter than fish from populations with a more limnetic score (r = −0.47, df = 43, P = 0.001).
Habitat Covariates
Of the 13 habitat variables measured, five yielded significant loadings on a principle component. The percent of the lake less than 1.52 m deep, the percent of the lake less than 3.04 m deep, and the mean lake depth all had significant loadings on PC1 (loadings = 0.93, 0.91, and −0.83 respectively), while alkalinity and calcium concentration had a significant loading on PC2 (loadings = 0.86, and 0.80 respectively). Principal component 1 was found to be significantly correlated with PMDS (r = −0.70, P = 0.007); however, PC2 was not (r = −0.50, P =0.084).
Predatory Fish
In lakes where northern pike had been recorded, stickleback had a significantly higher discriminant score (PMDS = 1.16, n = 15) than in lakes where pike were not recorded (PMDS = −0.57, n = 30, t = −2.08, df = 43, P = 0.022). No significant difference in PMDS was found between lakes stocked with salmonids (PMDS = 0.54, n = 19) and unstocked lakes (PMDS = −0.24, n = 26, t = −1.75, df = 43, P = 0.087).
DISCUSSION
Results of discriminant analysis indicate differences in the skull morphologies of known benthic and limnetic stickleback populations, and that these differences accurately separate the two groups. Stickleback with a lower discriminant score typically had shorter, deeper heads as has been reported previously for benthically feeding fish and likewise the skull morphology of fish with higher discriminant scores was characteristic of that expected for a limnetically feeding fish (Bentzen & McPhail, 1984; Bentzen, Ridgway & McPhail, 1984; McPhail, 1984; Bell & Foster, 1994a; Caldecutt & Adams, 1998). Cross-validation demonstrated that the separation of these two groups was independent of the individuals included in the training set. These results show that skull morphology is an accurate and effective means of differentiating between benthic and limnetic morphotypes of threespine stickleback.
We are unable test the applicability or comparability of this classification function in other contexts (e.g., with stickleback benthic-limnetic species pairs from British Columbia) due to the lack of comparable morphometric data. While the classification function presented here is specific to benthic-limnetic divergence in Cook Inlet populations of threespine stickleback, similar functions could be constructed for other systems and compared between systems. However, it should be noted that the effectiveness of this technique is maximized by using training sets specific to the region and traits of interest.
Comparisons of eye size between populations show that fish from populations with a lower (more benthic) PMDS have significantly larger eyes than populations with a higher (more limnetic) score. These results are contrary to what has been reported for threespine stickleback (e.g., McPhail, 1984; Schluter, 1993; Walker, 1997), and many other species of fish (e.g., Robinson & Wilson, 1994; Snorrason et al., 1994; Taylor, 1999) that show morphological variation along the benthic-limnetic axis. However, larger eyes have been observed in benthic morphotypes of some fish species (e.g., cichlids: Kassam et al., 2003a; Kassam et al., 2003b) and in a polymorphic population of threespine stickleback in Benka Lake, Alaska (W. Cresko, personal communication).
Larger eye size is associated with the need for increased visual sensitivity due to reduced light availability and/or the need for high spatial resolution (Walls, 1963; Archer, 1999; Motani et al., 1999; Land & Nilsson, 2002; Thomas et al., 2006). Larger eye size is generally associated with limnetic morphotypes because the prey are small. In contrast, large eyes are assumed to be unnecessary for benthic feeding because benthic prey are relatively large. However, not all benthic prey are large; in some lakes the benthic macroinvertebrate community is dominated by small water mites, Chironomidae, or oligochaets that are the size of many zooplankton (Hanson, Prepas & Mackay, 1989; Rasmussen, 1993). Additionally, the littoral environment is typically more complex and benthic prey are better concealed (Ivlev, Magill & Scott, 1964; Diggins, Summerfelt & Mnich, 1979; Crowder & Cooper, 1982). Thus, high visual acuity may be required to identify and target prey amongst the structural components (Kassam et al., 2003b). Finally, littoral habitats often have reduced light availability due to increased turbidity, dissolved compounds (staining), and/or shading (Utne, 1997; Steedman, Kushneriuk & France, 2001; Wetzel, 2001), necessitating larger eyes to retain adequate visual sensitivity. For these reasons larger eyes could be advantageous for benthic fish under some conditions.
