Abstract
Objective: Human embryonic stem cells (hESCs) have recently been reported as an unlimited source of mesenchymal stem cells (MSCs). The present study not only provides an identical and clinically compliant MSC source derived from hESCs (hESC-MSCs), but also describes the immunomodulative effects of hESC-MSCs in vitro and in vivo for a carbon tetrachloride (CCl4)-induced liver inflammation model. Methods: Undifferentiated hESCs were treated with Rho-associated kinase (ROCK) inhibitor and induced to fibroblast-looking cells. These cells were tested for their surface markers and multilineage differentiation capability. Further more, we analyzed their immune characteristics by mixed lymphocyte reactions (MLRs) and animal experiments. Results: hESC-MSCs show a homogenous fibroblastic morphology that resembles bone marrow-derived MSCs (BM-MSCs). The cell markers and differentiation potential of hESC-MSCs are also similar to those of BM-MSCs. Unlike their original cells, hESC-MSCs possess poor immunogenicity and can survive and be engrafted into a xenogenic immunocompetent environment. Conclusions: The hESC-MSCs demonstrate strong inhibitory effects on lymphocyte proliferation in vitro and anti-inflammatory infiltration properties in vivo. This study offers information essential to the applications of hESC-MSC-based therapies and evidence for the therapeutic mechanisms of action.
Keywords: Human embryonic stem cells, Mesenchymal stem cells, Differentiation, Immunomodulative effects
1. Introduction
Mesenchymal stem cells (MSCs) are multipotent progenitors traditionally found in bone marrow (Friedenstein et al., 1974). Human MSCs (hMSCs) were initially isolated and cultured by Haynesworth et al. (1992). Adult and fetal MSCs stained negatively for CD34 and CD45 and positively for CD73, CD105, and CD166 (Campagnoli et al., 2001; in′t Anker et al., 2003; Sabatini et al., 2005; da Silva Meirelles et al., 2006; Seeberger et al., 2006). MSCs were shown to have the ability to give rise to various mesenchymal tissues such as bone, cartilage, and adipose tissue (Pittenger et al., 1999). Recently, unique immunologic properties of MSCs have been described, such as their poor immunogenicity in vitro and in vivo (Klyushnenkova et al., 2005; Ryan et al., 2005), inhibition of the proliferation and cytotoxicity of natural killer (NK) cells (Spaggiari et al., 2008), suppression of T cell proliferation (di Nicola et al., 2002), and suppression of B cell proliferation and differentiation (Corcione et al., 2006). Taking advantage of their immune privileges, MSCs promise tremendous therapeutic potential. Koç et al. (2002) reported allogenic MSC infusion for the treatment of metachromatic leukodystrophy and Hurler syndrome. Ringdén et al. (2006) reported that MSCs were transplanted for the treatment of severe acute graft-versus-host disease. However, both laboratory and clinical studies have been characterized by the unclear sources and poor reproducibility of MSCs (Rao and Mattson, 2001).
Compared with MSCs, embryonic stem cells (ESCs), pluripotent stem cells derived from the inner cell mass of blastocysts of mammals, are believed to be more versatile (Thomson et al., 1998). Due to their excellent proliferative potential, human ESCs (hESCs) are considered as an alternative to overcome the limitations of hMSCs for regenerative medicine. Several cell types have been developed from hESCs (Carpenter et al., 2001; Kehat et al., 2003; Lavon et al., 2004), though the current differentiation methods are still unsatisfactory. Additionally the risks of tumor formation and immune rejection after hESC transplantation are always a concern (Grinnemo et al., 2006).
Most recently, derivation of MSCs from hESCs was reported by several groups (Barberi et al., 2005; Olivier et al., 2006; Lian et al., 2007). These hESC-derived MSCs (hESC-MSCs) represent an unlimited source of MSCs for cell-based therapy (Hwang et al., 2008; Chen et al., 2009; Karlsson et al., 2009). In this study, we describe an optimized protocol for deriving MSCs from hESCs and characterize the hESC-MSC population by observing cell-surface markers. Further, to address the significant potential of hESC-MSCs in clinical applications, the immunologic properties of these cells were evaluated.
2. Materials and methods
2.1. Cell culture and differentiation
hESC line HUES3 (passage 47) was cultured on mitomycin-C (Kyowa, Japan)-inactivated mouse embryonic fibroblasts (MEFs) in knockout Dulbecco’s modified Eagles medium (knockout-DMEM; Gibco, USA) supplemented with 20% (v/v) knockout serum replacement (SR; Gibco), 2 mmol/L l-glutamine (Gibco), 0.1 mmol/L β-mercaptoethanol (Sigma, USA), 1% (v/v) nonessential amino acids (Gibco), and 10 ng/ml human basic fibroblast growth factor (bFGF; Invitrogen, USA). For expansion, hESCs were subcultured by mechanical dissection every 5–7 d. Tissue culture plates and dishes (Falcon, Becton-Dickinson, USA) were coated with 5 g/L gelatin (Gibco). The cells were cultured at 37 °C in 5% CO2 with daily change of medium.
To induce hESCs differentiation toward hESC-MSCs, cells were pretreated with 10 μmol/L Rho-associated kinase (ROCK) inhibitor (Y-27632, Sigma) for 1 h. Then hESCs were completely dissociated with TrypLE™ Express (Gibco) and plated onto gelatin-coated dishes at 5×104 cm−2 in the medium containing 10 μmol/L Y-27632. After 12 h, the medium was replaced by complete medium (DMEM medium supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco)). After 3 d, non-adherent cells and debris were removed. The adherent cells were cultured continuously in complete medium and passaged using TrypLE™ Express (Gibco) when confluent. Homogeneous fibroblast-like cells could be obtained after 2–3 passages.
