Abstract
MicroRNAs (miRNAs) are endogenous small noncoding RNAs that regulate gene expression at the posttranscriptional level. Studies have shown that zebrafish miRNAs play a key role in embryo development, tissue fate establishment, and differentiation by interacting with specific targets, usually in the 3′UTR of the mRNA. Identification of the target sequence is fundamental to elucidating miRNA function. Since bioinformatics can predict hundreds of potential targets for each miRNA, experimental validation of the actual target site is required. Although recent studies have employed the HEK293 cell line to investigate mammalian miRNA targets, our results have shown that the cell line is not suitable for studies of zebrafish miR-430b miRNA. In this article we describe a convenient in vitro assay system that involves the use of zebrafish cell cultures and a luciferase reporter construct to evaluate miR-430b target sites. The cell culture-based assay could be used to validate target sequences of other zebrafish miRNAs.
Introduction
MicroRNAs (MiRNAs) are naturally occurring small RNAs, normally 18- to 25-nucleotides (nt) long, that target specific mRNAs for degradation or translational repression by binding to a complementary target sequence on the mRNA.1 MiRNAs are transcribed by RNA polymerase II initially in the form of primary transcripts (pri-miRNAs) that are processed into precursor miRNAs in the nucleus and finally mature miRNAs in the cytoplasm.2 Dicer is the enzyme that trims the precursor miRNA to the mature form. miRNAs recognize and associate with target mRNAs possessing a partial complementary sequence. An miRNA-induced silencing complex (miRISC) then forms and induces degradation or translational repression of the target mRNA.3,4 Components of the miRISC include members of the GW182 protein family, Dicer, TRBP, and the Argonaute protein family that serve as the core slicer.5,6 miRNAs play multiple roles in regulating gene expression usually by targeting 3′ untranslated regions, and rarely by targeting the protein coding region,7 5′ untranslated region8 or 5′ promoter sequence.9 Each miRNA may target more than one mRNA with each mRNA potentially being targeted by multiple miRNAs in a sequential10 or coordinated fashion.11 The interaction of an miRNA with its target is directed by a 6- to 8-nt seed sequence located on the 5′ end of the miRNA that recognizes the perfectly matched target sequence with no G:U wobble allowed.12 On the basis of this rule, most miRNAs are predicted to have ∼10–100 mRNA targets, making it necessary to experimentally verify each target sequence.
The zebrafish is an established model for studies of embryogenesis and human disease and provides an excellent system to investigate the function of miRNAs during organ development and pathogenesis.13–15 Since many miRNA families are conserved among vertebrates, studies of zebrafish miRNAs will contribute to a basic understanding of miRNA function and facilitate future pharmaceutical application.16,17 Approximately 360 different zebrafish miRNAs have been identified and sequenced, making it necessary to have available a convenient and efficient method to verify each of their targets.18,19
In recent studies, the identification of zebrafish miRNA targets has been achieved using an assay system in which embryos are injected with the miRNA along with a construct containing a reporter gene linked to the predicted candidate target sequence.20–22 Recognition of the target sequence results in miRNA-mediated mRNA decay or translational repression and a loss or decrease in reporter gene expression. One disadvantage of this approach is the need to introduce the reporter construct and miRNA into individual embryos by injection, making it less convenient than a cell culture-based assay system for large-scale screening of potential target sequences. To complement the existing embryo microinjection assay approach, we have developed an in vitro assay system using a well-characterized zebrafish cell line and a sensitive luciferase reporter construct. The cell line is easy to culture and the assay provides reproducible results. The in vitro assay can be used to conveniently verify miRNA targets, and it also provides a system to investigate the molecular mechanism of miRNA function in zebrafish.
Materials and Methods
Plasmid construction
The 3′UTRs of zgc63829 and gstm were cloned from total cDNA made from early zebrafish embryos and inserted into pGEMT-easy vector (primers listed in Table 1). pGEMT-3′nanos containing the nanos-3′UTR was previously constructed in our lab.23 The 3′UTR sequence was removed from each plasmid with EcoRI, blunted, and ligated into the XbaI site of pGL3-promoter (Promega) to form pGL3-3′zgc63829, pGL3-3′nanos, and pGL3-3′gstm each encoding the respective 3′UTR linked to luciferase cDNA under the control of the SV40 promoter. To construct pGL3-3xPT, 3xPT was removed from pCS2-eGFP-3xPT and ligated into the XbaI site of pGL3-promoter. Correct construction of each of the finished plasmids was confirmed by sequencing.
