Abstract
Eucommia ulmoides Oliv. (EuO), also known as Duzhong, native to China, has been reported to have antioxidative function, but its cellular mechanism is not fully examined yet. We investigated inhibitory effects of EuO leaf ethanol extracts on H2O2-induced apoptosis in rat osteoblastic MC3T3-E1 cells and underlying mechanisms. Locally-grown Duzhong leaves were extracted with ethanol. MC3T3-E1 cells were treated with EuO (6.25, 12.5, 25, 50, and 100 µg/ml) for 24 h, and then H2O2 (800 µmol/L) for an additional 24 h. Cell survival rate, percentage of apoptosis, and expressions of caspases 3, 6, 7, and 9 were examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, microscopic analysis, Western blotting, and reverse transcription polymerase chain reaction (RT-PCR). The final EuO leaf ethanol extract powder was detected to contain caffeotannic acid at 58 mg/g and geniposide at 3.45 mg/g by high performance liquid chromatography (HPLC). EuO remarkably restrained cell oxidative damage and increased cell survival rate in a dose-dependent manner: 0 µg/ml, 0.21; 6.25 µg/ml, 0. 28; 12.5 µg/ml, 0.31; 25 µg/ml, 0.48; 50 µg/ml, 0.54; and 100 µg/ml, 0.66 (P<0.05), with the half-effective concentration being around 25 µg/ml. MTT results were confirmed by microscopic analysis. Western blotting and RT-PCR analyses showed that the expressions of caspases 3, 6, 7, and 9 were significantly decreased in the EuO-treated cells compared with the control (EuO- and H2O2-free) (P<0.05), with the half-effective concentration of EuO ranging from 12.5 to 25 µg/ml. We conclude that the ethanol-extracted EuO leaf extracts promoted the growth of MC3T3-E1 cells, and suppressed the H2O2-induced apoptosis in a rat MC3T3-E1 osteogenic cell model, likely due to the inhibition of caspases’ activities. The results indicate that EuO is a potent antioxidant, which may contribute to its many cellular protective functions, including the promotion of bone growth.
Keywords: Eucommia ulmoides Oliv., Duzhong, Oxidative damage, H2O2, MC3T3-E1 cells, Free radicals, Apoptosis
1. Introduction
Eucommia ulmoides Oliv. (EuO), also known as Duzhong, native to China, is the only plant in the Eucommia genus of the Eucommiaceae family. The bark and leaf of EuO have been used in traditional Chinese medicine to treat lower back pain, knee pain, and the liver, kidney, and spleen disorders. EuO extracts have antihypertensive, antioxidative, and antigastric ulcer effects (Metori et al., 1997; Deyama et al., 2001; Yang et al., 2003). Water extracts of EuO leaves have been reported to have potent antimutagenic effects (Nakamura et al., 1997; Lee et al., 2005; Luo et al., 2009). EuO extracts have also been reported to enhance collagen synthesis (Metori et al., 1997; Li et al., 2000a; 2000b; Deyama et al., 2001). It is generally assumed that natural substances in EuO give rise to these beneficial effects. The chemical constituents of EuO leaves contain phenylpropanoid compounds such as sitosterol, ursolic acid, and chlorogenic acid; iridoids such as eucommiol, geniposidic acid, geniposide, and aucubin; and flavonoids such as kaempferol and quercetin.
Previous pharmacological action studies of EuO have much focused on its function in promoting osteogenesis. Li et al. (1998) reported that collagen synthesis in the aged-rat model was stimulated by the administration of hot water-extracts from EuO leaves. Ha et al. (2003) reported that EuO influences osteoblastic differentiation and prevents bone loss, and concluded that an EuO fraction with high polarity might contain active constituents stimulating the proliferation of osteoblasts. It is thought that some components of fractions of EuO cortex water-extract may participate in each step of the activation of osteoblasts, which, in turn, facilitates osteogenesis and suppresses osteoblast activity to inhibit osteolysis (Ha et al., 2003; Miyake et al., 2003; Prouillet et al., 2004). EuO also contains compounds, such as geniposidic acid, effective against aging (Li et al., 2000b).
The antioxidative effects of EuO have been another research focus. It is reported that EuO leaves contain antioxidative and free radical scavenging components. EuO could possibly act as a prophylactic agent to prevent free radical-related diseases (Cho et al., 2003; Yen and Hsieh, 2003), because it contains flavonoids, a principal contributor to antioxidant activity, that have the obvious capability of eliminating superoxide anion (O2 −) and hydroxy free radicals (·OH). When under stress conditions such as trauma, an organism will produce reactive oxygen species (ROS) such as O2 −, hydrogen peroxide (H2O2), and ·OH, which are catalyzed by superoxide dismutase (SOD), and also oxidation enzymes, resulting in cellular damage and apoptosis (Chen et al., 2006). Therefore, it is clear that EuO plays a role in influencing apoptosis. However, its mechanisms on the apoptotic process have not been widely examined.
