Summary
Mutations of the progranulin (GRN) gene are major cause of familial frontotemporal lobar degeneration with transactive response (TAR) DNA-binding protein of 43kDa (TDP-43) proteinopathy (FTLD-TDP). We studied the spatial patterns of TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCI) neuronal intranuclear inclusions (NII) in histological sections of the frontal and temporal lobe in eight cases of FTLD-TDP with GRN mutation using morphometric methods and spatial pattern analysis. In neocortical regions, the NCI were clustered and the clusters were regularly distributed parallel to the pia mater; 58% of regions analysed exhibiting this pattern. The NII were present in regularly distributed clusters in 35% of regions but also randomly distributed in many areas. In neocortical regions, the sizes of the regular clusters of NCI and NII were 400–800 µm, approximating to the size of the modular columns of the cortico-cortical projections, in 31% and 36% of regions respectively. The NCI and NII also exhibited regularly spaced clustering in sectors CA1/2 of the hippocampus and in dentate gyrus. The clusters of NCI and NII were not spatially correlated. The data suggest degeneration of the cortico-cortical and cortico-hippocampal pathways in FTLD-TDP with GRN mutation, the NCI and NII affecting different clusters of neurons.
Keywords: Frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP), TAR DNA-binding protein (TDP-43), Progranulin (GRN) mutation, Spatial topography
Introduction
Frontotemporal lobar degeneration (FTLD) is the second most frequent form of cortical dementia of early-onset after Alzheimer’s disease (AD) (Tolnay and Probst, 2002). The disorder is associated with a heterogeneous group of clinical syndromes including frontotemporal dementia (FTD), FTD with motor neuron disease (FTD/MND), progressive non-fluent aphasia (PNFA), semantic dementia (SD), and progressive apraxia (PAX) (Snowden et al., 2007).
FTLD with transactive response (TAR) DNA-binding protein of 43kDa (TDP-43) proteinopathy (FTLD-TDP), previously called FTLD with ubiquitin positive inclusions (FTLD-U), is characterized by variable neocortical and allocortical atrophy principally affecting the frontal and temporal lobes. Histologically, there is neuronal loss, microvacuolation in the superficial cortical laminae, and a reactive astrocytosis (Cairns et al., 2007a; Armstrong et al., 2010). A variety of TDP-43 immunoreactive changes are present in FTLD-TDP including neuronal cytoplasmic inclusions (NCI) (Davidson et al., 2007), neuronal intranuclear inclusions (NII) (Pirici et al., 2006), dystrophic neurites (DN) and, oligodendroglial inclusions (GI).
A proportion of cases of FTLD-TDP cases are familial (Cairns et al., 2007a); the majority of cases being caused by mutations of the progranulin (GRN) gene (Baker et al., 2006; Cruts et al., 2006; Mukherjee et al., 2006; Behrens et al., 2007; Rademakers and Hutton, 2007). A less prevalent disorder, FTLD with valosin-containing protein (VCP) gene mutation (Forman et al., 2006), also has TDP-43 immunoreactive inclusions and recently, variants in the ubiquitin associated binding protein 1 (UBAP1) gene (Rollinson et al., 2009) were shown to have TDP-43 inclusions. Several different frame-shift and premature termination mutations have been identified in FTLD-TDP with GRN mutation (Beck et al., 2008). Abnormal protein products may accumulate within the endoplasmic reticulum of the cell due to inefficient secretion or mutant RNA may have a lower expression within the cell at least in some mutants (Mukherjee et al., 2006). TDP-43 is a nuclear protein but in FTLD-TDP, TDP-43 is redistributed from the nucleus to the cytoplasm, is ubiquinated, hyperphosphorylated, and then cleaved to generate C-terminal fragments (Neumann et al 2007). These fragments accumulate to form the NCI and NII which may cause cell death.
Inclusions incorporating abnormal protein aggregates are a common histological feature of neurodegenerative disease; the majority characterised by tau (tauopathies) or α-synuclein (synucleinopathies) immunoreactivity (Goedert et al., 2001). In the neocortex of the tauopathies (Armstrong et al., 1998, 1999), synucleinopathies (Armstrong et al., 1997, 2004) and neuronal intermediate filament inclusion disease (NIFID) (Bigio et al., 2003; Cairns et al., 2003, 2004; Josephs et al., 2003; Armstrong and Cairns, 2006; Cairns and Ghoshal, 2010), the NCI occur in clusters which exhibit a regular periodicity parallel to the pia mater. suggesting the inclusions develop in relation to specific cortico-cortical and cortico-hippocampal projections. The objectives of the present study were to determine in cases of FTLD-TDP with GRN mutation: 1) whether the NCI and NII exhibit a similar spatial pattern, 2) the relative importance of the NCI and NII, and 3) whether the NCI and NII are spatially correlated.
