Abstract
Murine leukemia virus (MLV)–based replication-competent retrovirus (RCR) vectors have been shown to mediate efficient, selective, and persistent tumor transduction, thereby achieving significant therapeutic benefit in a wide variety of cancer models. To further augment the efficiency of this strategy, we have developed a delivery method employing a gutted adenovirus encoding an RCR vector (AdRCR); thus, tumor cells transduced with the adenoviral vector transiently become RCR vector producer cells in situ. As expected, high-titer AdRCR achieved significantly higher initial transduction levels in human cancer cells both in vitro and in vivo, as compared to the original RCR vector itself. Notably, even at equivalent initial transduction levels, more secondary RCR progeny were produced from AdRCR-transduced cells as compared to RCR-transduced cells, resulting in further acceleration of subsequent RCR replication kinetics. In pre-established tumor models in vivo, prodrug activator gene therapy with high-titer AdRCR could achieve enhanced efficacy compared to RCR alone, in a dose-dependent manner. Thus, AdRCR hybrid vectors offer the advantages of high production titers characteristic of adenovirus and secondary production of RCR in situ, which not only accelerates subsequent vector spread and progressive tumor transduction, but can also significantly enhance the therapeutic efficacy of RCR-mediated prodrug activator gene therapy.
Introduction
Gene therapy approaches for cancer have suffered from inadequate transduction efficiencies of conventional replication-defective vectors that have been used so far. Oncolytic virotherapy using replication-competent viruses (RCRs) represents an emerging technology that shows considerable promise as a novel treatment option.1 Recently, we2,3,4,5,6,7,8,9 and others10,11,12,13,14,15,16,17 have shown that RCR vectors based on murine leukemia virus (MLV) exhibit unique characteristics as virotherapy agents, mediating highly selective infection and stable gene transfer throughout entire solid tumor masses in vivo as viral replication proceeds, even after initial inoculation at multiplicities of infection (MOIs) as low as 0.01. Although noncytolytic by nature, RCR vectors engineered to carry the yeast cytosine deaminase (CD) prodrug activator gene demonstrated highly efficient and synchronized killing of infected tumor cells both in vitro and in vivo upon administration of its prodrug, 5-fluorocytosine (5-FC).4,5,7,8,18 Furthermore, as RCR vectors stably integrate into the host cell genome, residual infected cancer cells serve as a reservoir for long-term viral persistence and further viral spread, even to metastatic sites. For example, after a single stereotactic intratumoral injection of RCR vector delivering the CD prodrug activator gene into orthotopically xenografted human gliomas, viral spread was observed in the majority of secondary tumor foci that had migrated away from the primary tumor site, thereby enabling multiple cycles of 5-FC prodrug administration at periodic intervals to achieve 100% survival for >100 days, as compared to 0% survival of control groups in <40 days.4 Based on the successful results achieved in preclinical studies, a clinical trial of RCR-mediated prodrug activator gene therapy in patients with recurrent glioblastoma has recently been approved by the US Food and Drug Administration.
In a wide variety of human cancer cell lines, amphotropic (i.e., broad host species tropism, including human) MLV-based RCR vectors quickly spread in a logarithmic manner and infect >90% of the cell population within a few days after virus inoculation.4,5,6,7,8,10,12,13,14,15,16,18,19 However, in certain human cell lines such as MDA-MB-231 and MDA-MB-435 mammary carcinoma, HeLa cervical carcinoma, and Gli36 glioma cells, there is a prolonged lag time before viral replication reaches sufficient levels for logarithmic spread to proceed. In vitro, this can be overcome by increasing the initial input dose of RCR vector, but due to the low titers characteristic of retrovirus production, achieving adequate initial transduction levels in vivo to facilitate rapid propagation within solid tumor masses can present a challenge. Therefore, novel methods to further improve the efficiency of RCR vector delivery are desirable as studies move into human clinical trials.