Of the 13 habitat lake variables examined, five displayed significant loadings onto a PC. Principle component 1 included three significant variables relating to the depth profile and accounted for 34% of the variation between lakes. Discriminant score was positively correlated with PC1, indicating that shallow lakes (higher percentage of lake area less than 1.52 and 3.05 m deep, and a shallower mean depth) had lower discriminant scores and thus more benthic morphologies. This result was expected since shallow lakes have a greater relative littoral area and thus more benthic habitat than deeper lakes. In shallow lakes, benthic prey would almost certainly dominate, and zooplankton would most likely comprise only a small portion of the available prey.
Principle component 2 included two significant variables (alkalinity and calcium concentration), both representing the prevalence of dissolved cations in the water, and accounted for an additional 20% of the variation between lakes. Population mean discriminant score was not significantly correlated with PC2, suggesting that while these variables represent important habitat differences between lakes, they are not important determinants of the observed morphological variation.
While salmonids did not have a significant impact on stickleback skull morphology, the presence of invasive northern pike was correlated with higher (more limnetic) PMDS. The difference in the impact of these two predators likely results from their different foraging behaviors. Salmonids typically forage in both littoral and limnetic habitats (Sandlund et al., 1987; L’Abee-Lund, Langeland & Saegrov, 1992; Vander Zanden & Vadeboncoeur, 2002) while pike are specialized to forage in littoral environments (Chapman & Mackay, 1984; Vollestad, Skurdal & Qvenild, 1986). When littoral predators, such as pike, dominate a lake system, prey fish may be forced out of the littoral zone and into pelagic refugia (Grimm & Backx, 1990; Jacobsen & Perrow, 1998; Burks et al., 2002). Under these conditions, benthic feeding in the littoral zone carries the cost of increased predation risk and thus there may be increased selection for effective limnetic foraging. Therefore, the increased prevalence of stickleback with limnetic skull morphology in lakes known to contain invasive pike is likely the result of a predator driven habitat shift. It is noteworthy that pike in Cook Inlet Basin lakes tend to drive stickleback populations to extinction except in those lakes with limnetic refugia (Haught, 2009).
This study demonstrates the ability to distinguish benthic and limnetic morphotypes of threespine stickleback using a classification function based on skull morphology. Such a classification function can be used to identify populations that are divergent along the benthic-limnetic axis for studies of the influence of trophic morphology on genetics, life history, reproductive behavior, physiology, or other traits. This classification could be improved upon by the inclusion of other variables such as gill-raker and dental morphology, diet, and stable isotope ratios of tissues. Classification functions such as the one presented here allow for the reduction of the many, often complex, differences between morphotypes into a single score that reflects the relative position of a population along the benthic-limnetic axis, and are thus a powerful tool for examining the role trophic specialization plays in the divergence of species. As illustrated in this paper, these classification functions can also be used to gain insights into the ways in which populations evolve in response to ecological conditions, such as lake morphometry and exotic predators.