The goal of this study was to investigate antioxidative effects of EuO extracts and the underlying mechanisms. An experimental model of MC3T3-E1 cells, a rat osteogenesis cell line, with H2O2-induced apoptosis was established. Treated with various concentrations of EuO, MC3T3-E1 cells’ survival rate, percentage of apoptosis, and expressions of caspases 3, 6, 7, and 9 were examined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, Western blotting analysis, and reverse transcription polymerase chain reaction (RT-PCR).
2. Materials and methods
2.1. Cell culture and grouping
All cell culture medium components were purchased from GIBCO, USA, unless otherwise noted. Rat MC3T3-E1 osteogenic cells (Riken Cell Bank, Tsukuba, Ibaraki, Japan) were maintained in an incubator at 37 °C with a humidified atmosphere of 5% CO2 and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal calf serum and 100 U/ml penicillin or 100 µg/ml streptomycin. This study was divided into three groups: H2O2 treatment, H2O2+EuO treatment, and control (H2O2- and EuO-free). In the H2O2 group, 800 µmol/L of H2O2 was applied, and in the H2O2+EuO group, H2O2 (800 µmol/L) and various concentrations of EuO (6.25, 12.5, 25, 50, and 100 µg/ml) were applied.
2.2. Preparation of ethanol extracts of EuO leaves
We followed an ethanol extraction method in the preparation of EuO leaf extracts, as described by Luo et al. (2009). Briefly, EuO leaves (100 g) were treated with 80% ethanol (1 g:8 ml) and twice boiled under reflux for 1 h. After filtration, the supernatant was vacuum-dried at 50 °C, re-dissolved into distilled water, filtered, and vacuum-dried at 50 °C. After freeze-drying, the final EuO extracts were obtained and kept at 4 °C. The total caffeotannic acid and geniposide contents from the EuO leaf extracts were then determined with high performance liquid chromatography (HPLC) in comparison with standard preparation of caffeotannic acid and geniposide (Chinese Institute for the Control of Pharmaceutical and Biological Products, Beijing). The final EuO leaf ethanol extract powder contains caffeotannic acid at 58 mg/g and geniposide at 3.45 mg/g.
2.3. Determination of minimally optimal concentration of EuO for MC3T3-E1 cell growth
Minimally optimal concentration of EuO for MC3T3-E1 cell growth was determined by MTT assay using an MTT assay kit (Sigma, St. Louis, MO, USA). MC3T3-E1 cells (5×104 cells/well) were seeded in a 96-well plate, and treated with various concentrations of EuO (1 000, 500, 250, 125, and 62.5 µg/ml) 24 h after plating. Cells were incubated for an additional 72 h at 37 °C, and then treated with MTT (30 µl per well, 2 mg/ml) at 37 °C for 4 h. Cells were solubilized by adding 150 μl of dimethyl sulphoxide (DMSO), and the plate was shaken for 5–15 min to ensure that the MTT-deoxidized product was completely lysed. Absorbance was measured at 570 nm. The experiment was performed in triplicate and statistically analyzed by one-way analysis of variance (ANOVA). An optimized EuO concentration was determined.
2.4. Determination of H2O2-induced MC3T3-E1 cell apoptosis
The dosage of H2O2 that secures MC3T3-E1 cell apoptosis was also determined by MTT assay (Hansen et al., 1989). MC3T3-E1 cells (5×104 cells/well) were seeded onto a 24-well plate for 24 h, and H2O2 (1 000, 900, 800, 700, 600, 500, 400, 300, 200, 100, and 50 µmol/L; Sigma, St. Louis, MO, USA) were added to the plate. Cells were morphologically observed at 24 and 48 h with a microscope (Olympus, Japan). The dose of H2O2 that induced the half-value of MC3T3-E1 apoptosis was determined. The experiment was run in triplicate.