To address this issue, we have recently developed novel hybrid vectors (AdRCR) that are based on helper-dependent (“gutted”) adenoviruses (HDAd), in which all adenovirus coding sequences are fully deleted, and therefore have a cloning capacity of up to 36 kb. This allows the incorporation of complete retrovirus, lentivirus, or retrotransposon vector–encoding sequences,20,21,22,23,24 hence HDAd can be employed as a first-stage carrier for expression of encoded second-stage RCR vectors that are transiently produced in situ from adenovirus-infected cells.
Previously, we constructed and tested an HDAd for delivery of ecotropic (i.e., murine species-specific) RCR vector expressing the green fluorescent protein (GFP) reporter gene.24 The human adenovirus type 5–based HDAd could infect both human and murine cells, but only transiently, and was incapable of efficient replication in murine cells. Conversely, the ecotropic RCR vector was incapable of infecting human cells, but showed robust replication in murine cells. Thus, based on species-specific differential tropism, we demonstrated proof-of-concept for hybrid vectors that can achieve both the high titers characteristic of adenoviruses, and permanent gene delivery characteristic of retroviruses, via a two-step transduction mechanism.24 However, the potential for employing this strategy to enhance gene transfer efficiency in vivo, and to thereby potentiate the efficacy of therapeutic gene delivery in relevant experimental cancer models, has not yet been explored.
Accordingly, we have now developed AdRCR hybrid vectors for delivery and in situ production of amphotropic RCR capable of infecting human cancer cells. This report also represents the first use of AdRCR vectors to deliver RCR to human tumors in vivo, and for delivery of a therapeutic gene, the yeast CD prodrug activator gene. Our results demonstrate that the combined advantages of higher initial transduction by high-titer HDAd, followed by accelerated replicative spread of HDAd-derived secondary RCR, can achieve enhanced therapeutic efficiency of prodrug activator gene therapy in experimental cancer models in vivo.
Results
Production and validation of AdRCR hybrid vectors
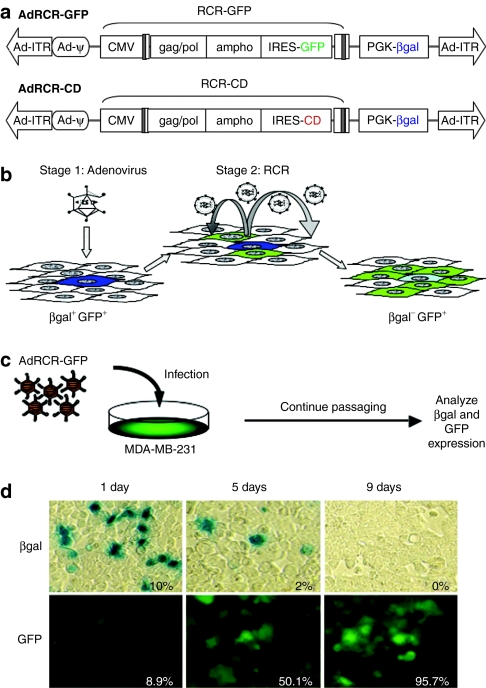
Hybrid vectors were designed and constructed by insertion of amphotropic MLV-based provirus sequences for RCR-GFP or RCR-CD,4,5,6,7 carrying the GFP marker gene or CD prodrug activator gene, respectively, into an HDAd backbone devoid of all adenovirus coding sequences (Figure 1a). These RCR-encoding HDAds, designated AdRCR-GFP and AdRCR-CD, were propagated using the FLPe/FRT and improved CRE/LoxP-based helper virus systems as previously described.25,26,27,28 The HDAd backbone of both hybrid vectors contains a phosphoglycerate kinase-1 (PGK) promoter-driven β-galactosidase (βgal) reporter gene cassette, which is entirely external to the RCR sequence and is not propagated along with the RCR vector, and thus serves as an independent marker of adenoviral transduction efficiency. The βgal titers of purified AdRCR vector preparations used in these experiments, as determined on 293 cells in the absence of detectable helper virus contamination, ranged from 7.0 × 109 to 3.8 × 1010 transducing units per ml (TU/ml).
Figure 1.