Materials and Methods
Cases
Eight cases of familial FTLD-TDP (see Table 1) with GRN mutation were obtained from the Departments of Neurology and Pathology and Immunology, Washington University, School of Medicine, St. Louis, Mo., USA. All cases exhibited frontotemporal lobar degeneration with neuronal loss, microvacuolation affecting the superficial cortical laminae, and reactive astrocytosis (Cairns et al., 2007b). None of the cases had coexisting motor neuron disease (FTLD-MND) (Josephs et al., 2005; Kersaitis et al., 2006) or hippocampal sclerosis (HS). Braak stage of the cases was based on the density and distribution of neurofibrillary tangles (NFT) (Braak et al., 1993).
Table 1.
Demographic features and gross brain weight of the eight cases of familial frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) caused by progranulin (GRN) mutation.
| Case | Gender | Age | Onset | Duration | Brain weight | Braak score |
|---|---|---|---|---|---|---|
| A | F | 74 | 68 | 6 | 975 | 0 |
| B | F | 84 | 69 | 15 | 570 | 4 |
| C | M | 67 | 52 | 15 | 960 | 0 |
| D | F | 67 | 58 | 9 | 880 | 2 |
| E | M | 63 | 57 | 6 | 1080 | 1 |
| F | M | 66 | 55 | 11 | 1050 | 1 |
| G | M | 79 | 71 | 8 | 1150 | - |
| H | F | 82 | 73 | 9 | 800 | 6 |
Braak score based on density and distribution of neurofibrillary tangles.
M: male; F: female.
Histological methods
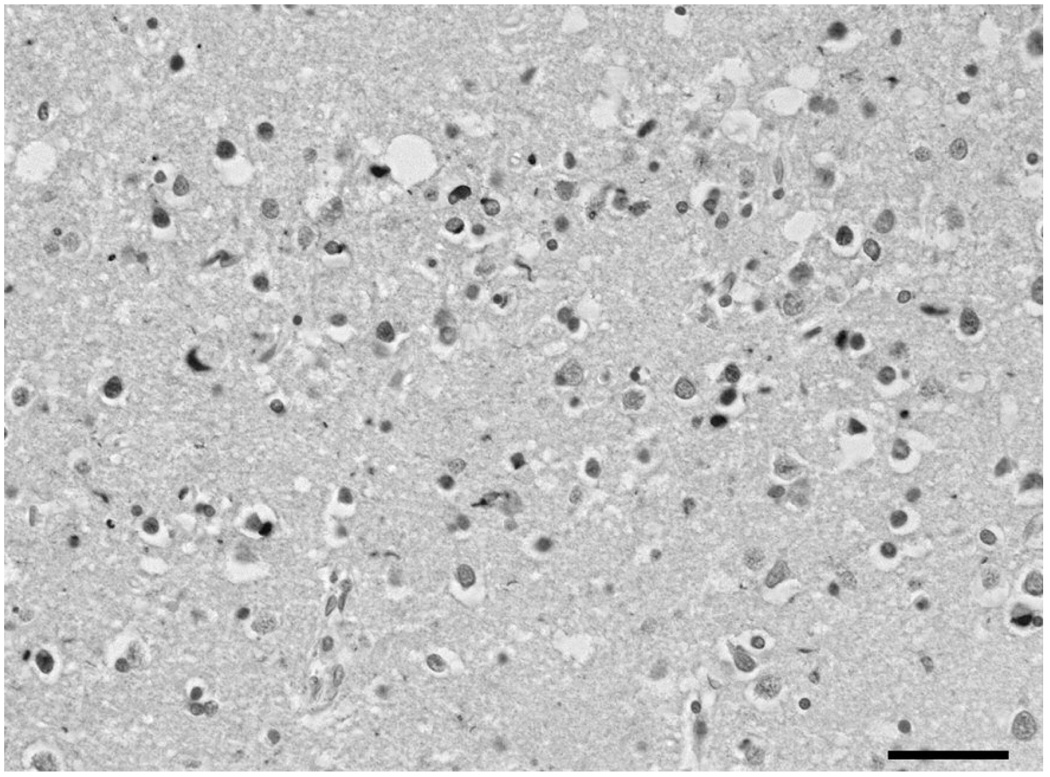
After death, the consent of the next of kin was obtained for brain removal, following local Ethical Committee procedures (Washington University School of Medicine, Human Studies Committee) and the 1995 Declaration of Helsinki (as modified Edinburgh, 2000). Tissue blocks were taken from the frontal cortex at the level of the genu of the corpus callosum to study the middle frontal gyrus (MFG) and the temporal lobe at the level of the lateral geniculate body to study the inferior temporal gyrus (ITG), parahippocampal gyrus (PHG), CA1/2 sectors of the hippocampus, and dentate gyrus (DG). Tissue was fixed in 10% phosphate buffered formal-saline and embedded in paraffin wax. Immunohistochemistry (IHC) was performed on 4–10 µm sections with a rabbit polyclonal antibody that recognizes TDP-43 (dilution 1:1000; ProteinTech Inc., Chicago, IL). Sections were also stained with haematoxylin. A low power view of the inclusions in the ITG is shown in Figure 1.