2.5. Cell viability after EuO and H2O2 treatments
Cell viability was estimated using MTT assay and microscopic morphology observation (Dimmeler et al., 1997). MC3T3-E1 cells (1×104 cells/well) were seeded in a 96-well plate and EuO (100 µl/well) at various concentrations in a safe and effective range (6.25, 12.5, 25, 50, and 100 µg/ml) was added. After incubation for 24 h, cells were washed with DMEM thrice, treated with H2O2 medium (800 µmol/L), and then incubated for another 24 h. Cells were washed once with phosphate buffer solution (PBS) and then incubated for an additional 6 h in the presence of 50 µl/well MTT (2 mg/ml). Subsequently, 150 µl of DMSO was added, and the plate was shaken for 15 min. The medium was removed and cells were lysed. The absorbance surveyed at 570 nm was used to calculate the relative cell viability ratio. At 48 h before MTT assay, a morphological observation of MC3T3-E1 cells was performed to assess cell viability. The interventional concentration of EuO in inhibiting H2O2-induced MC3T3-E1 cell apoptosis was thus determined.
2.6. Western blotting analysis
The expressions of caspases 3, 6, 7, and 9 were detected by Western blotting. Cytoplasmic extracts were prepared with 150 µl cell lysis buffer (Santa Cruz, CA, USA; lysis buffer:phenylmethylsulfonyl fluoride (PMSF):protease inhibitor cocktail=100:1:1) on ice for 30 min, then the centrifuged supernatant was collected, and the protein concentration was quantified using the detergent-compatible (DC) protein assay kit (Bio-Rad, Richmond, CA, USA). Proteins were mixed with 2× sodium dodecyl sulphate (SDS) sample buffer. A total of 40 µg of proteins were separated in a 10% (w/v) polyacrylamide gel and blotted on a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The blots were blocked for 2 h, and incubated with polyantibodies anti-caspases 3, 6, 7, and 9 (Santa Cruz, CA, USA) at 1:800, 1:800, 1:800, and 1:600, respectively, for 12 h. Subsequently, the membranes were washed in buffer (PBS with 0.1% (v/v) Tween-20) and then incubated with horseradish peroxidase link-coupled rat anti-rabbit-antibody (Chemicon, CA, USA) at 1:1000 in blocking buffer. In all experiments, ponceau staining was carried out to control equal loading and the bands were visualized by electrogenerated chemiluminescence (ECL) Western blotting system (GE Healthcare Biosciences, USA). Antibodies used in the Western blotting analysis were rat anti-goat caspase 3, rat anti-rabbit caspases 6, 7, and 9 polyclonal antibodies, anti-goat IgG, and anti-rabbit IgG. The target molecular weights for caspases 3, 6, 7, and 9 are 17, 34, 35, 32 kDa, respectively. The data shown are representative of at least three experiments.
2.7. RT-PCR
RNA was isolated using TRIzol (Invitrogen, San Mateo, CA, USA) following the manufacturer’s instructions. Complementary DNA (cDNA) was amplified and PCR was performed using a standardized program: 35 cycles of 95 °C for 5 min, 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 2 min for caspases 3, 6, and 7; 28 cycles of 95 °C for 5 min, 94 °C for 30 s, 57.7 °C for 30 s, and 72 °C for 6.5 min for caspase 9 and β-actin. The PCR products were electrophoresed through a 10 g/L agarose gel containing ethidium bromide and visualized by ultraviolet (UV) light. β-actin was used as an internal standard to verify equal loading in each experiment. The sequences of the primers are listed in Table 1. The target fragments are 136 (caspase 3), 132 (caspase 6), 122 (caspase 7), 425 (caspase 9), and 348 bp (β-actin).
Table 1.
Primer sequences
| Gene | Primer sequence* |
| Caspase 3 | 5′-AGCAGCTTTGTGTGTGTGATTCTAA-3′, 5′-AGTTTCGGCTTTCCAGTCAGAC-3′ |
| Caspase 6 | 5′-AGACAAGCTGGACAACGTGACC-3′, 5′-CCAGGAGCCATTCACAGTTTCT-3′ |
| Caspase 7 | 5′-GGAGGACTATGGTGATTGGAGC-3′, 5′-TAAGGCGCTGGTGGATATGG-3′ |
| Caspase 9 | 5′-GGATCCATGCCCAGACCAGTGGACATTGG-3′, 5′-GGTCTAGATTATGATGTTTTAAAGAAAAG-3′ |
| β-actin | 5′-TGGAATCCTGTGGCATCCATGAAAC-3′, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ |
Sense and antisense sequences, respectively
2.8. Statistical analysis
All experiments were run in triplicate. All data were presented as mean±standard deviation (SD). Statistical significance was examined by one-way ANOVA. A P value <0.05 was considered statistically significant.
3. Results
3.1. Influence of EuO on MC3T3-E1 cell growth
MTT assay results showed that EuO, at 62.5 µg/ml or lower, did not affect MC3T3-E1 cell growth, while 125 µg/ml EuO promoted the cell growth, and at 250, 500, and 1 000 µg/ml EuO depressed the cell growth (Table 2). A minimally optimal concentration range of EuO applied to MC3T3-E1 cells for the purpose of the current study requires that it does not inhibit, nor promote, cell growth. This concentration was determined to be <125 µg/ml.
