Abstract
Objective: This study was carried out to test the effects of methotrexate (MTX) and black seed oil (BSO) on pristane-induced arthritis (PIA) in rats. Methods: Inbred dark agouti (DA) rats were induced by a single subcutaneous injection of pristane, and then treated with MTX or BSO. Arthritis severity was evaluated macroscopically and microscopically. Plasma nitric oxide (NO) concentration was determined by the Griess method and cytokine mRNA expression in the spleen was detected by the real-time reverse transcription-polymerase chain reaction (RT-PCR). Results: The clinical arthritis severity was decreased after MTX treatment, while the BSO groups did not show significant changes compared with the disease group. The plasma NO level of the MTX group was significantly decreased compared with the disease group, but the BSO groups showed no difference from the disease group in plasma NO levels. The interferon-γ (IFN-γ) and interleukin-17A (IL-17A) mRNA expressions in the spleens were significantly decreased in the MTX group, but only showed a declining trend in the BSO groups compared with the disease group. Neither MTX nor BSO had an effect on the mRNA expressions of IL-4, transforming growth factor β (TGF-β), and tumor necrosis factor-α (TNF-α) in the spleen. Conclusions: MTX, but not BSO, can reduce the arthritis severity and decrease the mRNA expressions of IFN-γ and IL-17A in pristane-induced arthritis of rats.
Keywords: Methotrexate (MTX), Black seed oil (BSO), Pristane-induced arthritis (PIA), Interferon-γ (IFN-γ), Interleukin-17A (IL-17A)
1. Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease that principally affects diarthrodial joints, and the central pathology is synovitis (Helliwell et al., 2000; Yamamoto, 2009). The mechanism of RA is complicated and still unclear. The commonly used agents for RA are non-specific and have many adverse effects (Lee and Weinblatt, 2001; Klareskog et al., 2006; Pratt et al., 2009).
Methotrexate (MTX) is the most frequently used disease-modifying anti-rheumatic drug (DMARD). Although MTX can prevent de novo pyrimidine and purine synthesis, the mechanism of MTX in RA remains obscure (Alarcón, 2000; Cronstein, 2005; Wessels et al., 2007; McLean-Tooke et al., 2009). Black seed oil (BSO) obtained from Nigella sativa has long been used in many kinds of illnesses, such as asthma, headache, hypertension, eczema, and rheumatism (Salem, 2005; Shahzad et al., 2009). Tekeoglu et al. (2006) reported that thymoquinone (TQ), one of the main constituents of BSO, can suppress the clinical and radiological severities of adjuvant-induced arthritis in Sprague-Dawley and Wistar rats.
In recent years, many researchers have focused on imbalances within the immune system to explain the development of RA and its therapy. T helper cells and their cytokines are important factors in regulating immune balance. Interferon-γ (IFN-γ), interleukin-4 (IL-4), and interleukin-17A (IL-17A) represent the hallmarks for Th1, Th2, and Th17 cells, respectively (Mosmann and Coffman, 1989; Ivanov et al., 2006; Mucida et al., 2007; Bettelli et al., 2008).
In this study, we hypothesized that MTX or BSO can ameliorate pristane-induced arthritis (PIA) by regulating immune balance. Arthritis in dark agouti (DA) rats was induced by pristane and then treated with MTX or BSO. We observed the arthritis severity macroscopically and microscopically, and measured gene expressions of IFN-γ, IL-4, IL-17A, transforming growth factor β (TGF-β), and tumor necrosis factor-α (TNF-α) in the rat spleens.