ACKNOWLEDGMENTS
The authors would like to thank W. Cresko and K. Lum for providing the skull morphology landmark set and three anonymous reviewers for their comments on the manuscript. D. Adams, W. Aguirre and J. Rohlf provided technical assistance with geometric morphometrics. M.A. Bell provided specimens from Walby and Beaver House Lakes. All other stickleback were collected under Alaska Department of Fish and Game permit numbers SF-2001-062, SF-2002-002, SF-2003-019, SF-2004-012, SF-2005-020, SF-2006-017, SF-2007-026, SF-2008-059 and SF-2009-016. All work was approved by the UAA IACUC. Funding was provided by National Science Foundation grant DEB 0320076 and University of Alaska Anchorage Faculty Development Grants to FAvH, and a National Science Foundation Research Experiences for Undergraduates site award to UAA. Additional support came from the Alaska INBRE program, grant number 5P20RR016466 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
LITERATURE CITED
- Adams CE, Fraser D, Huntingford FA, Greer RB, Askew CM, Walker AF. Trophic polymorphism amongst Arctic charr from Loch Rannoch, Scotland. Journal of Fish Biology. 1998;52:1259–1271. [Google Scholar]
- Amundsen PA, Bohn T, Vaga GH. Gill raker morphology and feeding ecology of two sympatric morphs of European whitefish (Coregonus lavaretus) Annales Zoologici Fennici. 2004;41:291–300. [Google Scholar]
- Archer SN. Adaptive Mechanisms in the Ecology of Vision. Kluwer Academic Publisers; Boston, MA: 1999. [Google Scholar]
- Baker JA, Cresko WA, Foster SA, Heins DC. Life-history differentiation of benthic and limnetic ecotypes in a polytypic population of threespine stickleback (Gasterosteus aculeatus) Evolutionary Ecology Research. 2005;7:121–131. [Google Scholar]
- Bell MA, Andrews CA. Evolutionary consequences of postglacial colonization of freshwater by primatively anadromous fishes. In: Streit B, Stadler T, Lively CM, editors. Evolutionary Ecology of Freshwater Animals. Birkhauser Verlag; Boston, MA: 1997. pp. 323–363. [Google Scholar]
- Bell MA, Foster SA. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; New York, NY: 1994a. [Google Scholar]
- Bell MA, Foster SA. Introduction to the evoluionary biology of the threespine stickleback. In: Bell MAaF, S. A., editor. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford: 1994b. pp. 1–27. [Google Scholar]
- Bell MA, Ortí G. Pelvic reduction in theespine stickleback from Cook Inlet lakes: geographical distribution and intrapopulation variation. Copeia. 1994;2:314–325. [Google Scholar]
- Bell MA, Ortí G, Walker JA, Koenings JP. Evolution of pelvic reduction in threespine stickleback fish: a test of competing hypotheses. Evolution. 1993;47:906–914. doi: 10.1111/j.1558-5646.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Bentzen P, McPhail JD. Ecology and evolution of sympatric sticklebabcks (Gasterosteus): specialiation for alternative trophic niches in the Enos Lake species pair. Canadian Journal of Zoology. 1984;62:2280–2286. [Google Scholar]
- Bentzen P, Ridgway MS, McPhail JD. Ecology and evolution of sympatric sticklebacks (Gasterosteus): spatial segregation and seasonal habitat shifts in the Enos Lake species pair. Canadian Journal of Zoology. 1984;62:2436–2439. [Google Scholar]
- Bernatchez L, Chouinard A, Lu G. Integrating molecular genetics and ecology in studies of adaptive radiation: whitefish, Coregonus sp., as a case study. Biological Journal of the Linnean Society. 1999;68:173–194. [Google Scholar]
- Bernatchez L, Vuorinen JA, Bodaly RA, Dodson JJ. Genetic evidence for reproductive isolation and multiple origins of sympatric trophic ecotypes of whitefish (Coregonus) Evolution. 1996;50:624–635. doi: 10.1111/j.1558-5646.1996.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Blake RW. Fish functional design and swimming performance. The Journal of Fish Biology. 2004;65:1193–1222. [Google Scholar]
- Burks RL, Lodge DM, Jeppesen E, Lauridsen TL. Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshwater Biology. 2002;47:343–365. [Google Scholar]