Table 2.
Influence of EuO at various concentrations on MC3T3-E1 cell growth after 48 h culture by MTT assay
| EuO (µg/ml) | MTT absorbance |
|||
| No. 1 | No. 2 | No. 3 | Mean±SD | |
| 1 000 | 0.381 | 0.440 | 0.487 | 0.436±0.037** |
| 500 | 0.421 | 0.489 | 0.554 | 0.488±0.067** |
| 250 | 0.896 | 1.039 | 1.227 | 1.054±0.166 |
| 125 | 1.427 | 1.574 | 1.019 | 1.340±0.288* |
| 62.50 | 0.981 | 1.058 | 1.013 | 1.018±0.039 |
| 31.25 | 0.817 | 0.774 | 0.835 | 0.809±0.031 |
| Control | 1.021 | 1.110 | 0.952 | 1.028±0.079 |
P<0.05, compared with the control (H2O2- and EuO-free)
P<0.01, compared with the control (H2O2- and EuO-free)
3.2. MC3T3-E1 apoptosis induced by H2O2 at various concentrations
Both microscopic observation and MTT assay results show that MC3T3-E1 apoptosis was induced by H2O2 in a dose-response manner. At 400 µmol/L of H2O2, no marked morphological change was observed under a light microscope; at 800–1 000 µmol/L, cells started swelling; and at 1 000 µmol/L, cells underwent apoptosis (Fig. 1). Toxic effects of various concentrations of H2O2 on MC3T3-E1 cells were also observed, and H2O2 at 800 µmol/L was found to clearly cause the depression of succinic dehydrogenase activity in MC3T3-E1 cells (Fig. 2). The absorbance of H2O2 at 800 µmol/L by MTT assay was significantly lower than that of the control group (P<0.05), indicating that the dosage of H2O2 to induce a half-value of MC3T3-E1 apoptosis was around 800 µmol/L, which is close to the previously reported value (600 µmol/L) by Kim et al. (2003).
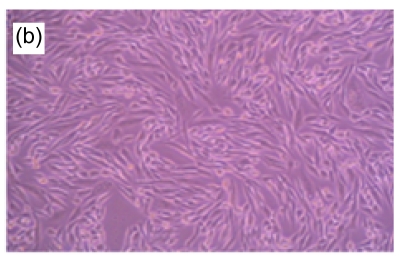
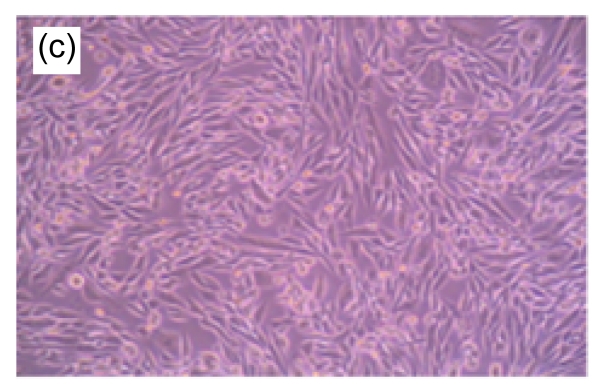
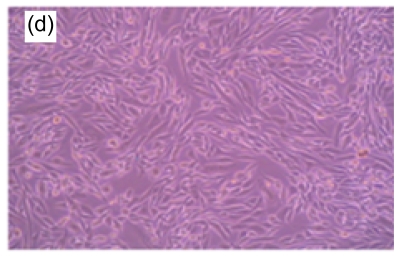
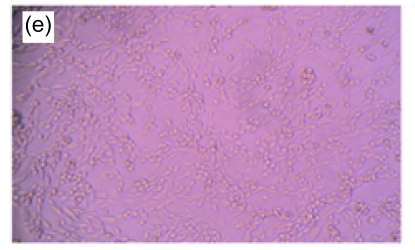
Fig. 1.
Morphologic changes of MT3C3-E1 cells in different concentrations of H2O2 and EuO after 48 h culture
(a) H2O2 at 800 µmol/L, showing apoptotic characteristics, such as cellular swelling, cell-rounding, microvillus disappearance, and cytoplasmic-condensation; (b) H2O2- and EuO-free control, with a normal fusiform shape; (c, d) EuO at 100 (c) and 50 (d) µg/ml, both with a normal fusiform shape; (e, f) EuO at 12.5 (e) and 6.25 (f) µg/ml both displaying apoptotic features, microvillus disappearance, and cytoplasmic-condensation. Magnification: 10×
Fig. 2.