2.2. Surface antigen analysis of hESC-MSCs
Cell-surface antigens on hESC-MSCs were assessed using fluorescence activated cell sorting (FACS). hESC-MSCs (passage 8) were collected and resuspended to 5×105 cells in 50 µl of phosphate buffered saline (PBS). The cells were incubated with phycoerythrin (PE)-conjugated rat anti-human CD105, CD45, CD166, CD73, and STRO-1, and PE-Cy7-conjugated rat anti-human CD34 (Becton-Dickinson) on ice for 30 min (Campagnoli et al., 2001; in′t Anker et al., 2003; Sabatini et al., 2005; da Silva Meirelles et al., 2006; Seeberger et al., 2006). For all samples, 1×104 cells were analyzed on an FACSCalibur (Becton-Dickinson). All data were analyzed using CellQuest 3.1 software (Becton-Dickinson).
2.3. Functional differentiation of hESC-MSCs
To further characterize these hESC-MSCs, osteogenic, adipogenic, and chondrogenic differentiations of hESC-MSCs were achieved under conditions described previously for adult MSCs (Pittenger et al., 1999).
For osteogenic differentiation, hESC-MSCs were seeded at 5×103 cells/cm2 in complete medium. At 50% confluence, the medium was supplemented with 50 μmol/L ascorbic acid-2-phosphate, 10 nmol/L dexamethasone, and 10 mmol/L β-glycerophosphate (all from Sigma). The medium was changed every 3 d for three weeks and the cells were analyzed for mineralization with von Kossa staining. For von Kossa staining, the cultures were washed twice with PBS, fixed in Histofix® solution for 30 min, rinsed twice with distilled water, stained with 50 g/L silver nitrate (Sigma) in the dark for 10 min, rinsed thrice with distilled water, and finally exposed to bright light for 30 min.
To induce adipogenic differentiation, cells were seeded at 1×104 cells/cm2. At confluence, cells were incubated with complete medium supplemented with 0.5 mmol/L indomethacin, 100 nmol/L dexamethasone, 10 µg/ml insulin, and 0.2 mmol/L 3-isobutyl-1-methylxanthine (all from Sigma). The medium was changed every 3 d for two weeks. Finally the cells were analyzed for lipid content with oil-red O staining. For oil-red O staining, cells were washed thrice with PBS, fixed in 40 g/L paraformaldehyde for 15 min, and stained with oil-red O solution (Sigma) for 15 min.
Chondrogenic differentiation of hESC-MSCs was induced in a pellet culture system. Briefly, 5×105 cells were placed in a 15-ml polypropylene tube (Falcon), and centrifuged to a pellet. The pellet was cultured at 37 °C with 5% CO2 in 1 ml of complete medium containing 10 ng/ml transforming growth factor-β3 (TGF-β3; PeproTech, USA), 50 µg/ml ascorbic acid-2-phosphate (Sigma), 100 nmol/L dexamethasone (Sigma). The medium was changed every 3 d for three weeks. After induction, the pellets were stained with toluidine blue (Sigma) to detect pericellular sulfated glycosaminoglycan (GAG) deposition.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted by using TRIzol reagent (Invitrogen). Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, USA) and random primers (Promega) were used for first strand complementary DNA (cDNA) synthesis according to the manufacturer’s instructions. PCR was carried out with Taq DNA polymerase (TaKaRa, Japan), and the PCR products were visualized by electrophoresis with a 15 g/L agarose gel containing 0.5 μg/ml ethidium bromide. The target genes, primer sequences, and product sizes are listed in Table 1.
Table 1.
Oligonucleotide primers used in the RT-PCR
| Gene | Primer sequence (5′→3′) | GenBank accession No. | Product size (bp) |
| ALP | Forward: CCCGTGGCAACTCTATCTT | NM_000478 | 553 |
| Reverse: GGGCGGCAGACTTTGGTT | |||
| Osteocalcin | Forward: AGGGCAGCGAGGTAGTGA | NM_199173 | 150 |
| Reverse: CCTGAAAGCCGATGTGGT | |||
| PPARγ | Forward: GCAGAGCAAAGAGGTGGC | NM_015869 | 470 |
| Reverse: AGGACTCAGGGTGGTTCA | |||
| Aggrecan | Forward: AGGAGACAGAGGGACACGTC | NM_013227 | 249 |
| Reverse: TCCACTGGTAGTCTTGGGCAT | |||
| Perlecan | Forward: ACAGCCACCAGTCACCCACG | NM_005529 | 279 |
| Reverse: GCTTCATCAGTTCGGTCCTCACA | |||
| β-actin | Forward: AGCAAGCAGGAGTATGACG | NM_001101 | 354 |
| Reverse: TTTAGGATGGCAAGGGAC |
ALP: alkaline phosphotase; PPARγ: peroxisome proliferator-activated receptor γ
2.5. Mixed lymphocyte reactions (MLRs)
Mouse splenocytes were isolated by mincing and tearing spleens through a stainless steel mesh into PBS. Mononuclear cells (MNCs) were collected by Ficoll density gradient centrifugation. Cells were washed thrice with PBS and resuspended in RPMI-1640 medium supplemented with 2 mmol/L l-glutamine, 0.1 mmol/L β-mercaptoethanol, 1% (v/v) nonessential amino acids, and 10% (v/v) FBS (Azpiroz et al., 1999). After incubation, the cells were harvested as responder lymphocytes. The effect of hESC-MSCs on lymphocyte growth (viability and proliferation) was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrzolium bromide (MTT) absorbance and generating growth curves.
For MTT absorbance assay, the hESC-MSCs treated with mitomycin-C were plated onto 96-well plates at different densities. After incubation for 12 h 1×105 cells/ml lymphocytes suspended in culture medium with or without 10 μg/ml concanavalin A (ConA) were plated in triplicate into the plates. On the day of assay, 2 mg/ml MTT dissolved in PBS was added to each well. After 5 h incubation, the medium was removed and 200 µl dimethyl sulphoxide (DMSO) was added to each well. Then the plates were shaken in the dark for 15 min, and the absorbance was recorded at 570 nm.
For growth curve analysis, 3.2×106 lymphocytes were plated onto 24-well plates in culture medium with or without mitomycin-C treated hESC-MSCs. The total number of lymphocytes in triplicate wells was counted everyday for 5 d.