Table 1.
Primers Used in the Study
| zgc63829-3′UTR |
| zgc63829-f: 5′-CTT-CCA-CCA-ATA-GAG-GAA-CTA-GGA-3′ |
| zgc63829-r: 5′-CAG-ACC-ATA-TAA-TCT-GTT-CCC-ATA-3′ |
| gstm-3′UTR |
| gstm-f: 5′-GGA-GTG-AAA-CTA-ACA-ACC-ATT-GCC-AAC-AAC-3′ |
| gstm-r: 5′-TGA-GAC-AAT-CCA-GAA-CCA-CAA-TCT-3′ |
| dicer-1 |
| dicer1-f: 5′-CTT-GCA-ATC-ACT-GGC-CAT-CCT-TAT-CG-3′ |
| dicer1-r: 5′-GCA-GCT-CCC-GAA-CCA-TGA-TTC-CTG-CA-3′ |
| ago-1 |
| ago1-f: 5′-ATG-GGA-TGG-AGC-CGG-GAC-CAT-CTG-3′ |
| ago1-r: 5′-GGA-CCC-TCT-GCG-AGT-CAG-TTA-GTG-TC-3′ |
| ago-2 |
| ago2-f: 5′-ATG-TAT-CCC-ATT-GGA-GCA-GCT-GGT-G-3′ |
| ago2-r: 5′-CCA-ATG-GGT-AAA-GGC-ATG-GCA-GTG-TAG-AG-3′ |
| vasa |
| vasa-f: 5′-TGT-GGA-CGT-GAG-TGG-CAG-CAA-TC-3′ |
| vasa-r: 5′-CTA-GAT-AGC-GCA-CTT-TAC-TCA-GG-3′ |
| β-actin |
| actin-f: 5′-AGA-CAT-CAG-GGT-GTC-ATG-GTT-GGT-3′ |
| actin-r: 5′- TGG-TCT-CGT-GGA-TAC-CGC-AAG-ATT-3′ |
HEK293 cell culture and transient transfection
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum in a humidified incubator (37°C, 5% CO2). Plasmid and miRNA transfections were performed in 48-well plates (3 × 104 cells/well) using the Lipofectamine2000 reagent (Invitrogen) according to the manufacturer's protocol.
Zebrafish cell culture and transient transfection
The zebrafish spleen stromal-derived cell line, ZSSJ,24 and an embryonic fibroblast cell line (ZF) were cultured in L15 medium supplemented with 10% bovine serum (28°C, ambient air). miR-430b (5′AAAGUGCUAUCAAGUUGGGdGdT) was commercially synthesized (IDT Company) and control miRNA was purchased from Santa Cruz Biotechnology. To perform the transfection experiments, the ZSSJ or ZF cells were seeded at 60%–80% confluency in 12-well plates. On the second day the medium was changed and transfection complex consisting of the Lipofectamine2000 reagent (2 μL), miRNA (50 nM), pCMV-lacZ (250 ng; Clontech), pGL3 plasmid (500 ng), and OPTI-MEMI (280 μL total volume; Gibco) was added to each well. After 5–6 h the transfection complex was replaced with a fresh L-15 culture medium containing 10% bovine serum and after an additional 48 h the cells were lysed (Reporter Lysis Buffer, 250 μL; Promega) and the supernatant was frozen. Each of the transfections was performed in triplicate. To measure transfection efficiency, fluorophore-tagged miRNA or pEGFP-N1 (Clontech) encoding the green fluorescent protein (EGFP) was introduced into the cells as described above and the number of fluorescent cells was observed using a Nikon fluorescence microscope (Eclipse TE200) equipped with a digital camera (Diagnostic Instrument).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted from zebrafish tissues and cell cultures by the addition of Trizol reagent (1 mL; Invitrogen) followed by TURBO DNase treatment (2 μL for 1 h; AMBION). cDNA synthesis was performed using the Invitrogen SuperScript First-Strand Synthesis System for RT-PCR kit according to manufacturer's instructions. The primer sequences used for dicer1, argonaute1 (ago1), argonaute2 (ago2), vasa, and β-actin RT-PCR are listed in Table 1.