Toxic effects of different concentrations of H2O2 on MC3T3-E1 cells by MTT assay
Data are expressed as mean±SD. * P<0.05, ▲ P>0.05, compared with the control (H2O2- and EuO-free)
3.3. Effect of EuO on H2O2-induced MC3T3-E1 cell apoptosis
To obtain a definitive quantification of the effect of EuO at various concentrations on H2O2-induced MC3T3-E1 cell apoptosis, the absorbance of cells was detected by MTT method (Fig. 3). A significant decrease in absorbance of MC3T3-E1 in serum-free medium treated with H2O2 (800 µmol/L) was observed, compared with the H2O2- and EuO-free group (0.21 vs. 0.66, P<0.05). The absorbance of MC3T3-E1 cells treated with the combination of H2O2 (800 µmol/L) and EuO at five different concentrations significantly increased in a dose-dependent manner (0 µg/ml, 0.21; 6.25 µg/ml, 0.28; 12.5 µg/ml, 0.31; 25 µg/ml, 0.48; 50 µg/ml, 0.54; and 100 µg/ml, 0.66; P<0.05). The half-inhibitory concentration of EuO on H2O2-induced MC3T3-E1 cell apoptosis was around 25 µg/ml, indicating that EuO is a potent antioxidant against H2O2 oxidation.
Fig. 3.
Effect of EuO on the toxicity of H2O2 by MTT assay
EuO antagonizes the toxicity of H2O2 (800 µmol/L). Group 1: positive control; Group 2: blank; Group 3: 100 μg/ml EuO+800 μmol/L H2O2; Group 4: 50 μg/ml EuO+800 μmol/L H2O2; Group 5: 25 μg/ml EuO+800 μmol/L H2O2; Group 6: 12.5 μg/ml EuO+800 μmol/L H2O2; Group 7: 6.25 μg/ml EuO+800 μmol/L H2O2. Data are expressed as mean±SD. * P<0.05, ▲ P>0.05, compared with the control (H2O2- and EuO-free)
A morphological observation of MC3T3-E1 cells was performed to confirm the apoptotic process. Non-induced MC3T3-E1 cells grew normally, with cells adhering to the bottom of the plate. The shapes of the endochylema were fusiform, polygonal, and irregular with round cell nuclei. After H2O2 (800 µmol/L) treatment, a high proportion of cells showed apoptosis-like changes such as being detached and cytoplasmic condensation, leading to cellular swelling, rounding, microvillus disappearing, cytoplasmic condensing, and vacuole appearing. In contrast, in EuO treatment groups, the morphology of cells suggested that the H2O2-induced apoptosis decreased in a dose-dependent manner (Fig. 1).
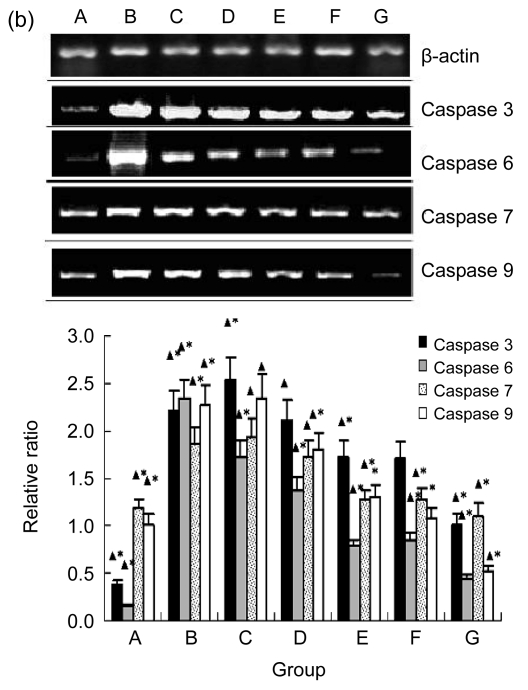
3.4. Inhibitory effect of EuO on expressions of caspases 3, 6, 7, and 9 in both mRNA and protein levels of H2O2-induced MC3T3-E1 cells
Western blotting analysis (Fig. 4a) and RT-PCR (Fig. 4b) both showed that the expressions of caspases 3, 6, 7, and 9 in H2O2-induced MC3T3-E1 cells treated with EuO were downregulated in a dose-dependent manner. It was estimated that the half-inhibitory concentration of EuO on most caspases tested in both Western blotting and RT-PCR analyses were between 12.5 to 25 µg/ml, while EuO at 50 or 100 µg/ml completely inhibited all caspases examined. These results indicate that EuO potently antagonized the apoptotic process of H2O2-treated MC3T3-E1 cells and that the caspase pathways were downregulated by EuO in both the mRNA and protein levels.