2.6. Experimental animals and transplantation
We used 6-week-old male BALB/c mice in compliance with the institutional guidelines. For immunization, approximately 2×106 hESCs or hESC-MSCs were injected triweekly by intraperitoneal injection. At 7 d after the fourth injection, the mice were eye-bled, and sera were isolated for immunocytochemistry analysis. For the acute CCl4-induced liver inflammation study, 5.0 ml/kg body weight of 10% CCl4 solution in mineral oil was administered by intraperitoneal injection (Sakaida et al., 2004). The following day, mice underwent cell transplantation and 1×107 hESCs or hESC-MSCs were transplanted into the injured liver of BALB/c mice via the caudal vein. One week after transplantation, the liver sections were analyzed by immunohistochemistry.
2.7. Immunofluorescence staining
For immunocytochemistry analysis, cells were fixed with 40 g/L paraformaldehyde for 30 min, washed with PBS, and then blocked with 50 g/L bovine serum albumin (BSA) at room temperature. Serum samples, mentioned above, rabbit anti-human Nanog antibody (Abcam, USA), and mouse anti-human SSEA-4 antibody (R&D, USA) were used as the primary antibodies. FITC-labeled goat anti-mouse antibody, FITC-labeled goat anti-rabbit antibody (Epitomics, http://www.epitomics.com), and PE-labeled goat anti-mouse antibody were used as the secondary antibodies, respectively. All the samples were counterstained with Hoechst 33258 (Sigma) for 5 min. Cells were visualized under fluorescence microscopy (Olympus IX-70, Japan).
For immunohistochemistry analysis, the liver tissues were fixed by perfusion with 40 g/L paraformaldehyde and cryoprotected in 300 g/L sucrose for 3 d. Histological analyses of liver tissues were conducted by serial tissue section and stained with rabbit anti-human Ki-67 and rabbit anti-mouse CD3 antibodies (Epitomics). The secondary antibody was Dylight 594-labeled goat anti-rabbit antibody (Chemicon, USA).
2.8. Statistical analysis
Statistical comparisons between hESCs and hESC-MSCs were made using unpaired Student’s t-test in SPSS 12.0. Probability values (P) <0.05 were considered statistically significant.
3. Results
3.1. Mesenchymal differentiation of hESCs
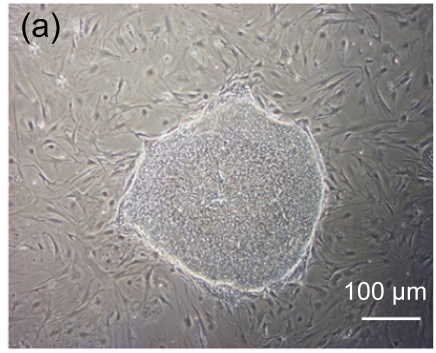
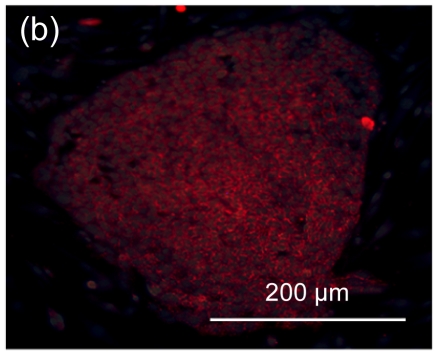
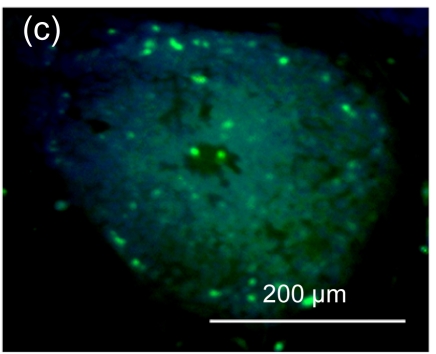
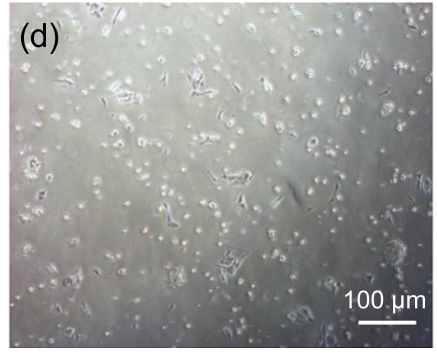
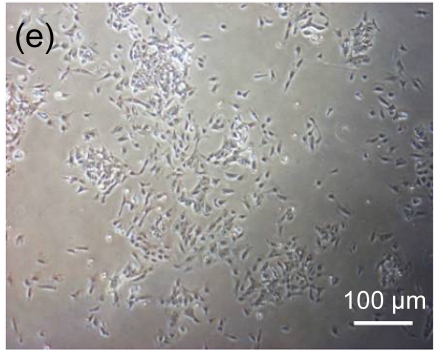
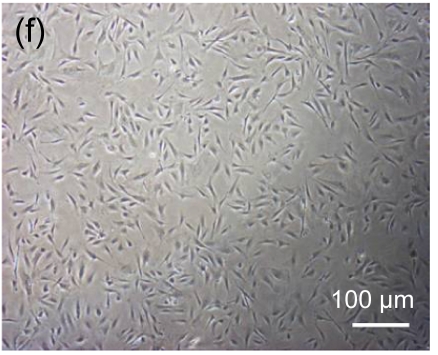
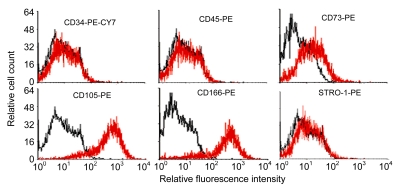
Undifferentiated hESCs showed typical compact colony morphology and stained positively for SSEA-4 and Nanog (Figs. 1a–1c). After 10 d of mesenchymal induction, the differentiated hESCs showed a homogenous fibroblastic morphology that resembled adult MSCs (Figs. 1d–1f). These fibroblast-like cells exhibited many surface markers similar to adult MSCs, including positive staining for CD73, CD105, CD166, and STRO-1, and negative staining for hematopoietic markers such as CD34 and CD45 (Fig. 2).