Luciferase and β-galactosidase assay
Firefly luciferase activity was measured with the use of the Luciferase Assay System (Promega). Cell lysate (40 μL) and LAR II (100 μL) were added into individual wells of a 96-well plate followed by an immediate reading in a luminometer (Thermo Labsystems). The transfection efficiency was normalized to β-galactosidase, measured using a commercial assay system (Galactosidase Enzyme Assay System with Reporter Lysis Buffer; Promega). Briefly, in individual wells of a 96-well plate, cell lysate (50 μL) was incubated (37°C) with 2 × Buffer containing ONPG substrate (50 μL) until a faint yellow color was observed (∼2–3 h). The reaction was stopped by the addition of 150 μL of 1 M Na2CO3 and the absorbance (420 nm) was immediately measured.
Statistical analysis
Results were analyzed by Student's t-test, and data are presented as means ± standard deviation with significance at p < 0.05.
Results
Zebrafish miRNA–mRNA interactions cannot be recapitulated in HEK293 cells
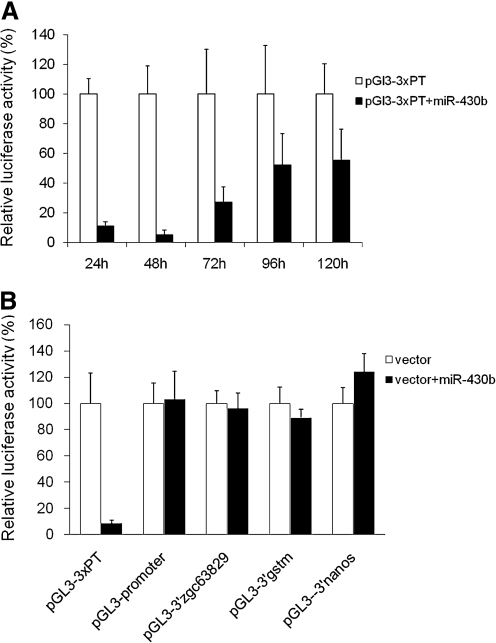
In previous studies the HEK293 cell line was used as an in vitro assay system to investigate human and mouse miRNA–mRNA interactions.25,26 Individual miRNAs were cotransfected into the HEK293 cells along with a luciferase reporter construct containing the luciferase gene linked to a potential miRNA target sequence, usually in the 3′UTR.27,28 Recognition of the target sequence by the miRNA resulted in decreased luciferase expression that was quantified with a luminometer. To determine if the HEK293 cells could be used to investigate zebrafish miRNA–mRNA interactions, we introduced zebrafish miR-430b into the cells along with a luciferase reporter construct containing various known miR-430b target sequences. When the pGL3-3xPT luciferase construct containing three tandem copies of the perfect target sequence for miR-430b29 was introduced into the HEK293 cells, luciferase expression was 90% and 95% lower than the controls after 24- and 48-h post-transfection, respectively (Fig. 1A). To determine if the HEK293 cells could also support miR-430b-mediated knockdown when a confirmed in vivo target sequence was used in the assay, miR-430b was introduced into the cells along with known target sequences from zgc63829-, gstm-,29 and nanos-3′UTRs.30 The results showed that luciferase expression was not reduced when each of the confirmed target sequences were assayed (Fig. 1B), indicating that the HEK293 cell line is not a suitable assay system for studies of zebrafish miR-430b-mediated suppression of gene expression.
FIG. 1.
HEK293 cells fail to support the interaction of zebrafish miR-430b with known mRNA targets. (A) Time course of miR-430b-mediated inhibition of luciferase expression using the reporter construct pGL3-3xPT, which carries three tandom copies of the perfect target sequence. pGL3-3xPT and miR-430b were cotransfected into the HEK293 cells and luciferase expression was measured at the times indicated post-transfection. (B) miR-430b-mediated inhibition of luciferase expression using reporter constructs, pGl3-3′zgc63829, pGl3-3′gstm, and pGl3-3′nanos that carry 3′UTRs containing known miR-430b target sequences. Luciferase expression was measured at 48 h post-transfection (hpt). pGL3-3xPT and pGL-3 promoter were used as positive and negative controls, respectively, and the transfection efficiency was normalized to β-galactosidase expression from pCMV-lacZ. Each bar on the graph represents the average of four separate transfections.