2. Materials and methods
2.1. Animals
DA rats (originated from Zentralinstitut für Versuchstierzucht, Hannover, Germany) were bred in the animal house under specific pathogen free (SPF) conditions with a 12 h:12 h of light/dark cycle and air-conditioning. The rats were housed in polystyrene cages with standard rodent chow and water ad libitum. Rats at the age of 8–12 weeks were randomly divided into seven groups matched for sex and age: four rats in the control group (NC) and eight rats in each of BSO prevention low dose group (PL), BSO prevention high dose group (PH), BSO treatment low dose group (TL), BSO treatment high dose group (TH), MTX treatment group (MTX), and disease group (D). Rats were anesthetized intraperitoneally by 20 mg/ml pentobarbital sodium (0.15 ml/100 g body weight (BW)) at 26 d post-induction. Blood samples were collected into heparin sodium anticoagulation tubes (Jiangxi Sophisticated Medical Equipment Co., China) by abdominal aorta punctures, and then were centrifuged at 3 000 r/min for 20 min, and stored at −80 °C. Left posterior paws were removed and fixed in 40 mg/ml paraformaldehyde. Spleens were collected and stored at −80 °C. All experiments had been approved by the Institutional Animal Ethics Committee of Xi’an Jiaotong University, China.
2.2. Induction and evaluation of arthritis
Arthritis was induced by a single subcutaneous injection of 150 μl pristane (Sigma-Aldrich, USA) at the base of the rat tail. Rats in the NC group were injected with 150 μl phosphate buffered saline (PBS) instead.
A macroscopic scoring system was used to monitor arthritis development once every two days after pristane injection in all four limbs. The redness and swelling of each joint were observed, and the maximum score was 15 points per limb.
Rat left posterior paws were fixed and decalcified, then stained with hematoxylin and eosin (H&E). A pathological scoring system was used to evaluate the severity of arthritis. Synovitis, inflammation, and repair each were given the maximum of 3 points.
2.3. Treatment with BSO and MTX
The rats were given BSO (Quinessence Aromatherapy Ltd., UK) at different time points by intragastric administration. We began to give the medicine one day before induction in the prevention groups, and started to administrate the medicine at 13 d after induction in the treatment groups, as our previous observations showed that the average onset of PIA was at 14 d after induction (Meng et al., 2010). Both groups were given the medicine continuously for 5 d. Two dose groups of BSO for both prevention and treatment were used, with 2.5 and 5 ml/kg BW for low and high doses, respectively. MTX (Pfizer Pharmaceuticals Ltd., Australia) was given by intramuscular injection at the dose of 1 mg/kg BW once at 13 d after induction. PBS was used in control rats.
2.4. Plasma nitric oxide (NO) detection
Plasma NO level was assayed by the Griess reaction. Briefly, 100 μl blood plasma was mixed with 80 μl of 375 mmol/L ZnSO4 and 120 μl of 275 mmol/L NaOH, and then centrifuged at 13 000 r/min for 20 min. The supernatant was obtained and added with 400 mg of copper-plated cadmium and 100 μl of 0.2 mol/L glycine buffer (pH=7.5). The mixture was shaken for 2.5 h at room temperature. Then 100 µl of supernatant of each well was added into 96-well plates, and reacted with 100 µl Griess reagent (1 mg/ml N-(1-naphthyl) ethylenediamine dihydrochloride:10 mg/ml sulfanilamide) to develop color. The optical density (OD) was measured at 545 nm wavelength by a microplate reader (Thermo Electron Co., Finland). The NO concentration was obtained by calculating OD values against a standard curve from gradient concentrations of sodium nitrite.
2.5. RNA isolation and mRNA expression detection
Total RNA was isolated from the spleens using the TRIzol method (Invitrogen, USA). The complementary DNA (cDNA) was obtained by reverse transcription kits according to the manufacturer’s protocol (RevertAid, Fermentas Life Sciences, Canada). The IFN-γ, IL-17A, IL-4, TGF-β, and TNF-α mRNA expression levels in the spleen were determined by the real-time reverse transcription-polymerase chain reaction (RT-PCR), and the primers used are shown in Table 1. Then cDNA expression was determined by SYBR Green II Supermix (TaKaRa, China) using the iQ5 real-time PCR detection system (Bio-Rad, USA).
Table 1.