- Caldecutt WJ, Adams DC. Morphometrics of trophic osteology in the threespine stickleback, Gasterosteus aculeatus. Copeia. 1998:827–838. [Google Scholar]
- Carroll AM, Wainwright PC, Huskey SH, Collar DC, Turingan RG. Morphology predicts suction feeding performance in Centrarchid fishes. Journal of Experimental Biology. 2004;207:3873–3881. doi: 10.1242/jeb.01227. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Mackay WC. Direct observation of habitat utilization by northern pike. Copeia. 1984:255–258. 1984. [Google Scholar]
- Crowder LB, Cooper WE. Habitat structural complexity and the interaction between bluegills and their prey. Ecology. 1982;63:1802–1813. [Google Scholar]
- Day T, McPhail JD. The effect of behavioural and morphological plasticity on foraging efficiency in the threespine stickleback (Gasterosteus sp) Oecologia. 1996;108:380–388. doi: 10.1007/BF00334665. [DOI] [PubMed] [Google Scholar]
- del Giorgio PA, Gasol JM. Biomass distribution in freshwater plankton communities. The American Naturalist. 1995;146:135–152. [Google Scholar]
- Diggins MR, Summerfelt RC, Mnich MA. Altered feeding electivity of the bluegill from increased prey accessibility following macrophyte removal. Proceedings of the Oklahoma Academy of Science. 1979;59:4–11. [Google Scholar]
- Drenner R, Hambright KD, Vinyard GL, Gophen M. Particle ingestion by Tilapia galilaea is not affected by removal of gill rakers and microbranchiospines. Transactions of the American Fisheries Society. 1987;116:272–276. [Google Scholar]
- Ehlinger TJ, Wilson DS. Complex foraging polymorphism in bluegill sunfish. Proceedings of the National Academy os Sciences. 1988;85:1878–1882. doi: 10.1073/pnas.85.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry-Graham LA, Bolnick DI, Wainwright PC. Using functional morphology to examine the ecology and evolution of specialization. Integrative and Comparative Biology. 2002;42:265–277. doi: 10.1093/icb/42.2.265. [DOI] [PubMed] [Google Scholar]
- Foster SA, Scott RJ, Cresko WA. Nested biological variation and speciation. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1998;353:207–218. [Google Scholar]
- Gerking SD. Feeding Ecology of Fish. Academic Press; San Diego, CA: 1994. [Google Scholar]
- Gerstner CL. Maneuverability of four species of coral-reef fish that differ in body and pectoral-fin morphology. Canadian Journal of Zoology. 1999;77:1102–1110. [Google Scholar]
- Gibson RN. Development, morphometry and particle retention capability of the gill rakers in the herring, Clupea harengus L. Journal of Fish Biology. 1988;32:949–962. [Google Scholar]
- Gíslason D, Ferguson MM, Skúlason S, Snorrason SS. Rapid and coupled phenotypic and genetic divergence in Icelandic Arctic char (Salvinus alpinus) Canadian Journal of Fisheries and Aquatic Sciences. 1999;56:2229–2234. [Google Scholar]
- Gosline WA. Functional Morphology and Classification of Teleostean Fishes. University of Hawaii Press; Honolulu, HI: 1973. [Google Scholar]
- Grey J. Ontogeny and dietary specialisation in brown trout (Salmo trutta L.) from Loch Ness, Scotland, examined using stable isotopes of carbon and nitrogen. Ecology of Freshwater Fish. 2001;10:168–176. [Google Scholar]
- Grimm MP, Backx J. The restoration of shallow eutrophic lakes, and the role of northern pike, aquatic vegetation and nutrient concentration. Hydrobiologia. 1990;200:557–566. [Google Scholar]
- Gross HP, Anderson JM. Geographic variation in the gillrakers and diet of european threespine sticklebacks, Gasterosteus aculeatus. Copeia. 1984;1984:87–97. [Google Scholar]
- Guiguer K, Reist JD, Power M, Babaluk JA. Using stable isotopes to confirm the trophic ecology of Arctic charr morphotypes from Lake Hazen, Nunavut, Canada. Journal of Fish Biology. 2002;60:348–362. [Google Scholar]
- Hair JF, Black WC, Anderson RE, Babin BJ. Multivariate Data Analysis. Prentice Hall; Upper Saddle River, NJ: 2009. [Google Scholar]