Fig. 1.
Morphologic observation of hESCs during differentiation into MSC-like cells
(a) hESCs were cultured on mitomycin-C inactivated MEFs; (b, c) Undifferentiated hESCs stained positively for SSEA-4 and Nanog, respectively; (d) hESCs treated with 10 μmol/L Y-27632 were plated into gelatin-coated dishes at low density in DMEM supplemented with 10% FBS; (e) On Day 4, non-adherent cells and debris were removed, and the adherent cells were cultured continuously in complete medium; (f) After 10 d, the cells showed a homogenous fibroblastic morphology that resembled adult MSCs
Fig. 2.
Expression of cell surface markers related to
hESC-MSCs hESC-MSCs exhibited many surface markers similar to adult MSCs, including positive staining for CD73, CD105, CD166, and STRO-1, and negative staining for hematopoietic markers such as CD34 and CD45
3.2. Differentiation potential of hESC-MSCs
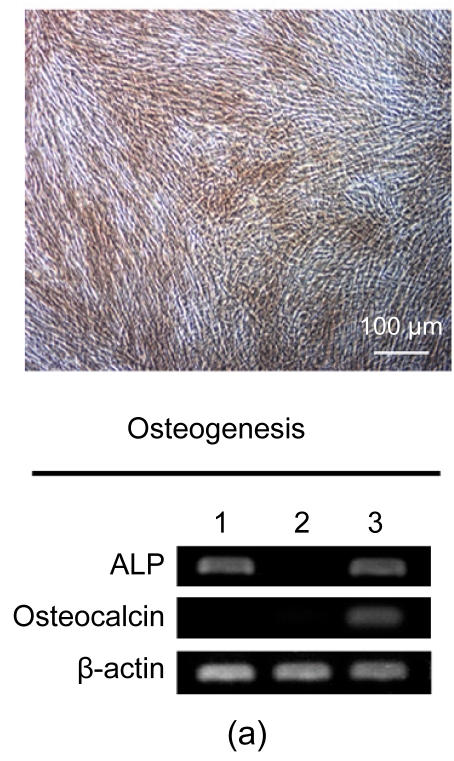
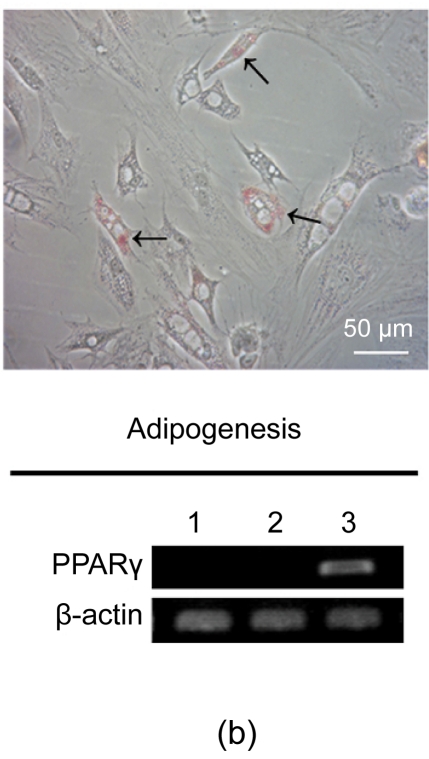
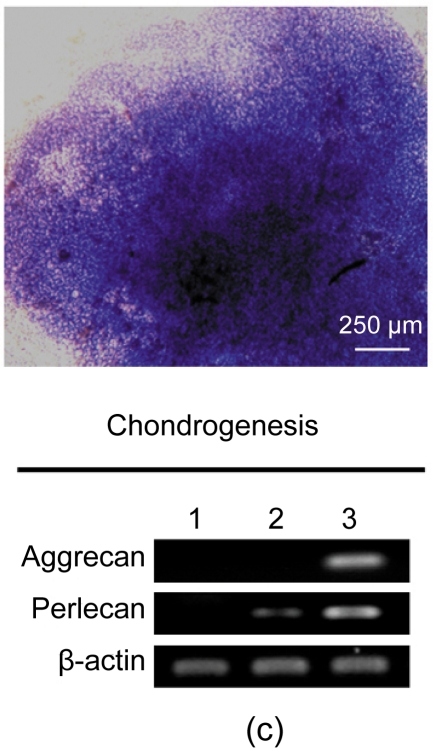
After three weeks, osteogenesis of hESC-MSCs was demonstrated by calcium deposition in the matrix visualized with von Kossa staining and increased expression of alkaline phosphotase (ALP) and osteocalcin. As shown in Fig. 3a, undifferentiated hESCs expressed high levels of ALP compared with the hESC-MSCs, while after osteogenic induction the differentiated hESC-MSCs restored the expression of ALP. Lipid droplets were detectable by oil-red O staining after two weeks of adipocytic induction and peroxisome proliferator-activated receptor γ (PPARγ), a marker of adipocytic differentiation, was detected by RT-PCR (Fig. 3b). After three weeks of induction, chondrogenic differentiation of hESC-MSCs was achieved. More than 80% of all cells stained positively with toluidine blue, showing the GAG biosynthesis in the cell pellets. The expressions of chondrogenic genes, aggrecan and perlecan, two components of extracellular matrix selectively expressed by chondrocytes, were confirmed by RT-PCR (Fig. 3c).
Fig. 3.