Components of the miRNA pathway are expressed in zebrafish cell lines
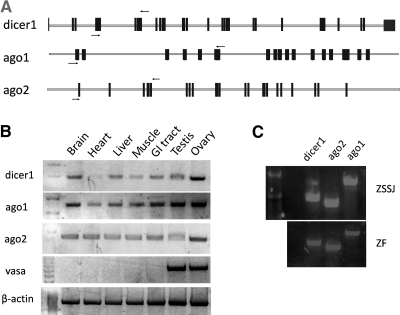
As an alternative to the HEK293 cells, we investigated whether two zebrafish cell lines could serve as an in vitro assay system to validate miR-430b target sequences. The ZF and ZSSJ cell lines24 were initiated from late-stage zebrafish embryos and adult spleen tissue respectively and have been maintained in culture for >30 passages. As a first step toward determining their suitability as an in vitro assay system, RT-PCR experiments were performed to determine if the cell lines express key enzymes of the miRNA biogenesis and gene knockdown pathways, including Dicer1,4 AGO1, and AGO2.31 Using several sets of PCR primers the results showed that dicer1, ago1, and ago2 are expressed in multiple tissues of adult zebrafish (Fig. 2B) as well as in the two established cell lines (Fig. 2C). The results indicate that the cell lines have the capacity to form the miRISC complex and may provide a suitable in vitro system to investigate zebrafish miRNA–mRNA interaction.
FIG. 2.
ZSSJ and ZF cell lines express dicer1, ago1, and ago2. (A) Schematic showing the intron (line) and exon (box) organization of zebrafish dicer1, ago1, and ago2 and the location of primers (arrows) used for RT-PCR analysis. Primers were designed to amplify exons 6 and 9 of dicer1; 1 and 6 of ago1 and 1 and 4 of ago2. (B) RT-PCR analysis of dicer1, ago1, and ago2 expression in adult zebrafish tissues and (C) ZSSJ and ZF cell cultures. β-actin was used as a positive control and vasa as a gonad-specific control.
The zebrafish cell lines have high miRNA transfection efficiencies
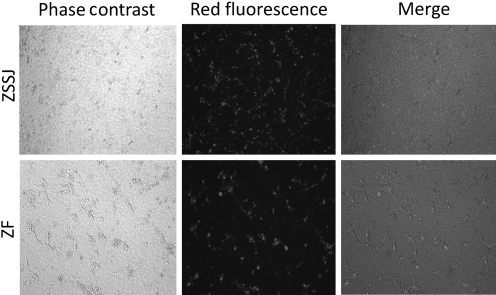
In addition to expressing the required miRISC components, if the cell lines are to provide a suitable in vitro assay system for the investigation of miRNA targets, a large percentage of the cells must have the capacity to reproducibly incorporate exogenously applied miRNA. Homogeneous uptake of the miRNA into a large percentage of cells is required to make it feasible to identify and validate target sequences following introduction of a reporter construct into a much smaller percentage of the cells. To determine the transfection efficiency of the ZF and ZSSJ cell lines for small RNAs, we introduced red fluorophore-labeled miRNA into the cells using the Lipofectamine2000 reagent. General observation of the overall percentage of fluorescent cells revealed that a transfection efficiency of >90% was achieved (Fig. 3), indicating that the cell lines could be used to investigate miRNA-mediated repression of reporter gene expression.
FIG. 3.
miRNA transfection efficiency of ZF and ZSSJ cells. ZF and ZSSJ cell cultures were transfected with siRNA coupled with a red fluorophore in a 12-well plate. Cell fluorescence was observed under a Nikon fluorescence microscope equipped with a digital camera. The pictures were taken 48 hpt.
ZSSJ cells support the interaction of zebrafish miR-430b with its mRNA target
Introduction of pEGFP-N1 and the firefly luciferase reporter construct, pGL3-promoter, into the ZF and ZSSJ cells using the Lipofectamine2000 reagent revealed that plasmid transfection frequency (GFP-positive cells) was 60% higher and luciferase expression approximately fivefold greater in ZSSJ than in ZF cells (data not shown). Since the level of luciferase expression was also less variable in the ZSSJ cells, we decided to use this cell line to investigate miR-430b-mediated repression of reporter gene expression.