- Hanson JM, Prepas EE, Mackay WC. Size distribution of the macroinvertebrate community in a freshwater lake. Canadian Journal of Fisheries and Aquatic Sciences. 1989;46:1510–1519. [Google Scholar]
- Hatfield T, Schluter D. Ecological speciation in sticklebacks: environment-dependent hybrid fitness. Evolution. 1999;53:866–873. doi: 10.1111/j.1558-5646.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Haught SB. Unpublished Master os Science. University of Alaska Anchorage; 2009. Threespine stickleback extirpation and evolution in the face of northern pike invasion. [Google Scholar]
- Hendry AP, Bolnick DI, Berner D, Peichel CL. Along the speciation continuum in stickleback. Journal of Fish Biology. 2009;75:2000–2036. doi: 10.1111/j.1095-8649.2009.02419.x. [DOI] [PubMed] [Google Scholar]
- Hindar K, Ryman N, Stahl G. Genetic differentiation among local populations and morphotypes of Arctic charr, Salvelinus alpinus. Biological Journal of the Linnean Society. 1986;27:269–285. [Google Scholar]
- Huckins CJF. Functional linkages among morphology, feeding performance, diet, and competitive ability in molluscivorous sunfish. Ecology. 1997;78:2401–2414. [Google Scholar]
- Hulsey CD, Mims MC, Streelman JT. Do constructional constraints influence cichlid craniofacial diversification? Proceedings of the Royal Society B. 2007;274:1867–1875. doi: 10.1098/rspb.2007.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivlev VS, Magill DW, Scott D. Experimental Ecology of the Feeding of Fishes. Yale University Press; New Haven, CT: 1964. [Google Scholar]
- Jacobsen L, Perrow MR. Predation risk from piscivorous fish influencing the diel use of macrophytes by planktivorous fish in experimental ponds. Ecology of Freshwater Fish. 1998;7:78–86. [Google Scholar]
- Jastrebski CJ, Robinson BW. Natural selection and the evolution of replicated trophic polymorphisms in pumpkinseed sunfish (Lepomis gibbosus) Evolutionary Ecology Research. 2004;6:285–305. [Google Scholar]
- Jones JR, Bell MA, Baker JA, Koenings JP. General limnology of lakes near Cook Inlet, southcentral Alaska. Lake and Reservoir Management. 2003;19:141–149. [Google Scholar]
- Jonsson B, Jonsson N. Polymorphism and speciation in Arctic charr. Journal of Fish Biology. 2001;58:605–638. [Google Scholar]
- Jonsson B, Skúlason S. Polymorphic segregation in Arctic charr Salvelinus alpinus (L.) from Vatnshlidarvatn, a shallow Icelandic lake. Biological Journal of the Linnean Society. 2000;69:55–74. [Google Scholar]
- Karve AD, von Hippel FA, Bell MA. Isolation between sympatric anadromous and resident threespine stickleback species in Mud Lake, Alaska. Environmental Biology of Fishes. 2008;81:287–296. [Google Scholar]
- Kassam DD, Adams DC, Ambali AJD, Yamaoka K. Body shape variation in relation to resource partitioning within cichlid trophic guilds coexisting along the rocky shore of Lake Malawi. Animal Biology. 2003a;53:59–70. [Google Scholar]
- Kassam DD, Adams DC, Hori M, Yamaoka K. Morphometric analysis on ecomorphologically equivalent cichlid species from Lakes Malawi and Tanganyika. Journal of Zoology. 2003b;260:153–157. [Google Scholar]
- Koenings JP, Edmundson JA, Kyle GB, Edmundson JM. Limnology field and laboratory manual: methods for assessing aquatic production. Alaska Department of Fish and Game: FRED Division Report; 1987. p. 212. (Series 71). [Google Scholar]
- L’Abee-Lund JH, Langeland A, Saegrov H. Piscivory by brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.) in Norwegian lakes. Journal of Fish Biology. 1992;41:91–101. [Google Scholar]
- Land MF, Nilsson D-E. Animal Eyes. Oxford University Press; New York, NY: 2002. [Google Scholar]
- Langeland A, Nøst T. Gill raker structure and selective predation on zooplankton by particulate feeding fish. Journal of Fish Biology. 1995;47:719–732. [Google Scholar]
- Lavin PA, McPhail JD. The evolution of freshwater diversity in the threespine stickleback (Gasterosteus aculeatus): site-specific differentation of trophic morphology. Canadian Journal of Zoology. 1985;63:2632–2638. [Google Scholar]