Fig. 1.
Low power view of the inclusions in the temporal cortex (lamina II) in familial frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) with progranulin (GRN) mutation. TDP-43 immunohistochemistry, haematoxylin. Scale bar: 30µm.
Morphometric methods
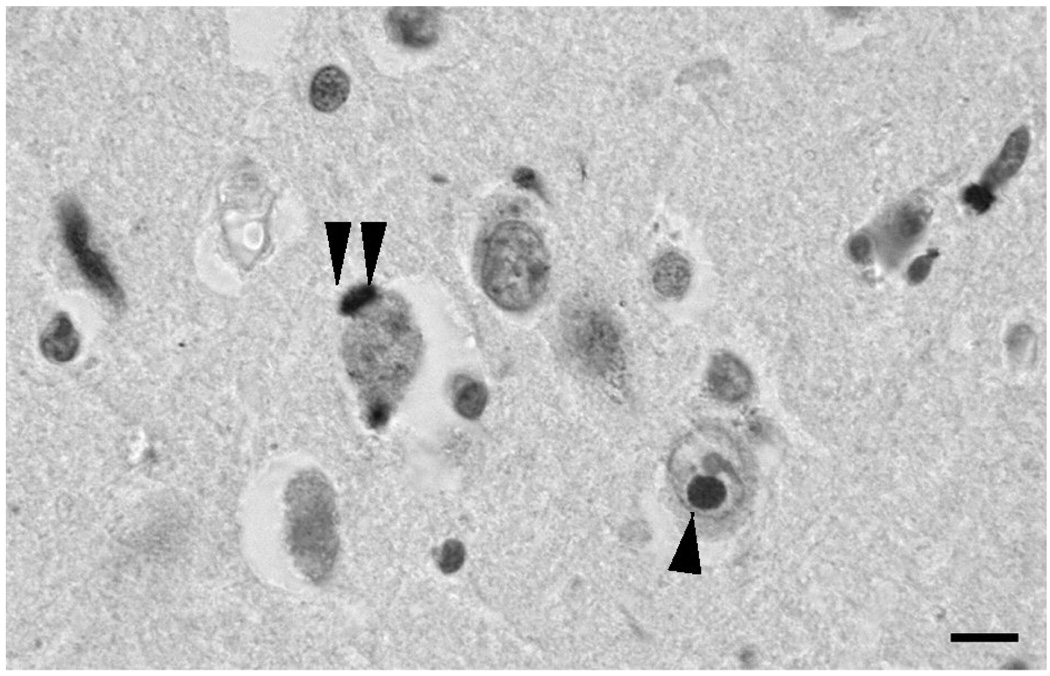
In neocortical regions, the NCI and NII were counted along strips of tissue (1600 to 3200 µm in length) located parallel to the pia mater, in 250×50 µm sample fields arranged contiguously. The sample fields were located in the upper (II/III) and lower (V/VI) laminae; the short edge of the sample field being orientated parallel with the pia mater and aligned with guidelines marked on the slide. In the hippocampus, the NCI and NII were counted from sector CA1 to CA2, the short dimension of the contiguous sample field being aligned with the alveus. NCI also occur in the dentate gyrus fascia in FTLD-TDP (Woulfe et el., 2001; Kovari et al., 2004; Mackenzie et al., 2006) and were counted with the sample field aligned with the upper edge of the granule cell layer. The number of NCI and NII were counted in each sample field. The majority of the NCI conformed to the C-type (Yaguchi et al., 2004) (Fig. 2) comprising large, intensely stained circular or crescent-shaped inclusions while the NII were present in nuclei with little normal TDP-43 immunolabelling (Pirici et al., 2006).