Fig. 4.
Inhibitory effect of EuO on protein expressions and transcriptions of caspases 3, 6, 7, and 9 in H2O2-induced MC3T3-E1 cells
(a) Western blotting results and relative ratio of protein levels. The molecular weights detected for caspases 3, 6, 7, and 9 were 17, 34, 35, and 32 kDa, respectively; (b) RT-PCR results and relative ratio of protein levels. The fragments detected for caspases 3, 6, 7, and 9 and β-actin were 136, 132, 122, 425, and 348 bp, respectively. EuO significantly decreased the protein expressions of caspases 3, 6, 7, and 9 in H2O2 (800 µmol/L)-induced MC3T3-E1 cells, and the inhibition effect was positively correlated with the concentration of EuO. Group A: control; Group B: H2O2 at 800 µmol/L; Groups C–G: H2O2 at 800 µmol/L and EuO at 6.25, 12.5, 25, 50, and 100 µg/ml, respectively. Data are expressed as mean±SD. * P<0.05, compared with the control (H2O2- and EuO-free); ▲ P<0.05, compared with H2O2 treatment alone
4. Discussion
In the present study, we used the leaves taken from EuO grown in Zhejiang Province, which possess high medicinal qualities, and produced the leaf extract by ethanol. Such produced EuO leaf extract powder was used to examine its influence on the growth of MC3T3-E1 cells, antioxidative effect against H2O2-induced MC3T3-E1 cell apoptosis, and underlying mechanisms. We were able to confirm that EuO is an enhancer for the growth of MC3T3-E1 cells and a potent antioxidant against H2O2-induced oxidative damage. We were also able to reveal for the first time that the inhibition of apoptosis of MC3T3-E1 cells induced by H2O2 may be via suppressing activations of caspases 3, 6, 7, and 9.
It has been reported that EuO promotes the growth of bone and muscle, which was further confirmed by our results. In this study, we found that EuO leaf extracts (125 µg/ml) enhanced the growth of MC3T3-E1 cells. Yao et al. (2005) reported that EuO leaf extracts have a stimulatory effect on differentiation of preosteoblast MC3T3-E1 cells. Chen and Luo (2009) reported that EuO extracts induced differentiation of goat bone marrow mesenchymal stem cells into osteoblasts and inhibited adipogenic differentiation. A new composite composed of tricalcium phosphate, gelatin, and Chinese medicine (containing EuO) as a bone substitute was evaluated and found to effectively increase the rate of tissue regeneration of damaged bones (Yao et al., 2005). Our previous study also indicated that the compound of calcium phosphate cement and EuO extracts promoted proliferation and adhesion of MC3T3-E1 osteoblasts in vitro (Lin et al., 2008).
The pharmacological effects of EuO leaves on bone and muscle are closely related to collagen metabolism (Li et al., 1998). Granuloma formation and collagen synthesis were significantly increased by the administration of methanol extracts of EuO cortex (Li et al., 2000a). A number of active EuO components, such as chlorogenic acid, geniposide, geniposidic acid, and aucubin, can influence osteoblast differentiation and slow bone loss. The EuO leaf ethanol extracts produced in the current experiments contained 58 mg/g of caffeotannic acid and 3.45 mg/g of geniposide.
EuO has been reported to be a strong antioxidant agent. Yen and Hsieh (2003) have shown that EuO leaf extract exhibited a rather significant inhibitory effect on DNA damage induced by H2O2 in lymphocytes in a concentration-dependent manner. Ohmori et al. (2005) assessed the antioxidant activity of EuO and found that the inhibitory effects of EuO on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals and low-density lipoprotein (LDL) oxidation were noticeable in the format of Duzhong tea in vitro. In the current H2O2-induced MC3T3-E1 apoptosis model, we have shown that the inhibitory effect of EuO on the apoptosis increased in a dose-dependent manner, with the half-inhibitory concentration being around 25 µg/ml by MTT assay, indicating that EuO is a potent antioxidant against H2O2 oxidation.
EuO’s antioxidant effects may lie in its inhibition on the cascade reaction of caspases. Western blotting and RT-PCR analyses showed that, when treated with EuO, the initiator caspase 9 and effectors caspases 3, 6, and 7 were drastically downregulated both in mRNA and protein levels in H2O2-induced MC3T3-E1 apoptotic model, with the half-effective concentration of EuO ranging from 12.5 to 25 µg/ml. Cell morphology also showed that H2O2 induced typical apoptotic features in MC3T3-E1 cells, which were inhibited by EuO in a dose-dependent manner. These results not only show that EuO is a potent antioxidant against H2O2-induced oxidative damage, but also suggest that the inhibition of caspases’ activities is likely the underlying explanation.