Osteogenic, adipogenic, and chondrogenic differentiations of hESC-MSCs
(a) After three weeks, osteogenesis was demonstrated by calcium deposition in the matrix visualized with von Kossa staining and increased expression of ALP and Osteocalcin; (b) Lipid droplets (arrows) were detectable by oil-red O staining after two weeks of adipocytic induction and PPARγ, a marker of adipocytic differentiation, was detected by RT-PCR; (c) While after three weeks of induction, chondrogenic differentiation of hESC-MSCs was achieved. More than 80% of all cells stained positively with toluidine blue. The expressions of chondrogenic genes, aggrecan and perlecan, were confirmed by RT-PCR. Lane 1: hESCs; Lane 2: hESC-MSCs; Lane 3: Induced cells
3.3. Growth inhibitory effect of hESC-MSCs on lymphocytes
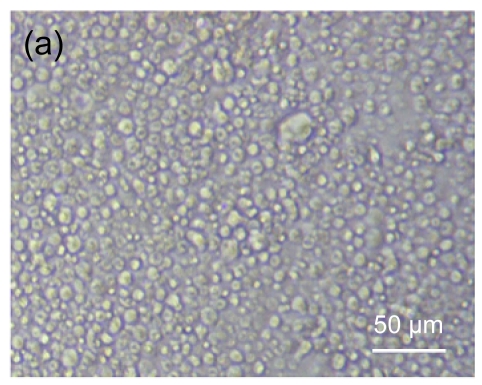
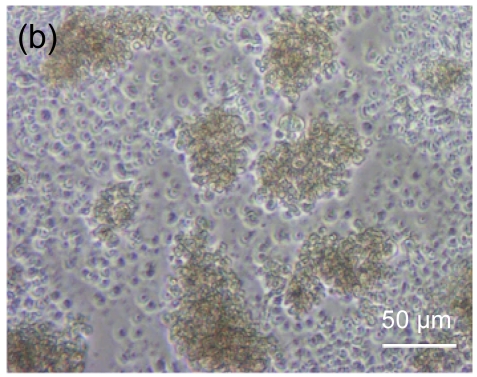
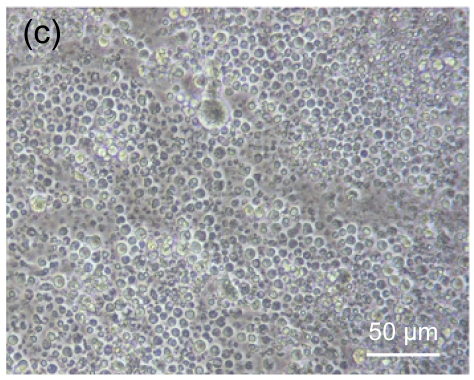
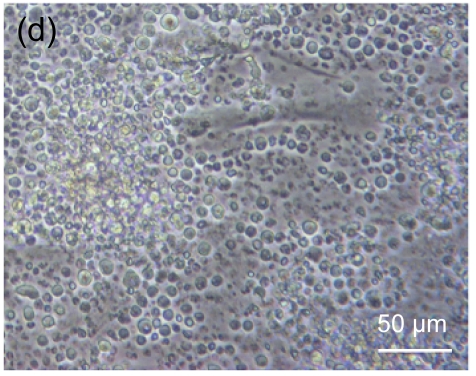
Fig. 4 shows the morphology of lymphocytes co-cultured with mitomycin-C treated hESC-MSCs for 3 d. Resting lymphocytes were scattered and round in shape (Fig. 4a). Lymphocytes showed clustering with the stimulation of ConA (Fig. 4b). Compared with controls, resting lymphocytes co-cultured with hESC-MSCs resembled naive lymphocytes, showing a scattered and round morphology (Fig. 4c), while ConA-stimulated lymphocytes co-cultured with hESC-MSCs showed a unique morphology, neither clustering nor scattering (Fig. 4d).
Fig. 4.
Morphologic observation of lymphocytes co-cultured with mitomycin-C inactivated hESC-MSCs
(a) Resting lymphocytes were scattered and round; (b) Lymphocytes showed clustering with stimulation by ConA; (c) Resting lymphocytes co-cultured with hESC-MSCs resembled naive lymphocytes, showing a scattered and round appearance; (d) ConA-stimulated lymphocytes co-cultured with hESC-MSC showed a unique morphology, neither clustering nor scattering
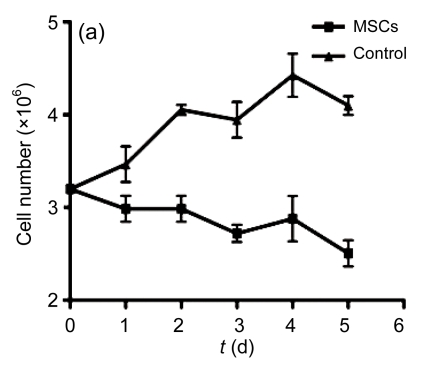
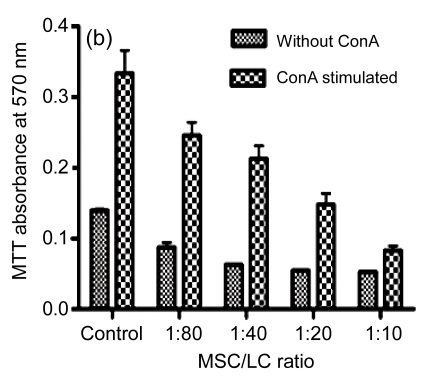
Furthermore, hESC-MSCs suppressed the proliferation of lymphocytes in a dose-dependent fashion, and hESC-MSCs inhibited lymphocyte proliferation effectively, even in low concentrations (Fig. 5b). The growth curve of lymphocytes (LCs) at 1:80 ratio (MSC/LC) is shown in Fig. 5a, indicating a time-dependent reduction of lymphocytes when co-cultured with hESC-MSCs.
Fig. 5.