Primers used for amplification of cytokines by real-time RT-PCR
| Cytokine | Primer sequence | Amplified length (bp) | Annealing temp. (°C) |
| IFN-γ | Sense: CCCTCTCTGGCTGTTACTGC | 149 | 65 |
| Antisense: TTTCGTGTTACCGTCCTTTTG | |||
| IL-17A | Sense: CTACCTCAACCGTTCCACTT | 191 | 65 |
| Antisense: ACTTCTCAGGCTCCCTCTTC | |||
| IL-4 | Sense: TGCACCGAGATGTTTGTACCAGA | 92 | 63 |
| Antisense: TTGCGAAGCACCCTGGAAG | |||
| TGF-β | Sense: GGACTACTACGCCAAAGAAG | 294 | 64 |
| Antisense: TCAAAAGACAGCCACTCAGG | |||
| TNF-α | Sense: TCAGCCTCTTCTCATTCCTGC | 202 | 60 |
| Antisense: TTGGTGGTTTGCTACGACGTG |
2.6. Statistical analysis
Data were expressed as mean±standard error of mean (SEM). Mann-Whitney test was used to analyze all the experiments. The correlation analysis between two parameters was performed by Pearson method. A P value <0.05 was considered statistically significant.
3. Results
3.1. Effects of MTX and BSO on clinical arthritis severity
The clinical arthritis severity was evaluated by a macroscopic scoring system once every two days. The arthritis severity started to decrease after MTX treatment, while that of rats in the BSO groups only had declining trends, but no significant changes compared with the disease group, regardless of whether the BSO group was prevention or treatment, low or high dosage (Fig. 1a ).
Fig. 1.
Evaluation of clinical and pathological arthritis scores and plasma NO levels of rats in each group
(a) Clinical arthritis score of each group rats after pristane induction; (b) Pathological evaluation of ankle joints by H&E staining. A pathological scoring system was adopted to evaluate the severity of arthritis. ‘Pathology’ indicated total pathological arthritis scores of ankle, which include synovitis, destruction, and repair; (c) Plasma NO level of each group rats detected by the Griess reaction method. Mann-Whitney test was used to analyze the data: * P<0.05 compared with the NC group. All data are expressed as mean±SEM. NC: normal control group; D: disease group; PL: BSO prevention low dose group; PH: BSO prevention high dose group; TL: BSO treatment low dose group; TH: BSO treatment high dose group; MTX: MTX treatment group
3.2. Effects of MTX and BSO on ankle joint pathology
Left posterior paw of each rat was harvested at 26 d after immunization and stained with H&E. The ankle joint was evaluated by a pathological scoring system, which considers synovitis, joint destruction, and repair. The pathological score was correlated with the clinical arthritis score (r=0.573, P=0.007). There was no difference of pathological scores among the D, PL, PH, TL, TH, and MTX groups (Fig. 1b). Thus, both BSO and MTX did not affect ankle joint pathology at 26 d after pristane injection.
3.3. Effects of MTX and BSO on plasma NO levels in PIA rats
Plasma NO levels of the D, PL, PH, and TL groups were significantly increased compared with those of the NC group. Plasma NO levels of the MTX group were significantly decreased compared with those of the D group, and there was no difference in plasma NO levels between the NC and MTX groups (Fig. 1c). We concluded that MTX could decrease plasma NO levels in PIA rats.
3.4. Effects of MTX and BSO on the mRNA expressions of IFN-γ and IL-17A in rat spleens
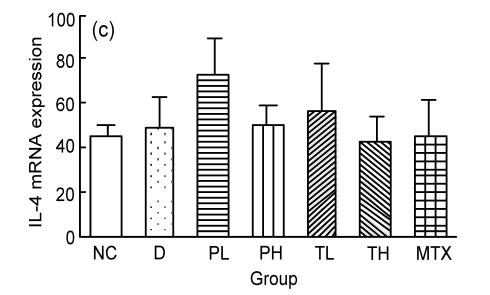
Spleen total RNA was isolated and reverse-transcribed into cDNA. The IFN-γ and IL-17A mRNA expressions were significantly decreased in the MTX group, but in the BSO groups, only IL-17A showed declining trends compared with the disease group (Figs. 2a and 2b). The IL-17A mRNA expression was correlated with repair (r=0.835, P=0.001), while IFN-γ was correlated with plasma NO levels (r=0.511, P=0.002). Neither MTX nor BSO affected the expressions of IL-4, TGF-β, and TNF-α (Figs. 2c, 2d, and 2e).