Fig. 2.
Neuronal cytoplasmic inclusions (NCI) (double arrow) and neuronal intranuclear inclusions (NII) (single arrow) in the upper laminae of the parahippocampal gyrus (PHG) in familial frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) with progranulin (GRN) mutation. TDP-43 immunohistochemistry, haematoxylin. Scale bar: 10 µm.
Data analysis
The data were analysed by spatial pattern analysis (Armstrong, 1993a, 1997, 2006). This method uses the variance-mean ratio (V/M) of the data to determine whether the inclusions were distributed randomly (V/M = 1), regularly (V/M < 1), or were clustered (V/M > 1) along the strip of tissue studied. Counts of inclusions in adjacent sample fields were then added together successively to provide data for increasing field sizes, e.g., 50×250 µm, 100×250 µm, 200×250 µm etc., up to a size limited by the length of the strip sampled. V/M is plotted against field size to determine first, whether the clusters of inclusions were regularly or randomly distributed and second, to estimate the mean cluster size parallel to the tissue boundary. A V/M peak indicated the presence of regularly spaced clusters (Armstrong, 1993a, 1997, 2006). The statistical significance of a peak was tested using the ‘t’ distribution. Pearson’s correlation coefficient ‘r’ was used to test the degree of correlation between the densities of NCI and NII (Armstrong, 2003).
Results
Examples of the spatial patterns exhibited by the NCI and NII in individual brain regions are shown in Fig 3. In the upper laminae of the MFG, there was a V/M peak at a field size of 400 µm suggesting the presence of clusters of NCI, 400 µm in diameter, regularly distributed parallel to the pia mater. In the upper laminae of the PHG, the NII exhibited a V/M peak at a field size of 200 µm suggesting the presence of clusters of NII, 200 µm in diameter, also regularly distributed parallel to the pia mater.
Fig. 3.
Examples of the spatial patterns exhibited by neuronal cytoplasmic inclusions (NCI) in the upper laminae of the middle frontal gyrus (MFG) and neuronal intranuclear inclusions (NII) in the upper laminae of the parahippocampal gyrus (PHG) in familial frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) with progranulin (GRN) mutation *: Peak significant at P <0.05.
A summary of the spatial patterns observed in all regions and cases is shown in Table 2. In the neocortex, the commonest spatial pattern exhibited by the NCI was the presence of clusters of lesions regularly distributed parallel to the pia mater, a pattern observed in 11/19 (58%) of regions analysed. In neocortical regions exhibiting regular clustering, the cluster size of the NCI was in the size range 400–800µm in 4/13 (31%) regions, less than 400 µm in 8/13 (62%) regions, and greater than 800 µm in 1/13 (8%) of regions. In remaining regions, lesions were distributed at random, were regularly distributed, or were present in larger clusters. Similarly, the NII were present in regularly distributed clusters in the neocortex in 11/31 (35%) regions. The regularly distributed clusters of the NII were in the size range 400–800 µm in 4/11 (36%) of regions, less than 400 µm in 6/11 (55%) of regions, and greater than 800 µm in 1/11 (9%) of regions. A random distribution of the NII was present in 12/31 (39%) of analyses. Regularly spaced clustering of NCI and NII was also present in sectors CA1/2 of the hippocampus and in the dentate fascia. Chi-square (χ2) contingency tests suggested that the NCI and NII exhibited a similar range of spatial patterns (χ2 = 2.73, 3DF, P > 0.05).
Table 2.