- Liem KF. Ecomorphology of the teleostean skull. In: Hanken J, Hall BK, editors. The Skull: Functional and Evolutionary Mechanisms. University of Chicago Press; Chicago, IL: 1993. pp. 422–452. [Google Scholar]
- Logan MS, Iverson SJ, Ruzzante DE, Walde SJ, Macchi PJ, Alonso MF, Cussac VE. Long term diet differences between morphs in trophically polymorphic Percichthys trucha (Pisces: Percichthyidae) populations from the southern Andes. Biological Journal of the Linnean Society. 2000;69:599–616. [Google Scholar]
- Lu G, Bernatchez L. Correlated trophic specialization and genetic divergence in sympatric lake whitefish ecotypes (Coregonus clupeaformis): support for the ecological speciation hypothesis. Evolution. 1999;53:1491–1505. doi: 10.1111/j.1558-5646.1999.tb05413.x. [DOI] [PubMed] [Google Scholar]
- Magnuson JJ, Heitz JG. Gill raker apparatus and food selectivity among mackerels, tunas, and dolphins. Fish. Bull. 1971;69:361–370. [Google Scholar]
- Malmquist HJ, Snorrason SS, Skúlason S, Jonsson B, Sandlund OT, Jónasson PM. Diet Differentiation in Polymorphic Arctic Charr in Thingvallavatn, Iceland. The Journal of Animal Ecology. 1992;61:21–35. [Google Scholar]
- McCarthy ID, Fraser D, Waldron S, Adams CE. A stable isotope analysis of trophic polymorphism among Arctic charr from Loch Ericht, Scotland. Journal of Fish Biology. 2004;65:1435–1440. [Google Scholar]
- McKaye KR, Kocher T, Reinthal P, Harrison R, Kornfield I. Genetic evidence for allopatric and sympatric differentiation among color morphs of a Lake Malawi Cichlid fish. Evolution. 1984;38:215–219. doi: 10.1111/j.1558-5646.1984.tb00273.x. [DOI] [PubMed] [Google Scholar]
- McPhail JD. Ecology and evolution of sympatric sticklebacks (Gasterosteus): morphological and genetic evidence for a species pair in Enos Lake, British Columbia. Canadian Journal of Zoology. 1984;62:1402–1408. [Google Scholar]
- McPhail JD. Speciation and the evolution of reproductive isolation in the sticklebacks (Gasterosteus) of south-western British Columbia. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford, UK: 1994. pp. 399–437. [Google Scholar]
- Meyer A. Cost of morphological specialization: feeding performance of the two morphs in the trophically polymorphic cichlid fish, Cichlasoma citrinellum. Oecologia. 1989;80:431–436. doi: 10.1007/BF00379047. [DOI] [PubMed] [Google Scholar]
- Mittelbach GG, Osenberg CW, Wainwright PC. Variation in resource abundance affects diet and feeding morphology in the pumpkinseed sunfish (Lepomis gibbosus) Oecologia. 1992;90:8–13. doi: 10.1007/BF00317802. [DOI] [PubMed] [Google Scholar]
- Motani R, Rothschild BM, Wahl W., Jr Large eyeballs in diving ichthyosaurs. Nature. 1999;402:747. doi: 10.1016/s0002-9394(00)00518-3. [DOI] [PubMed] [Google Scholar]
- Nagel L, Schluter D. Body size, natural selection, and speciation in sticklebacks. Evolution. 1998;52:209–218. doi: 10.1111/j.1558-5646.1998.tb05154.x. [DOI] [PubMed] [Google Scholar]
- Norton SF. Capture success and diet of cottid fishes: the role of predator morphology and attack kinematics. Ecology. 1991;72:1807–1819. [Google Scholar]
- Norton SF, Brainerd EL. Convergence in the feeding mechanics of ecomorphologically similar species in the Centrarchidae and Cichlidae. Journal of Experimental Biology. 1993;176:11–29. [Google Scholar]
- Odling-Smee LC, Boughman JW, Braithwaite VA. Sympatric species of threespine stickleback differ in their performance in a spatial learning task. Behavioral Ecology and Sociobiology. 2008;62:1935–1945. [Google Scholar]
- Omori M, Hamner WM. Patchy distribution of zooplankton: behavior, population assessment and sampling problems. Marine Biology. 1982;72:193–200. [Google Scholar]
- Ostbye K, Amundsen PA, Bernatchez L, Klemetsen A, Knudsen R, Kristoffersen R, Naesje TF, Hindar K. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times. Molecular Ecology. 2006;15:3983–4001. doi: 10.1111/j.1365-294X.2006.03062.x. [DOI] [PubMed] [Google Scholar]