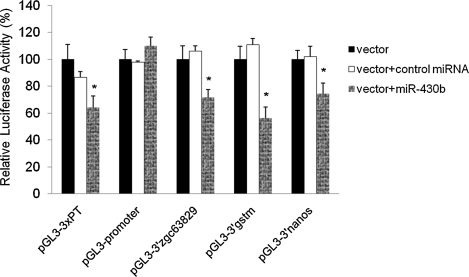
To conduct the assay, ZSSJ cells were cotransfected with a luciferase reporter construct containing an miR-430b target sequence along with either miR-430b or control miRNA. The relative luciferase activity was calculated by dividing the amount of luciferase expression measured in each of the experimental groups by the level found in the cells transfected with the reporter alone, which was set as 100%. A decrease in reporter gene expression was interpreted as resulting from control miRNA- or miR-430b-mediated inhibition of gene expression. As showed in Figure 4, control miRNA did not cause a significant inhibition of luciferase expression for each of the targets. Cells transfected with miR-430b exhibited a 36% decrease in luciferase expression when the reporter construct contained a repeat of three perfect target sequences (pGL3-3xPT). No decrease in luciferase expression was observed for the pGL3-promoter construct that did not contain a known miR-430b target site. When reporter plasmids containing the 3′UTRs of zgc63829, gstm, or nanos were introduced into the cells along with miR-430b, luciferase expression was decreased 28%, 46%, and 24%, respectively, which was significantly different from cells transfected with control miRNA or the reporter plasmid alone (Fig. 4). The results indicate that ZSSJ cells provide a suitable in vitro environment for zebrafish miR-430b to recognize the target sequence and inhibit translation of the mRNA.
FIG. 4.
ZSSJ cells support the interaction of zebrafish miR-430b and mRNA target sequences. ZSSJ cells were transfected with miR-430b or control miRNA along with one of three luciferase reporter constructs, pGl3-3′zgc63829, pGl3-3′gstm, or pGl3-3′nanos, that carry known 3′UTR target sequences for miR-430b. Luciferase expression was measured 48 hpt. pGL3-3xPT and pGL-3 promoter were used as positive and negative controls, respectively. The transfection efficiency was normalized to β-galactosidase expression from pCMV-lacZ. Each bar represents the average of three separate transfections. *A significant difference (p < 0.05).
Discussion
Results demonstrating that miR-430b-mediated inhibition of gene expression can be recapitulated in the zebrafish ZSSJ cell line indicate that the cells may serve as a suitable in vitro assay system to validate other zebrafish miRNA targets and to study miRNA–mRNA interactions. However, since this study focused solely on miR-430b, it is not known how well the ZSSJ cells will work with other zebrafish miRNAs. Also, the failure of HEK293 cells to support miR-430b-mediated inhibition does not necessarily preclude the use of this cell line for studies of other zebrafish miRNAs. ZSSJ cells express the core components of miRISC and the cells efficiently incorporate exogenous small miRNAs that are introduced by transfection. Using the ZSSJ cells and the luciferase reporter plasmid containing one of three different zebrafish 3′UTR target sequences, it was possible to reproducibly measure relative levels of miR-430b-mediated reporter gene inhibition. The convenience of performing the in vitro assay using the ZSSJ cells and the ability to reproducibly measure intermediate levels of reporter gene inhibition should make the assay ideal for the rapid screening and validation of a large number of zebrafish miRNA target candidates. Since the sequence of individual miRNAs and their specific targets are only partially complimentary, methods are needed to perform detailed investigations of each miRNA–mRNA interaction to identify key sequences that impart specificity. The ZSSJ assay would provide an in vitro approach for introducing specific sequence alterations and base substitutions in the miRNA seed and the mRNA target sequence, making it possible to characterize the sequence requirements for miRNA target specificity.
Another potential application of the in vitro assay is to perform a functional analysis of the individual zebrafish miRISC core components. Access to the complete zebrafish genomic sequence will allow the identification and cloning of each component of the zebrafish miRISC complex based on homology with other species. Once the individual miRISC components are available, the wildtype or mutated proteins can be added to the assay system to examine their effect on miRNA function.
Acknowledgments
We thank Drs. Niels Bols for the ZSSJ cells, Antonio Giraldez for pCS3-GFP-3xPT, Gregory Hannon for pCMV-hAGO1 and pCMV-hAGO2, Xing Tong for pCMV-lacZ, Tentsao Wong for pGEMT-3′nanos, Joe Ogas for fluorophore miRNA, Shawn Donkin and Henry Chang for the use of their luminometers, and Darl Swartz for the use of his plate reader. The study was supported by NIH (R01GM069384).
Disclosure Statement
No competing financial interests exist.