While exploring the mechanism of EuO in antagonizing H2O2-induced apoptosis in MC3T3-E1 cells, a direct scavenging effect of EuO on H2O2 must be considered. The polyphenols isolated from EuO displayed an effective radical-scavenging activity, which may trigger other agents in eliminating free radicals (Cho et al., 2003). EuO may also eliminate ·OH in solution (Chen et al., 2002; Luo et al., 2009). However, in the cell viability experiment of the current study, we applied EuO to the cells 24 h prior to the administration of H2O2, in order to minimize the possibility of a direct interaction between an oxidant and antioxidant.
It should be noted that there are a number of factors affecting EuO’s biological and pharmaceutical functions and effects, such as the plant growth conditions, biological parts, and the extraction methods. Yen and Hsieh (2003) reported that the inhibitory effect of EuO leaf extract on DNA damage induced by H2O2 in lymphocytes was more significant than that of roasted EuO cortex. Luo et al. (2009) found that enzyme-assisted water extract is more effective than the ethanol extracts of EuO leaves for antioxidant extraction. Nishida et al. (2003) reported that “crude drug” of EuO (150 µg/ml) induced apoptosis of HL-60 cells. This discrepancy is likely due to a high concentration of EuO applied, as we also observed that EuO at higher doses (250–1 000 µg/ml) were toxic to MC3T3-E1 cells. This could be also due to a different source and/or process of the “crude drug”. In the present study, the EuO leaves were from locally-grown EuO and the extracts were made by ethanol extraction.
In conclusion, ethanol EuO leaf extracts enhanced the growth of MC3T3-E1, and suppressed the H2O2-induced apoptosis in a rat MC3T3-E1 osteogenic cell model. This effect is likely due to the inhibition of caspases’ activities. EuO is a potent antioxidant, a characteristic which may contribute to its many cellular protective functions, including the promotion of bone growth.
Acknowledgments
The authors thank Mr. Hang-ping YAO (State Key Laboratory for Infectious Diseases Diagnosis and Treatment, the First Affiliated Hospital, School of Medicine, Zhejiang University, China) for his technical assistance.
Footnotes
Project (No. 2007C33030) supported by the Science and Technology Program of Zhejiang Province, China
References
- 1.Chen GH, Tian YL, Yang GL, Wang XY, Zhao HR. Determination of eliminating ratio of Chinese traditional medicine for hydroxyl radical by fluorescent spectrophotometry. Spectrosc Spect Anal. 2002;22(4):634–636. (in Chinese) [PubMed] [Google Scholar]
- 2.Chen WC, Luo J. Extracting solution from Eucommia ulmoides Oliv. induces differentiation of goat bone marrow mesenchymal stem cells into osteoblasts and inhibits adipogenic differentiation. J Clin Rehab Tissue Eng Res. 2009;13(10):1960–1964. (in Chinese) [Google Scholar]
- 3.Chen WH, Lu LG, Zeng MD, Xu ZN, Liu M, Mao YM, Fang JY. Effect of magnesium isoglycyrrhizinate on the proliferation and oxidative stress of rat hepatic stellate cells in vitro. Chin J Hepatol. 2006;14(6):426–430. (in Chinese) [PubMed] [Google Scholar]
- 4.Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1,1-diphenyl-2-picrylhydrazyl radical. Phytomedicine. 2003;10(6-7):544–551. doi: 10.1078/094471103322331520. [DOI] [PubMed] [Google Scholar]
- 5.Deyama T, Nishibe S, Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin. 2001;22(12):1057–1070. [PubMed] [Google Scholar]
- 6.Dimmeler S, Haendeler J, Nehls M, Zeiher AM. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1β-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med. 1997;185(4):601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha H, Ho J, Shin S, Kim H, Koo S, Kim IH, Kim C. Effects of Eucommiae Cortex on osteoblast-like cell proliferation and osteoclast inhibition. Arch Pharm Res. 2003;26(11):929–936. doi: 10.1007/BF02980202. [DOI] [PubMed] [Google Scholar]