Spatial pattern of the TDP-43 immunoreactive neuronal cytoplasmic inclusions (NCI) and neuronal intranuclear inclusions (NII) in areas of the frontal and temporal lobe in eight cases of frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) caused by progranulin (GRN) mutation.
| Brain region and lamina | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | FC | ITG | PHG | HC | DG | ||||
| II/III | V/VI | II/III | V/VI | II/III | V/VI | ||||
| A | NCI | 400 | - | - | - | - | - | - | - |
| NII | R | Reg | 50,200 | R | 200 | Reg | - | - | |
| B | NCI | 400 | - | - | - | - | - | R | >400 |
| NII | R | - | - | - | >400 | - | - | - | |
| C | NCI | - | R | - | - | - | - | - | 50,400 |
| NII | >800 | R | R | Reg | 200 | Reg | 200 | 100 | |
| D | NCI | 400 | 400 | 200 | R | - | - | - | - |
| NII | 50,400 | 400 | 200 | 100 | - | - | R | - | |
| E | NCI | R | 200 | - | - | - | - | - | >800 |
| NII | R | 100 | - | - | - | - | - | - | |
| F | NCI | 100 | Reg | 100 | - | - | - | - | R |
| NII | 400 | R | R | - | - | 200 | - | 50 | |
| G | NCI | >800 | - | R | - | 100 | - | - | 50 |
| NII | R | - | Reg | R | - | - | - | - | |
| H | NCI | 100 | 100 | 100 | Reg | - | - | 200 | 200 |
| NII | R | Reg | - | - | - | R | 100 | - | |
Data represent the dimensions (µm), measured parallel to the tissue boundary, of regularly distributed clusters of NCI and NII with the following exceptions: R: Random distribution; RG: Regular distribution of individual inclusions. Data preceded by ‘>’ indicate large scale clustering of at least the dimension indicated and without regular spacing. Where two figures are present, clustering occurred at two scales, i.e., smaller clusters were aggregated into larger ‘superclusters’. (−) indicates that density of inclusions was too low to determine spatial pattern. Chi-square (χ2) contingency tables: Comparison of totals for cortical regions versus CA1/2 and DG; NCI (χ2 = 2.88, P > 0.05); NII (χ2 = 2.79, P > 0.05), Comparison between histological features: χ2 = 2.73, 2DF, P > 0.05. familial frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) with progranulin (GRN) mutation. MFG: Middle frontal gyrus; ITG: Inferior temporal gyrus; PHG: Parahippocampal gyrus; HC: sectors CA1/2 of the Hippocampus; DG: Dentate gyrus.
In a total of 41 regions, there were positive correlations between the densities of the NCI and NII in only 2/41 (5%) of regions. Hence, the clusters of NCI and NII were distributed independently of each other in the majority of regions.
Discussion
The NCI were clustered and the clusters frequenctly exhibited a regular periodicity parallel to the tissue boundary. The NII were present in regularly clustered in about a third of regions but were also randomly distributed in many areas. The spatial patterns of the NCI are similar to those of comparable inclusions in the tauopathies (Armstrong, 1993b; Armstrong et al., 1998, 1999) synucleinopathies (Armstrong et al., 1997, 2004), and in NIFID (Armstrong and Cairns, 2006) suggesting common pattern of degeneration in these disorders (Armstrong et al., 2001).
In the tauopathies, synucleinopathies, and in NIFID, the spatial patterns of the NCI suggest degeneration of specific cortico-cortical and cortico-hippocampal projections (Hiorns et al., 1991; De Lacoste and White, 1993; Armstrong et al., 2001; Delatour et al., 2004). The cells of origin of these projections are approximately 500–800 µm in width, traverse the laminae in columns, and are regularly distributed along the cortex (Hiorns et al., 1991). In FTLD-TDP with GRN mutation, the NCI and NII were regularly distributed parallel to the pia mater in 58% and 35% of cortical regions respectively consistent with their development in relation to the cortico-cortical pathways. Moreover, the estimated size of the NCI or NII clusters approximated to the assumed dimension of the cells of origin of these projections in 31% and 36% of regions respectively.
In some neocortical regions, the NCI and NII occurred in clusters 50–200µm in diameter; smaller than the size of the cortico-cortical columns and a similar size to the clusters of NCI in NIFID (Armstrong and Cairns, 2006). Hence, in some regions, TDP-43 pathology appears to affect only a subset of the neurons within a column. In some neocortical regions, the smaller clusters of NCI and NII were aggregated into larger ‘superclusters’ and in other areas, clusters >800 µm in diameter were present. Hence, smaller clusters may give rise to larger accumulations of lesions as the disease develops (Armstrong, 1993b). In addition, in the majority of regions, the clusters of NCI and NII were not spatially correlated with each other suggesting they affect different clusters of neurons.