Suppression of the lymphocyte (LC) proliferation by hESC-MSCs
(a) At ratio 1:80 (MSC/LC), the growth curve of lymphocytes is shown, indicating a time-dependent reduction of lymphocytes when co-cultured with hESC-MSCs; (b) hESC-MSCs inhibited the lymphocyte proliferation in a dose-dependent fashion
3.4. Immunomodulative effect of hESC-MSCs in vivo
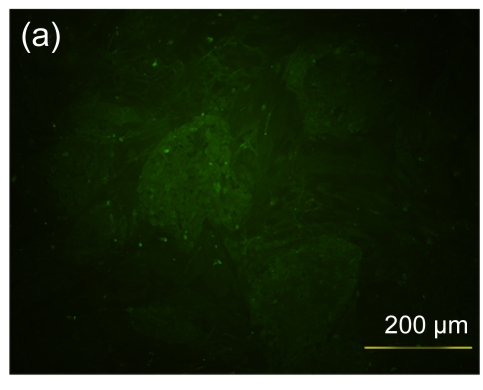
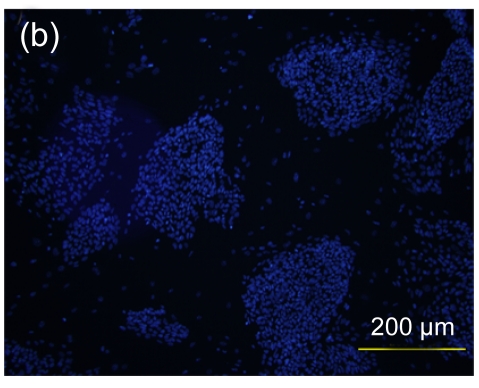
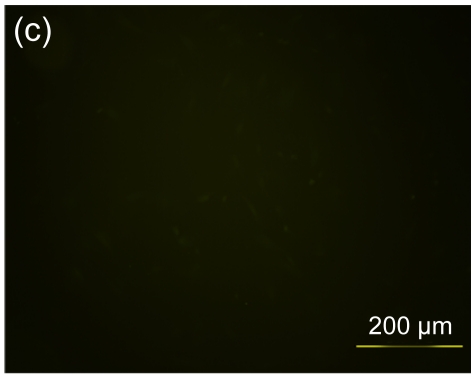
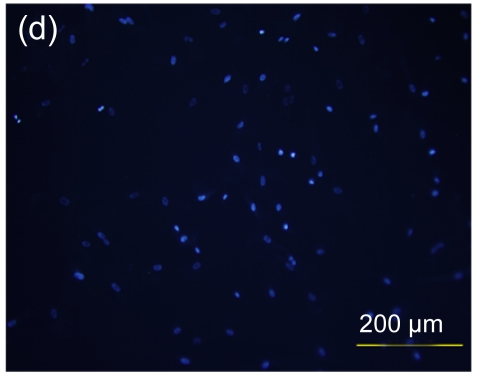
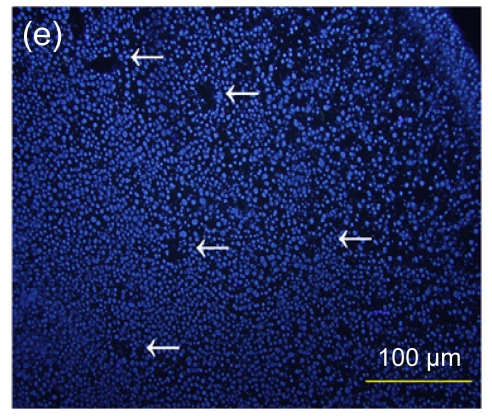
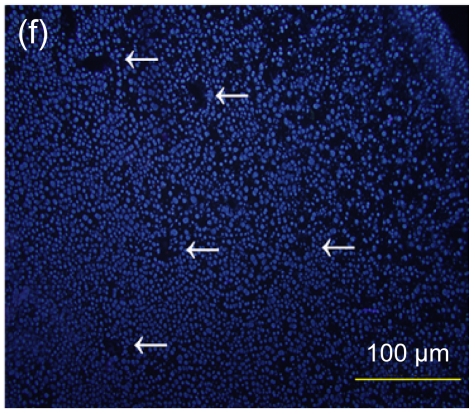
Several experimental studies have demonstrated that hMSCs can survive and be engrafted into an immunocompetent environment (Ryan et al., 2005; Niemeyer et al., 2008). We found that the hESC-MSCs have the same ability. We detected the antibody production of mice immunized with hESCs or hESC-MSCs. The hESC-MSCs failed to induce antibody production in immunocompetent BALB/c mice (Fig. 6). In the xenograft model, as shown in Fig. 7, cells which stained positively with human specific Ki-67 immunostaining mainly accumulated in portal areas where CCl4-induced necrosis and inflammation occurred.
Fig. 6.
Antibody production of hESC or hESC-MSC induction in BALB/c mice
(a) hESCs succeeded in inducing antibody production in immunocompetent BALB/c mice; (b) hESC nuclei were counterstained with Hoechst 33258; (c) hESC-MSCs failed to induce antibody production; (d) hESC-MSC nuclei were counterstained with Hoechst 33258
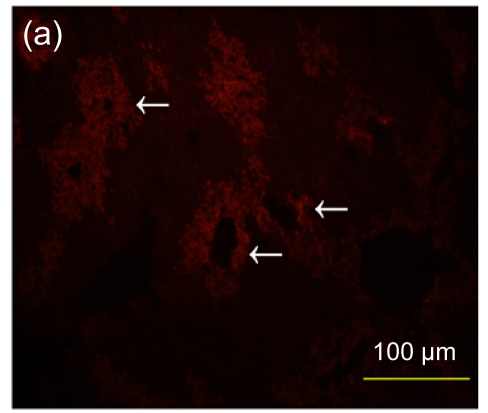
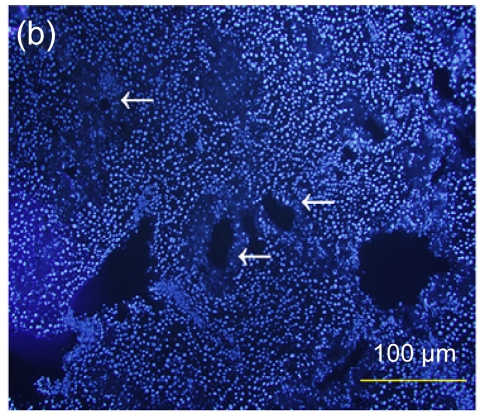
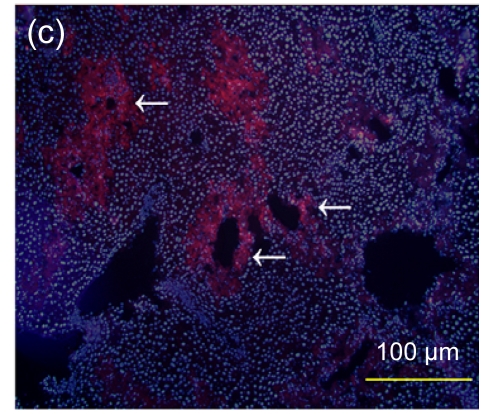
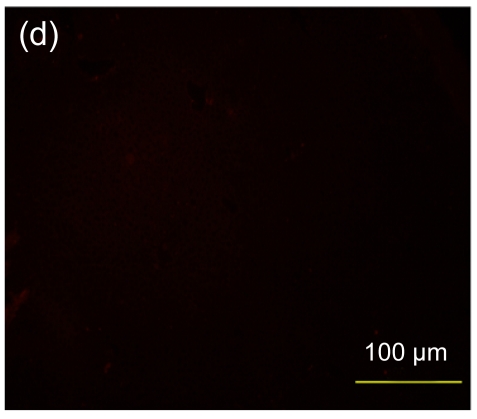
Fig. 7.