Fig. 2.
Effect of MTX on IFN-γ (a), IL-17A (b), IL-4 (c), TGF-β (d), and TNF-α (e) mRNA expressions in PIA rat spleens by real-time quantitative PCR
MTX can decrease IFN-γ and IL-17A mRNA expressions in the spleens. Mann-Whitney test was used to analyze the data: * P<0.05 compared with the D group. All data are expressed as mean±SEM. NC: normal control group; D: disease group; PL: BSO prevention low dose group; PH: BSO prevention high dose group; TL: BSO treatment low dose group; TH: BSO treatment high dose group; MTX: MTX treatment group
4. Discussion
Our results indicate that MTX ameliorates the clinical arthritis severity and decreases the IFN-γ and IL-17A mRNA expressions in PIA rat spleens. The clinical arthritis score and the mRNA expressions of IFN-γ and IL-17A had declining trends (P>0.05) in the BSO groups.
RA is a chronic polyarthritis disorder, and PIA is one of its animal models, a T cell-dependent model which can be used for mechanism and therapy research (Lu et al., 2002; Holmberg et al., 2006; Olofsson and Holmdahl, 2007; Smolen and Aletaha, 2009). It has been shown that T cells isolated from the PIA rat spleens transfer severe arthritis, which indicates that the critical arthritogenic change induced by pristane is present in the spleen (Holmberg et al., 2006). Therefore, we focused on the spleen to study the immune reaction after pristane injection.
NO reflects an immune-activated state with upregulated activity of inducible nitric oxide synthase (iNOS) by inflammatory mediators (Reddy et al., 2005). In RA patients, the activity of iNOS is increased and the plasma NO level is higher, in comparison with the normal control (Savnik et al., 2001; Romas et al., 2002). The synovial fluid nitrite, a breakdown product of NO, was also increased in RA patients. Synovial fluid nitrite concentration was significantly correlated with serum nitrite concentration in patients with RA (Farrell et al., 1992). Previously, we have found that the plasma NO level is also increased in PIA rat models (Meng et al., 2010). In this study, our results showed that MTX reduced plasma NO levels in PIA rats, indicating that MTX can decrease the inflammatory level of the disease.
MTX is commonly known to directly inhibit dihydrofolate reductase, thymidylate synthase, and 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, and also to indirectly inhibit methylenetetrahydrofolate reductase. Thus, it mainly prevents de novo pyrimidine and purine syntheses (Celtikci et al., 2009; Patiño-García et al., 2009; Shimura et al., 2009). It has been reported that MTX efficiently suppresses PIA when given after the onset of arthritis, but the mechanism remains obscure (Lange et al., 2005). Recently, many studies have shown that MTX can inhibit cell proliferation and induce apoptosis in a dose- and time-dependent manner, and this effect is different in different cell types (de Lathouder et al., 2004; Wessels et al., 2007). MTX can also influence cytokine production. MTX inhibits the upregulations of IL-6, IFN-γ, and IL-17A in the coculture of T cells and fibroblasts (Miranda-Carus et al., 2004). Seitz et al. (2003) cultured the whole blood of healthy donors and RA patients, and found that MTX inhibited the productions of IL-4, IL-6, IL-13, IFN-γ, TNF-α, and granulocyte-macrophage colony-stimulating factors which were secreted by activated T cells. Interestingly, MTX did not affect cytokines produced by monocytes (Gerards et al., 2003). Thus, MTX plays the role of inhibiting activated T cells.
As we have shown here, MTX can decrease the over expressions of IFN-γ and IL-17A in PIA rats, which is consistent with the findings from in vitro studies (Seitz et al., 2003). Traditionally, we know that Th1 cells, which mainly secrete IFN-γ, play an important role in the pathogenesis of RA (Herman et al., 2008). Th17, a new designated T-helper cell type marked by IL-17A, IL-17F, and IL-22 secretions, has been shown to be closely related to RA (Harrington et al., 2005; Park et al., 2005). MTX can ameliorate RA by inhibiting the activation of not only Th1 but also Th17.