In conclusion, the spatial patterns of the TDP-43 immunoreactive NCI and NII in FTLD-TDP with GRN mutation are similar to those reported in the tauopathies (Armstrong, 1993, Armstrong et al., 1998, 1999), synucleinopathies (Armstrong et al., 1997, 2004), and NIFID (Armstrong and Cairns, 2006). Both NCI and NII contribute to the degeneration of cortical columns in the frontal and temporal lobes in FTLD-TDP. The similarity in the spatial pattern of inclusions in the neocortex of different dementias suggests cortico-cortical breakdown is a feature common to these disorders and result in the formation of aggregated inclusions within neurons incorporating various molecular residues (Armstrong et al., 2008). Differences in clinical syndromes between disorders may depend on the selective vulnerability of different neocortical regions and the pattern of spread of the pathology (Table 3). Hence, tauopathies such as Pick’s disease (PD) together with NIFID and FTLD-TDP have high densities of inclusions in the DG while PD, corticobasal degeneration (CBD), multiple system atrophy and FTLD-TDP have few or no inclusions in the CA sectors of the hippocampus.
Table 3.
Comparison of the abundance and distribution of inclusions in regions of frontal and temporal cortex, in the tauopathies, synucleinopathies, neuronal intermediate filament inclusion disease (NIFID) and frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP).
| Disorder | Inclusion | FC | ITG | PHG | HC | DG |
|---|---|---|---|---|---|---|
| Pick’s disease | NCI | + | + | + | + | ++ |
| Corticobasal degeneration | NCI | + | ++ | ++ | + | + |
| Progressive supranuclear palsy | NCI | + | + | + | ++ | + |
| Dementia with Lewy bodies | NCI | ++ | ++ | ++ | + | 0 |
| Multiple system atrophy | GCI | + | + | + | + | 0 |
| NIFID | NCI | ++ | ++ | ++ | ++ | ++ |
| FTLD-TDP | NCI | ++ | ++ | ++ | 0 | ++ |
NCI: neuronal cytoplasmic inclusions; GCI: Glial cytoplasmic inclusions; FC: Frontal cortex; ITG: Inferior temporal gyrus; PHG: Parahippocampal gyrus; HC: sectors CA1/2 of the Hippocampus; DG: Dentate gyrus; ++: frequent; +: moderately frequent; O: rare or absent
Acknowledgements
We thank clinical, genetic, pathology, and technical staff for making information and tissue samples available for this study and we thank the families of patients whose generosity made this research possible. Support for this work was provided the National Institute on Aging of the National Institute of Health, P50-AG05681.
Footnotes
The authors report no conflicts of interest.
References
- Armstrong RA. The usefulness of spatial pattern analysis in understanding the pathogenesis of neurodegenerative disorders, with particular reference to plaque formation in Alzheimer’s disease. Neurodegeneration. 1993a;2:73–80. [Google Scholar]
- Armstrong RA. Is the clustering of neurofibrillary tangles in Alzheimer's patients related to the cells of origin of specific cortico-cortical projections? Neurosci. Lett. 1993b;160:57–60. doi: 10.1016/0304-3940(93)90916-9. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. Analysis of spatial patterns in histological sections of brain tissue. J. Neurosc. Meth. 1997;73:141–147. doi: 10.1016/s0165-0270(96)02221-2. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. Measuring the degree of spatial correlation between histological features in thin sections of brain tissue. Neuropathology. 2003;23:245–253. doi: 10.1046/j.1440-1789.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. Methods of studying the planar distribution of objects in histological sections of brain tissue. J. Micros. (Oxf.) 2006;221:153–158. doi: 10.1111/j.1365-2818.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ. Topography of α-internexin-positive neuronal aggregates in 10 patients with neuronal intermediate filament inclusion disease. Eur. J. Neurol. 2006;13:528–532. doi: 10.1111/j.1468-1331.2006.01284.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. Dementia with Lewy bodies: Clustering of Lewy bodies in human patients. Neurosci. Lett. 1997;224:41–44. doi: 10.1016/s0304-3940(97)13451-6. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. Clustering of Pick bodies in Pick’s disease. Neurosci. Lett. 1998;242:81–84. doi: 10.1016/s0304-3940(98)00052-4. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. Clustering of cerebral cortical lesions in patients with corticobasal degeneration. Neurosci. Lett. 1999;268:5–8. doi: 10.1016/s0304-3940(99)00309-2. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Cairns NJ, Lantos PL. What does the study of spatial patterns tell us about the pathogenesis of neurodegenerative disorders? Neuropathology. 2001;21:1–12. doi: 10.1046/j.1440-1789.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. Spatial patterns of α-synuclein positive glial cytoplasmic inclusions in multiple system atrophy. Move. Disord. 2004;19:109–112. doi: 10.1002/mds.10637. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. What determines the molecular composition of abnormal protein aggregates in neurodegenerative disease? Neuropathology. 2008;28:351–365. doi: 10.1111/j.1440-1789.2008.00916.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Ellis W, Hamilton RL, Mackenzie IRA, Hedreen J, Gearing M, Montine T, Vonsattel J-P, Head E, Lieberman AP, Cairns NJ. Neuropathological heterogeneity in frontotemporal lobar degeneration with TDP-43 proteinopathy: a quantitative study of 94 cases using principal components analysis. J. Neural Transm. 2010;117:227–239. doi: 10.1007/s00702-009-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, Warrington EK, Stevens J, Revesz T, Hoton J, Al-Sarraj S, King A, Scabill R, Warren JD, Rossor MN, Collinge J, Mead S. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutation in a large UK series. Brain. 2008;131:706–720. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MI, Mukherjee O, Tu PH, Liscic RM, Grinberg LT, Carter D, Paulsmeyer K, Taylor-Reinwald L, Gitcho M, Norton JB, Chakraverty S, Goate AM, Morris JC, Cairns NJ. Neuropathologic heterogeneity in HDDD1: a familial frontotemporal lobar degeneration with ubiquitin-positive inclusions and progranulin mutation. Alz. Dis. Assoc. Disord. 2007;21:1–7. doi: 10.1097/WAD.0b013e31803083f2. [DOI] [PubMed] [Google Scholar]
- Bigio EH, Lipton AM, White CL, Dickson DW, Hirano A. Frontotemporal dementia and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol. App. Neurobiol. 2003;29:239–253. doi: 10.1046/j.1365-2990.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur. Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Ghoshal N. FUS A new actor on the frontotemporal lobar degeneration stage. Neurology. 2010;74:354–356. doi: 10.1212/WNL.0b013e3181ce5dd6. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Perry RH, Jaros E, Burn D, McKeith IG, Lowe JS, Holton J, Rossor MN, Skullerud K, Duyckaerts C, Cruz-Sanchez FF, Lantos PL. Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci. Lett. 2003;341:177–180. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IRA, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Lee VMY, Trojanowski JQ. Alpha-internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am. J. Pathol. 2004;164:2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Steiber A, Xu Y, Forman MS, Trojanowski JQ, Lee VMY, Mackenzie IRA. TDP-43 familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 2007a;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa K, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe J, Holm I, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA. Neuropathologic diagnostic and nosological criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007b;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselink I, van der ZJ, Engelborgs S, Wils H, Pirici D, Radamakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, van den BM, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IRA, Pickering Brown S, Du Plessis D, Neary D, Snowden JS, Mann DMA. Ubiquinated pathological lesions in frontotemporal lobar degeneration contain TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- De Lacoste M, White CL., III The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol. Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- Delatour B, Blanchard V, Pradier L, Duyckaerts C. Alzheimer pathology disorganizes cortico-cortical circuitry: direct evidence from a transgenic animal model. Neurobiol. Dis. 2004;16:41–47. doi: 10.1016/j.nbd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestrvik A, Smith TN, Tu PH, Watts GDJ, Markesbery WR, Smith CD, Kimonis VE. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J. Neuropathol. Exp. Neurol. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Serpell LC, Berriman J, Smith MJ, Jakes R, Crowther RA. From genetics to pathology: tau and alpha-synuclein assemblies in neurodegenerative diseases. Phil. Trans. Roy. Soc. Lond. B. Biol. Sci. 2001;356:213–227. doi: 10.1098/rstb.2000.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiorns RW, Neal JW, Pearson RCA, Powell TPS. Clustering of ipsilateral cortico-cortical projection neurons to area 7 in the rhesus monkey. Proc. Roy. Soc. Lond. 1991;246:1–9. doi: 10.1098/rspb.1991.0117. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Braendgaard H, Ozawa T, Fox NC, Petersen RC, Pearl GS, Ganguly M, Rosa P, Laursen H, Parisi JE, Waldemar G, Quinn NP, Dickson DW, Revesz T. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Knopman DS, Whitwell JL, Boeve BF, Parisi JE, Petersen RC, Dickson DW. Survival in the two variants of tau negative FTLD: FTLD-U versus FTLD-MND. Neurology. 2005;65:645–647. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- Kersaitis C, Holliday GM, Xuereb JH, Pamphlett R, Bak TH, Hodges JR, Kril JJ. Ubiquitin-positive inclusions and progression of pathology in FTD and MND identifies a group with mainly early pathology. Neuropathol. Appl. Neurobiol. 2006;32:83–91. doi: 10.1111/j.1365-2990.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- Kovari E, Gold G, Giannakopoulos P, Bouras C. Cortical ubiquitin positive inclusions in frontotemporal dementia without motor neuron disease: a quantitative immunocytochemical study. Acta Neuropathol. 2004;108:207–212. doi: 10.1007/s00401-004-0881-8. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Baker M, Pickering-Brown S, Hsinng GYR, Lindholm C, Dwosh E, Cannon A, Rademakers R, Hutton M, Feldman HH. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- Mukherjee O, Pastor P, Cairns NJ, Chakraaverty S, Kauwe JSK, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Grinberg LT, Liscic RM, Armendariz J, Morris JC, Goate AM. HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann. Neurol. 2006;60:314–322. doi: 10.1002/ana.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Igaz LM, Kwong LK, Nakashima-Yasuda H, Kolb SJ, Dreyfuss G, Kretzschmar HA, Trojanowski JQ, Lee VMY. Absence of heterogeneous nuclear riboproteins and survival neuron protein (TDP-43) positive inclusions in frontotemporal lobar degeneration. Acta Neuropathol. 2007;113:543–548. doi: 10.1007/s00401-007-0221-x. [DOI] [PubMed] [Google Scholar]
- Pirici D, Vandenberghe R, Rademakers R, Dermant B, Cruts M, Vennekens K, Cuijt I, Lubke U, Centerick C, Martin JJ, Van Broeckhoven C, Kumar-Singh S. Characterization of ubiquinated intraneuronal inclusions in a novel Belgian frontotemporal lobar degeneration family. J. Neuropathol. Exp. Neurol. 2006;65:289–301. doi: 10.1097/01.jnen.0000205147.39210.c7. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Hutton M. The genetics of frontotemporal lobar degeneration. Curr. Neurol. Neurosci. Rep. 2007;7:434–442. doi: 10.1007/s11910-007-0067-6. [DOI] [PubMed] [Google Scholar]
- Rollinson S, Rizzu P, Sikkink S, Baker M, Halliwell N, Snowden J, Traynor BJ, Ruano D, Cairns N, Rohrer JD, Mead S, Collinge J, Rossor M, Akay E, Gueireiro R, Rademakers R, Morrison KE, Pastor P, Alonso E, Martinez-Lage P, Graff-Radford N, Neary D, Henlink P, Mann DMA, Van Swieten J, Pickering-Brown SM. Ubiquitin associated protein 1 is a risk factor for frontotemporal lobar degeneration. Neurobiol. Aging. 2009;30:656–665. doi: 10.1016/j.neurobiolaging.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Tolnay M, Probst A. Frontotemporal lobar degeneration- tau as a pied piper? Neurogenetics. 2002;4:63–75. doi: 10.1007/s10048-002-0140-x. [DOI] [PubMed] [Google Scholar]
- Woulfe J, Kertesz A, Munoz DG. Frontotemporal dementia with ubiquinated cytoplasmic and intranuclear inclusions. Acta Neuropathol. 2001;102:94–102. doi: 10.1007/s004010000346. [DOI] [PubMed] [Google Scholar]
- Yaguchi M, Fujita Y, Amari M, Takatama M, Al-Sarraj S, Leigh PN, Okamoto K. Morphological differences of intraneural ubiquitin positive inclusions in the dentate gyrus and parahippocampal gyrus of motor neuron disease with dementia. Neuropathology. 2004;24:296–301. doi: 10.1111/j.1440-1789.2004.00567.x. [DOI] [PubMed] [Google Scholar]