Immunofluorescence staining of human specific Ki-67 protein
(a) The positively stained cells mainly accumulated in portal areas (arrows) of the hESC-MSC transplanted mouse liver; (b) Cell nuclei in (a) were counterstained with Hoechst 33258; (d) No positive staining was found in the nontransplanted group; (e) Cell nuclei in (d) were counterstained with Hoechst 33258; (c) Merged image of (a) and (b); (f) Merged image of (d) and (e)
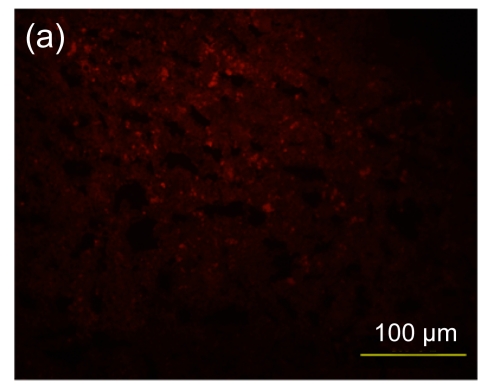
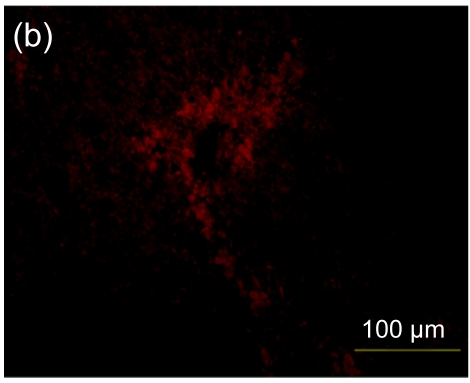
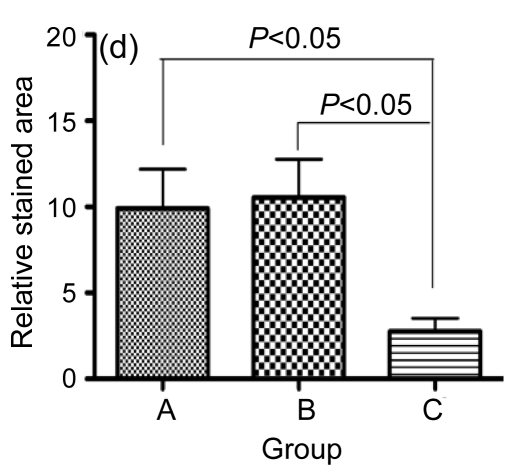
Furthermore, in the hESC-MSC transplant group, there was a significant decrease in positive staining for CD3, indicating that the inflammatory infiltration to the injured area was decreased (Fig. 8c). In the hESC transplant group (Fig. 8b), there was a minor accumulation of CD3+ cells in the inflammatory infiltration area compared with the control group (Fig. 8a). The sectional areas of inflammation were directly measured using National Institutes of Health (NIH) Image J software and differences between groups are shown in Fig. 8d.
Fig. 8.
Immunofluorescence staining of mouse CD3+ cells
A significant decrease in positive staining indicates that inflammatory infiltration was ameliorated in the hESC-MSC transplanted mice (Group C) (c), compared with nontransplanted mice (Group A) (a). A minor increase in inflammatory infiltration was found in the hESC transplanted mice (Group B) (b). (d) Quantitative and statistical analyses
4. Discussion
Embryonic and adult stem cells are two sources of donor cells utilized in stem cell-based therapies. ESCs have the potential to differentiate into any kind of body cell and can proliferate in vitro infinitely. However, they are difficult to control and have a risk for graft rejection (Grinnemo et al., 2006). Adult stem cells with multilineage differentiation capability are relatively safer in application, but are difficult to obtain in large quantities. Moreover, invasive procedures are required to obtain adult stem cells (Pittenger et al., 1999; Rao and Mattson, 2001). Based on others’ work and our findings, MSCs derived from hESCs may be a possible alternative to solve these problems. The hESC-MSCs were remarkably similar to MSCs derived from other sources, including having a homogenous fibroblastic morphology, an immunophenotype similar to adult MSCs, and a multilineage differentiation potential.