BSO has shown anti-inflammatory effects in asthma, experimental allergic encephalomyelitis, colitis, etc. (Abbas et al., 2005; Salem, 2005). BSO can play the immunomodulatory role in vivo. We previously reported that BSO ameliorated allergic asthma by inhibiting T cell proliferation in E3 rats. The mRNA and protein expression levels of IL-4, IL-5, and IL-6 were reduced to normal in BSO treated group rats (Shahzad et al., 2009). In this study, the BSO groups had declining trends of IL-17A, but no effect on IL-4 and IFN-γ expressions. Budancamanak et al. (2006) and Tekeoglu et al. (2006) reported that TQ had a treatment effect on adjuvant induced or collagen induced arthritis rat models. This observation may be caused by the use of different arthritis models and different medicinal components.
In conclusion, our findings support that MTX inhibits activated Th1 and Th17 cells in the treatment of PIA, but BSO has little effect on this model.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30630058, 30801027, and 30571725), and the Shaanxi Province International Cooperation Foundation of China (No. 2007-KW-06)
References
- 1.Abbas AT, Abdel-Aziz MM, Zalata KR, Al-Galel Tel DA. Effect of dexamethasone and Nigella sativa on peripheral blood eosinophil count, IgG1 and IgG2a, cytokine profiles and lung inflammation in murine model of allergic asthma. Egypt J Immunol. 2005;12(1):95–102. [PubMed] [Google Scholar]
- 2.Alarcón GS. Methotrexate use in rheumatoid arthritis. A clinician’s perspective. Immunopharmacology. 2000;47(2-3):259–271. doi: 10.1016/S0162-3109(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453(7198):1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budancamanak M, Kanter M, Demirel A, Ocakci A, Uysal H, Karakaya C. Protective effects of thymoquinone and methotrexate on the renal injury in collagen-induced arthritis. Arch Toxicol. 2006;80(11):768–776. doi: 10.1007/s00204-006-0094-0. [DOI] [PubMed] [Google Scholar]
- 5.Celtikci B, Lawrance AK, Wu Q, Rozen R. Methotrexate-induced apoptosis is enhanced by altered expression of methylenetetrahydrofolate reductase. Anti-Cancer Drugs. 2009;20(9):787–793. doi: 10.1097/CAD.0b013e32832f4aa8. [DOI] [PubMed] [Google Scholar]
- 6.Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev. 2005;57(2):163–172. doi: 10.1124/pr.57.2.3. [DOI] [PubMed] [Google Scholar]
- 7.de Lathouder S, Gerards AH, Dijkmans BA, Aarden LA. Two inhibitors of DNA-synthesis lead to inhibition of cytokine production via a different mechanism. Nucleosides Nucleotides Nucleic Acids. 2004;23(8-9):1089–1100. doi: 10.1081/NCN-200027365. [DOI] [PubMed] [Google Scholar]
- 8.Farrell AJ, Blake DR, Palmer RMJ, Moncada S. Increased concentrations of nitrite in synovial-fluid and serum samples suggest increased nitric-oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51(11):1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology. 2003;42(10):1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 10.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 11.Helliwell PS, Hetthen J, Sokoll K, Green M, Marchesoni A, Lubrano E, Veale D, Emery P. Joint symmetry in early and late rheumatoid and psoriatic arthritis: comparison with a mathematical model. Arthritis Rheum. 2000;43(4):865–871. doi: 10.1002/1529-0131(200004)43:4<865::AID-ANR18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M. Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin Exp Rheumatol. 2008;26(2):317–323. [PubMed] [Google Scholar]
- 13.Holmberg J, Tuncel J, Yamada H, Lu S, Olofsson P, Holmdahl R. Pristane, a non-antigenic adjuvant, induces MHC class II-restricted, arthritogenic T cells in the rat. J Immunol. 2006;176(2):1172–1179. doi: 10.4049/jimmunol.176.2.1172. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Klareskog L, Padyukov L, Ronnelid J, Alfredsson L. Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol. 2006;18(6):650–655. doi: 10.1016/j.coi.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Lange F, Bajtner E, Rintisch C, Nandakumar KS, Sack U, Holmdahl R. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Ann Rheum Dis. 2005;64(4):599–605. doi: 10.1136/ard.2004.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 18.Lu S, Nordquist N, Holmberg J, Olofsson P, Pettersson U, Holmdahl R. Both common and unique susceptibility genes in different rat strains with pristane-induced arthritis. Eur J Hum Genet. 2002;10(8):475–483. doi: 10.1038/sj.ejhg.5200832. [DOI] [PubMed] [Google Scholar]