- Peichel CL, Nereng KS, Ohgi KA, Cole BLE, Colosimo PF, Buerkle CA, Schluter D, Kingsley DM. The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414:901–905. doi: 10.1038/414901a. [DOI] [PubMed] [Google Scholar]
- Peres-Neto PR, Magnan P. The influence of swimming demand on phenotypic plasticity and morphological integration: a comparison of two polymorphic charr species. Oecologia. 2004;140:36–45. doi: 10.1007/s00442-004-1562-y. [DOI] [PubMed] [Google Scholar]
- Protasov VR. Vision and Near Orientation in Fish. Israel Program for Scientific Translations; Jerusalem, Israel: 1970. [Google Scholar]
- Proulx R, Magnan P. Contribution of phenotypic plasticity and heredity to the trophic polymorphism of lacustrine brook charr (Salvelinus fontinalis M.) Evolutionary Ecology Research. 2004;6:503–522. [Google Scholar]
- Purnell MA, Hart PJB, Baines DC, Bell MA. Quantitative analysis of dental microwear in threespine stickleback: a new approach to analysis of trophic ecology in aquatic vertebrates. Journal of Animal Ecology. 2006;75:967–977. doi: 10.1111/j.1365-2656.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- Purnell MA, Hart PJB, Baines DC, Bell MA. Correlated evolution and dietary change in fossil stickleback. Science. 2007;317:1887. doi: 10.1126/science.1147337. [DOI] [PubMed] [Google Scholar]
- Rasmussen JB. Patterns in the size structure of littoral zone macroinvertebrate communities. Canadian Journal of Fisheries and Aquatic Sciences. 1993;50:2192–2207. [Google Scholar]
- Robinson BW, Schluter D. Natural selection and the evolution of adaptive genetic variation in northern freshwater fishes. In: Mousseau TA, Sinervo B, Endler JA, editors. Genetic Variation in the Wild. Vol. 65. Oxford University Press; New York, NY: 2000. p. 94. [Google Scholar]
- Robinson BW, Wilson DS. Character release and displacement in fishes: a neglected literature. The American Naturalist. 1994;144:596–627. [Google Scholar]
- Robinson BW, Wilson DS. Genetic variation and phenotypic plasticity in a trophically polymorphic population of pumpkinseed sunfish (Lepomis gibbosus) Evolutionary Ecology. 1996;10:631–652. [Google Scholar]
- Rohlf FJ. tpsDIG2. version 2.12 Department of Ecology and Evolution, State University of New York at Stony Brook; 2008a. [Google Scholar]
- Rohlf FJ. tpsRelw. version 1.46 Department of Ecology and Evolution, State University of New York at Stony Brook; 2008b. [Google Scholar]
- Sandlund OT, Jonsson B, Malmquist HJ, Gydemo R, Lindem T, Skúlason S, Snorrason SS, Jónasson PM. Habitat use of arctic charr Salvelinus alpinus in Thingvallavatn, Iceland. Environmental Biology of Fishes. 1987;20:263–274. [Google Scholar]
- Savvaitova KA. Patterns of diversity and processes of speciation in Arctic char. In: Klemetsen A, Jonsson B, Elliott JM, editors. Proceedings of the Third international charr symposium June 13-18 1994; Trondheim, Norway. Drottningholm, Sweden: Soetvattenslaboratoriet; 1995. pp. 81–91. [Google Scholar]
- Scheffer M. Ecology of Shallow Lakes. Chapman & Hall; New York, NY: 1998. [Google Scholar]
- Schluter D. Adaptive radiation in sticklebacks: size, shape, and habitat use efficiency. Ecology. 1993;74:699–709. [Google Scholar]
- Schluter D. Ecological speciation in postglacial fishes. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1996;351:807–814. [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. The American Naturalist. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Snorrason SS, Skúlason S, Jonsson B, Malmquist HJ, Jónasson PM, Sandlund O, Lindem T. Trophic specialization in Arctic charr Salvelinus alpinus (Pisces; Salmonidae): morphological divergence and ontogenetic niche shifts. Biological Journal of the Linnean Society. 1994;52:1–18. [Google Scholar]
- Snowberg LK, Bolnick DI. Assortative mating by diet in a phenotypically unimodal but ecologically variable population of stickleback. The American Naturalist. 2008;172:733–739. doi: 10.1086/591692. [DOI] [PubMed] [Google Scholar]