In previous studies, different protocols have been described to derive MSC-like cells from hESCs, including co-culture with mouse OP9 cells (Barberi et al., 2005), embryoid body differentiation (Hwang et al., 2008), spontaneous differentiation in monolayer (Trivedi and Hematti, 2008), selection of cell populations by FACS (Lian et al., 2007), and mechanical separation (Olivier et al., 2006). Here we developed a novel, simplified method to obtain a morphologically homogeneous hESC-MSC population. Our derivation protocol required neither xenogenic feeder nor manual selection and showed greater stability. The hESC-MSCs obtained in this study exhibited excellent proliferative capacity and immune privilege. The mechanism that allows pluripotent cells to transform into multipotent cells could help us understand early events of human embryonic development. ROCK inhibitor has been reported to decrease the dependence of hESC on cell-cell contact and permit survival of single hESC (Watanabe et al., 2007). The mesenchymal differentiation of hESCs induced by Y-27632 prompted that cell-cell contact plays an important role in the process of pluripotent to multipotent transformation.
The survival of implanted hMSCs and improvement in tissue function have been described in different animal models (Bruder et al., 1998; Liechty et al., 2000; Toma et al., 2002). Recently, several in vitro studies have provided further evidence that hMSCs possess unique immunologic properties, showing that hMSCs fail to elicit proliferation of allogeneic lymphocytes even in the presence of costimulatory molecules (Bartholomew et al., 2002; di Nicola et al., 2002; le Blanc et al., 2003; Tse et al., 2003). Likewise, hESC-MSCs have a strong immunosuppressive effect on lymphocytes in vitro. Trivedi and Hematti (2008) and Yen et al. (2009) have reported that hESC-MSCs can suppress proliferation of T lymphocytes derived from peripheral blood MNCs. Our findings from MLRs of xenogenic lymphocytes and hESC-MSCs further support their results.
Following transplantation, the in vivo immune responses are much more complicated than those in the in vitro model. Thus our aim was to find out whether hESC-MSCs could survive and play a role in a xenogenic immunocompetent environment in vivo. Interestingly, we found that only weak immune responses could be induced in hESC-MSC transplanted mice compared with hESCs. Furthermore the implanted hESC-MSCs could not only survive and be engrafted into the CCl4-induced injured liver, but also ameliorate the inflammatory infiltration of injured areas. In the process of stem cell-based therapy, transplanted stem cells play roles through four possible mechanisms: (1) homing to injured tissues; (2) inhibiting inflammation by cell-cell contact or cytokines production; (3) promoting survival of damaged cells through paracrine factors; and (4) differentiating to functional cells and replacing the damaged cells (Wang and Li, 2007; Mishra, 2008). Our results demonstrate the effects of hESC-MSC-based therapy in ameliorating inflammation. Future work should investigate whether transplanted hESC-MSCs can differentiate into functional tissues or work as nutritive cells through paracrine secretion. In addition, previous studies reported that cells derived from hESCs have the potential to cause tumors. We did not observe any formation of tumors in our studies, even though large numbers of hESC-MSCs were transplanted.
In summary, hESC-MSCs possess strong immunosuppressive properties, including the inhibition of lymphocyte proliferation triggered by ConA, survival in a xenogenic immunocompetent environment, and amelioration of inflammatory infiltration in injured tissues. With their broad immune privileges, hESC-MSCs may represent a new and unlimited cell source for therapeutic use.
Footnotes
Project (No. 2007CB947804) supported by the National Basic Research Program (973) of China
References
- 1.Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24(3):345–361. doi: 10.1016/S0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 2.Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2(6):e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 4.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16(2):155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 5.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.V98.8.2396. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter M, Inokuma M, Denham J, Mujtaba T, Chiu CP, Rao M. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172(2):383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, Cao T, Zheng M, Ouyang HW. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. 2009;27(6):1276–1287. doi: 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 8.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 10.di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 11.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Grinnemo KH, Kumagai-Braesch M, Mansson-Broberg A, Skottman H, Hao X, Siddiqui A, Andersson A, Stromberg AM, Lahesmaa R, Hovatta O, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13(5):712–724. doi: 10.1016/S1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 13.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 14.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. PNAS. 2008;105(52):20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.in′t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–852. [PubMed] [Google Scholar]
- 16.Karlsson C, Emanuelsson K, Wessberg F, Kajic K, Axell MZ, Eriksson PS, Lindahl A, Hyllner J, Strehl R. Human embryonic stem cell-derived mesenchymal progenitors–potential in regenerative medicine. Stem Cell Res. 2009;3(1):39–50. doi: 10.1016/j.scr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Kehat I, Amit M, Gepstein A, Huber I, Itskovitz-Eldor J, Gepstein L. Development of cardiomyocytes from human ES cells. Methods Enzymol. 2003;365(2):461–473. doi: 10.1016/S0076-6879(03)65032-9. [DOI] [PubMed] [Google Scholar]
- 18.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 19.Koç ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30(4):215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 20.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72(5):230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 21.le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 22.Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, et al. Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells. 2007;25(2):425–436. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 23.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 24.Mishra PK. Bone marrow-derived mesenchymal stem cells for treatment of heart failure: is it all paracrine actions and immunomodulation? J Cardiovasc Med (Hagerstown) 2008;9(2):122–128. doi: 10.2459/JCM.0b013e32820588f0. [DOI] [PubMed] [Google Scholar]
- 25.Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10(8):784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 26.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24(8):1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 27.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 28.Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122(7):713–734. doi: 10.1016/S0047-6374(01)00224-X. [DOI] [PubMed] [Google Scholar]
- 29.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 30.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2(1):8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85(8):962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 32.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 33.Seeberger KL, Dufour JM, Shapiro AM, Lakey JR, Rajotte RV, Korbutt GS. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 34.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2 . Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 35.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36(3):350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 39.Wang XJ, Li QP. The roles of mesenchymal stem cells (MSCs) therapy in ischemic heart diseases. Biochem Biophys Res Commun. 2007;359(2):189–193. doi: 10.1016/j.bbrc.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 41.Yen BL, Chang CJ, Liu KJ, Chen YC, Hu HI, Bai CH, Yen ML. Brief report–human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells. 2009;27(2):451–456. doi: 10.1634/stemcells.2008-0390. [DOI] [PubMed] [Google Scholar]