- 19.McLean-Tooke A, Aldridge C, Waugh S, Spickett GP, Kay L. Methotrexate, rheumatoid arthritis and infection risk–what is the evidence? Rheumatology. 2009;48(8):867–871. doi: 10.1093/rheumatology/kep101. [DOI] [PubMed] [Google Scholar]
- 20.Meng L, Zhu W, Jiang C, He X, Hou W, Zheng F, Holmdahl R, Lu S. Toll-like receptor 3 upregulation in macrophages participates in the initiation and maintenance of pristane-induced arthritis in rats. Arthritis Res Ther. 2010;12(3):R103. doi: 10.1186/ar3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173(2):1463–1476. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 22.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7(1):145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 23.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 24.Olofsson P, Holmdahl R. Pristane-induced arthritis in the rat. Arthritis Res. 2007;136(2):255–268. doi: 10.1007/978-1-59745-402-5_19. [DOI] [PubMed] [Google Scholar]
- 25.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patiño-García A, Zalacain M, Marrodan L, San-Julian M, Sierrasesumaga L. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009;154(5):688–693. doi: 10.1016/j.jpeds.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Pratt AG, Isaacs JD, Mattey DL. Current concepts in the pathogenesis of early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2009;23(1):37–48. doi: 10.1016/j.berh.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy SVB, Wanchu A, Khullar M, Govindrajan S, Bambery P. Leflunomide reduces nitric oxide production in patients with active rheumatoid arthritis. Int Immunopharmacol. 2005;5(6):1085–1090. doi: 10.1016/j.intimp.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Romas E, Sims NA, Hards DK, Lindsay M, Quinn JWM, Ryan PFJ, Dunstan CR, Martin TJ, Gillespie MT. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161(4):1419–1427. doi: 10.1016/S0002-9440(10)64417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13-14):1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold-Samsoe B, Boesen J, Bliddal H. Magnetic resonance imaging of the wrist and finger joints in patients with inflammatory joint diseases. J Rheumatol. 2001;28(10):2193–2200. [PubMed] [Google Scholar]
- 32.Seitz M, Zwicker M, Villiger PM. Pretreatment cytokine profiles of peripheral blood mononuclear cells and serum from patients with rheumatoid arthritis in different American college of rheumatology response groups to methotrexate. J Rheumatol. 2003;30(1):28–35. [PubMed] [Google Scholar]
- 33.Shahzad M, Yang X, Raza Asim MB, Sun Q, Han Y, Zhang F, Cao Y, Lu S. Black seed oil ameliorates allergic airway inflammation by inhibiting T-cell proliferation in rats. Pulm Pharmacol Ther. 2009;22(1):37–43. doi: 10.1016/j.pupt.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Shimura C, Satoh T, Takayama K, Yokozeki H. Methotrexate-related lymphoproliferative disorder with extensive vascular involvement in a patient with rheumatoid arthritis. J Am Acad Dermatol. 2009;61(1):126–129. doi: 10.1016/j.jaad.2008.10.051. [DOI] [PubMed] [Google Scholar]
- 35.Smolen JS, Aletaha D. Developments in the clinical understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11(1):204. doi: 10.1186/ar2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tekeoglu I, Dogan A, Demiralp L. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2006;20(10):869–871. doi: 10.1002/Ptr.1964. [DOI] [PubMed] [Google Scholar]
- 37.Wessels JAM, Huizinga TWJ, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2007;47(3):249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T. Cutaneous manifestations associated with rheumatoid arthritis. Rheumatol Int. 2009;29(9):979–988. doi: 10.1007/s00296-009-0881-z. [DOI] [PubMed] [Google Scholar]