References
- 1.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Lim LP. Glasner ME. Yekta S. Burge CB. Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YS. Nakahara K. Pham JW. Kim K. He Z. Sontheimer EJ, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 5.Cifuentes D. Xue H. Taylor DW. Patnode H. Mishima Y. Cheloufi S, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Tay Y. Zhang J. Thomson AM. Lim B. Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 8.Orom UA. Nielson FC. Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhance their translation. Mol Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Place R. Li L. Pookot D. Noonan E. Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua Z. Lv Q. Ye W. Wong CK. Cai G. Gu D, et al. MicroRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimi ST. Fulcher JA. Chang MH. Gov L. Wang S. Lee B. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinated regulation of dendritic cell differentiation. Blood. 2009;114:404–414. doi: 10.1182/blood-2008-09-179150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carthew RW. Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng L. Carter AD. Childs SJ. miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci U S A. 2009;106:17793–17798. doi: 10.1073/pnas.0903693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le MT. Teh C. Shyh-Chang N. Xie H. Zhou B. Korzh V, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu YF. Du TT. Dong M. Zhu KY. Jing CB. Zhang Y, et al. Mir-144 selectively regulates embryonic alpha-hemoglobin synthesis during primitive erythropoiesis. Blood. 2009;113:1340–1349. doi: 10.1182/blood-2008-08-174854. [DOI] [PubMed] [Google Scholar]
- 16.Silva MT. do Vale A. dos Santos NM. Fish and apoptosis: studies in disease and pharmaceutical design. Curr Pharm Des. 2008;14:170–183. doi: 10.2174/138161208783378734. [DOI] [PubMed] [Google Scholar]
- 17.Rossi JJ. New hope for a microRNA therapy for liver cancer. Cell. 2009;137:990–992. doi: 10.1016/j.cell.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Chen PY. Manninga H. Slanchev K. Chien M. Russo JJ. Ju J, et al. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 2005;19:1288–1293. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloosterman WP. Steiner FA. Berezikov E. de Bruijn E. van de Belt J. Verheul M, et al. Cloning and expression of new microRNAs from zebrafish. Nucleic Acids Res. 2006;34:2558–2569. doi: 10.1093/nar/gkl278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pase L. Lieschke GJ. Validating microRNA target transcripts using zebrafish assays. Methods Mol Biol. 2009;546:227–240. doi: 10.1007/978-1-60327-977-2_14. [DOI] [PubMed] [Google Scholar]
- 21.Mishima Y. Abreu-Goodger C. Staton AA. Stahlhut C. Shou C. Cheng C, et al. Zebrafish miR-1 and miR-133 shape muscle gene expression and regulate sarcomeric actin organization. Genes Dev. 2009;23:619–632. doi: 10.1101/gad.1760209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du TT. Fu YF. Dong M. Wang L. Fan HB. Chen Y, et al. Experimental validation and complexity of miRNA-mRNA target interaction during zebrafish primitive erythropoiesis. Biochem Biophys Res Commun. 2009;381:688–693. doi: 10.1016/j.bbrc.2009.02.122. [DOI] [PubMed] [Google Scholar]
- 23.Fan L. Jesung M. Wong TT. Crodian J. Collodi P. Zebrafish primordial germ cell cultures derived from vasa: RFP transgenic embryos. Stem Cell Dev. 2008;17:585–597. doi: 10.1089/scd.2007.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing JG. El-Sweisi W. Lee LE. Collodi P. Seymour C. Mothersill C, et al. Development of a zebrafish spleen cell line, ZSSJ, and its growth arrest by gamma irradiation and capacity to act as feeder cells. In Vitro Cell Dev Biol Anim. 2009;45:163–174. doi: 10.1007/s11626-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgantas RW., III Hildreth R. Morisot S. Alder J. Liu C. Heimfeld S. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2006;104:2750–2755. doi: 10.1073/pnas.0610983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan ZX. Lin QX. Yang M. Deng CY. Kuang SJ. Zhou DZ, et al. A quick and efficient approach for gene silencing by using triple putative microRNA-based short hairpin RNAs. Mol Cell Biochem. 2009;323:81–89. doi: 10.1007/s11010-008-9966-3. [DOI] [PubMed] [Google Scholar]
- 27.Clop A. Marcq F. Takeda H. Pirottin D. Tordoir X. Bibé B. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38:813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 28.Takagi S. Nakajima M. Kida K. Yamaura Y. Fukami T. Yokoi T. MicroRNAs regulate human hepatocyte nuclear factor 4 alpha, modulating the expression of metabolic enzymes and cell cycle. J Biol Chem. 2010;285:4415–4422. doi: 10.1074/jbc.M109.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giraldez AJ. Mishima Y. Rihel J. Grocock RJ. Van Dongen S. Inoue K, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 30.Mishima Y. Giraldez AJ. Takeda Y. Fujiwara T. Sakamoto H. Schier AF, et al. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr Biol. 2006;16:2135–2142. doi: 10.1016/j.cub.2006.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi SW. Zang JB. Mele A. Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]