- Steedman RJ, Kushneriuk RS, France RL. Littoral water temperature response to experimental shoreline logging around small boreal forest lakes. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1638–1647. [Google Scholar]
- Svanbäck R, Eklöv P. Morphology dependent foraging efficiency in perch: a trade-off for ecological specialization? Oikos. 2003;102:273–284. [Google Scholar]
- Taylor EB. Species pairs of north temperate freshwater fishes: evolution, taxonomy, and conservation. Reviews in Fish Biology and Fisheries. 1999;9:299–324. [Google Scholar]
- Thomas RJ, Szekely T, Powell RF, Cuthill IC. Eye size, foraging methods and the timing of foraging in shorebirds. Functional Ecology. 2006;20:157–165. [Google Scholar]
- Travis MP. Unpublished Doctor of Philosophy. Stony Brook University; 2007. The functional morphology and evolution of feeding modes in threespine stickleback. [Google Scholar]
- Uchii K, Okuda N, Yonekura R, Karube Z, Matsui K, Kawabata Z. Trophic polymorphism in bluegill sunfish (Lepomis macrochirus) introduced into Lake Biwa: evidence from stable isotope analysis. Limnology. 2007;8:59–63. [Google Scholar]
- USEPA . Survey of the Nation’s Lakes. Field Operations Manual. U.S. Environmental Protection Agency; Washington, DC: 2007. EPA 841-B-07-004. [Google Scholar]
- Utne ACW The effect of turbidity and illumination on the reaction distance and search time of the marine planktivore Gobiusculus flavescens. Journal of Fish Biology. 1997;50:926–938. [Google Scholar]
- Vander Zanden MJ, Vadeboncoeur Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology. 2002;83:2152–2161. [Google Scholar]
- Vermeij GJ, Covich AP. Coevolution of freshwater gastropods and their predators. The American Naturalist. 1978;112:833–843. [Google Scholar]
- Vollestad LA, Skurdal J, Qvenild T. Habitat use, growth, and feeding of pike (Esox lucius L.) in four Norwegian lakes. Archiv für Hydrobiologie. 1986;108:107–117. [Google Scholar]
- Von Hippel FA. Conservation of threespine and ninespine stickleback radiations in the Cook Inlet Basin, Alaska. Behaviour. 2008;145:693–724. [Google Scholar]
- Wainwright PC. Ecological explanation through functional morphology: the feeding biology of sunfishes. Ecology. 1996;77:1336–1343. [Google Scholar]
- Wainwright PC. Functional morphology of the pharyngeal jaw apparatus. In: Shadwick RE, Lauder GV, editors. Fish Biomechanics. Elsevier Academic Press; San Diego, CA: 2006. pp. 77–102. [Google Scholar]
- Walker JA. Ecological morphology of lacustrine threespine stickleback, Gasterosteus aculeatus L. (Gasterosteidae) body shape. Biological Journal of the Linnean Society. 1997;61:3–50. [Google Scholar]
- Walls GL. The Vertebrate Eye and its Adaptive Radiation. Hafner Publishing Co.; New York, NY: 1963. [Google Scholar]
- Webb PW. Body form, locomotion and foraging in aquatic vertebrates. American Zoologist. 1984;24:107–120. [Google Scholar]
- Werner EE. Species packing and niche complementarity in three sunfishes. The American Naturalist. 1977;111:553–578. [Google Scholar]
- Westneat MW. Evolution of levers and linkages in the feeding mechanisms of fishes. Integrative and Comparative Biology. 2004;44:378–389. doi: 10.1093/icb/44.5.378. [DOI] [PubMed] [Google Scholar]
- Willacker JJ. Unpublished Master of Science. University of Alaska Anchorage; 2009. Geometric morphometrics of threespine stickleback in the Cook Inlet Basin, Alaska. [Google Scholar]
- Wimberger PH. Trophic polymorphisms, plasticity, and speciation in vertebrates. In: Stouder DJ, Fresh KL, Feller RJ, editors. Theory and Application in Fish Feeding Ecology. University of South Carolina Press; Columbia, SC: 1994. pp. 19–44. [Google Scholar]
- Wright DI, O’Brien WJ, Luecke C. A new estimate of zooplankton retention by gill rakers and its ecological significance. Transactions of the American Fisheries Society. 1983;112:638–646. [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric Morphometrics for Biologists. Elsevier Academic Press; San Francisco: 2004. [Google Scholar]