- 8.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 9.Kim MH, Chung J, Yang JW, Chung SM, Kwag NH, Yoo JS. Hydrogen peroxide-induced cell death in a human retinal pigment epithelial cell line, ARPE-19. Korean J Ophthalmol. 2003;17(1):19–28. doi: 10.3341/kjo.2003.17.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Lee MK, Cho SY, Kim DJ, Jang JY, Shin KH, Park SA, Park EM, Lee JS, Choi MS, Lee JS, et al. Du-zhong (Eucommia ulmoides Oliv.) cortex water extract alters heme biosynthesis and erythrocyte antioxidant defense system in lead-administered rats. J Med Food. 2005;8(1):86–92. doi: 10.1089/jmf.2005.8.86. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Sato T, Metori K, Koike K, Che QM, Takahashi S. The promoting effects of geniposidic acid and aucubin in Eucommia ulmoides Oliver leaves on collagen synthesis. Biol Pharm Bull. 1998;21(12):1306–1310. doi: 10.1248/bpb.21.1306. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Kamo S, Metori K, Koike K, Che QM, Takahashi S. The promoting effect of eucommiol from Eucommiae cortex on collagen synthesis. Biol Pharm Bull. 2000;23(1):54–59. doi: 10.1248/bpb.23.54. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Metori K, Koike K, Kita F, Che QM, Sato T, Shirai W, Takahashi S. Granuloma maturation in the rat is advanced by the oral administration of Eucommia ulmoides Oliver leaf. Biol Pharm Bull. 2000;23(1):60–65. doi: 10.1248/bpb.23.60. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Li YQ, Li WH, Chen H. Effects of calcium phosphate cement/Eucommia ulmoides extracts compound on cell proliferation and adhesion of MC3T3E1 osteoblasts in vitro. J Zhejiang Univ (Med Sci) 2008;37(1):78–82. doi: 10.3785/j.issn.1008-9292.2008.01.014. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Tian C, Xu J, Sun Y. Studies on the antioxidant activity and phenolic compounds of enzyme-assisted water extracts from Du-zhong (Eucommia ulmoides Oliv.) leaves. J Enzyme Inhib Med Chem. 2009;24(6):1280–1287. doi: 10.3109/14756360902829741. [DOI] [PubMed] [Google Scholar]
- 16.Metori K, Furutsu M, Takahashi S. The preventive effect of ginseng with Du-zhong leaf on protein metabolism in aging. Biol Pharm Bull. 1997;20(3):237–242. doi: 10.1248/bpb.20.237. [DOI] [PubMed] [Google Scholar]
- 17.Miyake M, Arai N, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Promoting effect of kaempferol on the differentiation and mineralization of murine pre-osteoblastic cell line MC3T3-E1. Biosci Biotechnol Biochem. 2003;67(6):1199–1205. doi: 10.1271/bbb.67.1199. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Nakazawa Y, Onizuka S, Satoh S, Chiba A, Sekihashi K, Miura A, Yasugahira N, Sasaki YF. Antimutagenicity of Tochu tea (an aqueous extract of Eucommia ulmoides leaves): 1. The clastogen-suppressing effects of Tochu tea in CHO cells and mice. Mutat Res. 1997;388(1):7–20. doi: 10.1016/s1383-5718(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 19.Nishida S, Kikuichi S, Yoshioka S, Tsubaki M, Fujii Y, Matsuda H, Kubo M, Irimajiri K. Induction of apoptosis in HL-60 cells treated with medicinal herbs. Am J Chin Med. 2003;31(4):551–562. doi: 10.1142/S0192415X03001211. [DOI] [PubMed] [Google Scholar]
- 20.Ohmori R, Iwamoto T, Tago M, Takeo T, Unno T, Itakura H, Kondo K. Antioxidant activity of various teas against free radicals and LDL oxidation. Lipids. 2005;40(8):849–853. doi: 10.1007/s11745-005-1447-4. [DOI] [PubMed] [Google Scholar]
- 21.Prouillet C, Maziere JC, Maziere C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67(7):1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Kato K, Noguchi K, Dairaku N, Koike T, Iijima K, Imatani A, Sekine H, Ohara S, Sasano H, et al. Tochu (Eucommia ulmoides) leaf extract prevents ammonia and vitamin C deficiency induced gastric mucosal injury. Life Sci. 2003;73(25):3245–3256. doi: 10.1016/j.lfs.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Yao CH, Tsai HM, Chen YS, Liu BS. Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin, and Chinese medicine as a bone substitute. J Biomed Mater Res B Appl Biomater. 2005;75(2):277–288. doi: 10.1002/jbm.b.30294. [DOI] [PubMed] [Google Scholar]
- 24.Yen GC, Hsieh CL. Inhibitory effect of Eucommia ulmoides Oliv. on oxidative DNA damage in lymphocytes induced by H2O2 . Teratog Carcinog Mutagen. 2003;23(s1):23–34. doi: 10.1002/tcm.10047. [DOI] [PubMed] [Google Scholar]