Adenovirus–RCR hybrid vector (AdRCR) design and validation. (a) Structure of AdRCR vectors. Upper: hybrid vector AdRCR-GFP, a helper-dependent adenoviral vector encoding RCR-GFP. Lower: hybrid vector AdRCR-CD, encoding RCR-CD. The adenoviral backbone of both AdRCR vectors contains a βgal reporter cassette situated outside the RCR sequence, which therefore serves as an independent marker of adenoviral transduction. (b) Principle of two-stage transduction with AdRCR vectors. AdRCR-GFP is shown as an example: the first-stage HDAd vector infects primary target cells efficiently as an adenovirus, and transiently produces RCR vectors in situ (indicated by βgal+ GFP+ cells). The released second-stage RCR vector then infects surrounding secondary target cells and undergoes permanent genomic integration, resulting in stable gene expression of its associated transgene (indicated by βgal− GFP+ cells), and further replicative spread. (c) Experimental design to validate RCR production following AdRCR infection. MDA-MB-231 cells inoculated with AdRCR-GFP vector (multiplicity of infection of 0.1) were maintained in replicate culture and passaged, and examined for expression of βgal and GFP at serial time points. (d) Analysis of transduced cells following AdRCR infection. At serial time points as indicated, aliquots of transduced cells were analyzed for βgal expression by X-gal staining, and for GFP by fluorescence microscopy and flow cytometry. Inset numbers indicate quantitation of βgal+ cells by imaging software or GFP+ cells by flow cytometry. Ad-ITR, adenovirus inverted terminal repeat sequence; βgal, β-galactosidase gene; CD, cytosine deaminase; CMV, cytomegalovirus promoter; gag-pol/env, amphotropic MLV coding sequences; GFP, green fluorescent protein; IRES, internal ribosome entry site; PGK, phosphoglycerate kinase promoter; ψ, packaging signal; RCR, replication-competent retrovirus.
We first confirmed two-stage transduction by AdRCR-GFP using MDA-MB-231 human mammary carcinoma cells, which show low permissivity for RCR infection and replication (see Supplementary Data). The presence of the βgal reporter in AdRCR-GFP allows discrimination between untransduced cells (βgal−/GFP−), cells transduced by first-stage adenovirus expressing RCR-GFP (βgal+/GFP+), and cells transduced by second-stage RCR (βgal−/ GFP+) (Figure 1b). MDA-MB-231 cells were transduced with AdRCR-GFP at MOI 0.1, and passaged at various times postinoculation (Figure 1c). As expected, βgal expression from the first-stage adenovirus quickly decreased over time, from ~10% the day after AdRCR infection, to ~2% by day 5, and was undetectable by day 9, likely due to on-going cell division, which causes dilution of nonintegrating HDAd episomes (Figure 1d). Conversely, the percentage of GFP+ cells after AdRCR transduction did not diminish, but instead rapidly increased over time, from <9% on day 1, to ~50% on day 5, and >95% by day 9, consistent with stable integration and progressive spread of the secondary RCR vector.
Kinetics of RCR production and spread after AdRCR transduction in vitro
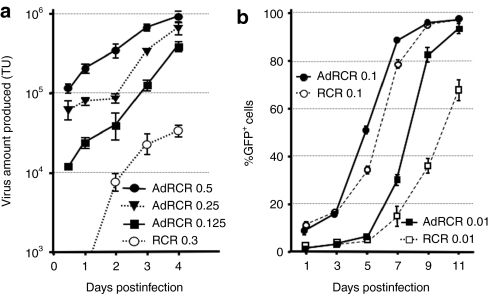
RCR production in situ was compared by harvesting conditioned medium from RCR- versus AdRCR-transduced MDA-MB-231 cells daily, and reinoculating naive cells in the presence of 3′-azido-3′-deoxythymidine to prevent further RCR spread. Notably, RCR was produced from AdRCR-transduced cells earlier, and at significantly higher titers, compared to cells transduced with RCR itself at similar MOIs (Figure 2a). In situ RCR production was undetectable until day 2 after RCR-GFP transduction at MOI 0.3, and remained in the 104 TU/ml range thereafter. In contrast, after AdRCR transduction at lower MOIs, >104 TU/ml RCR was already being produced within 12 hours, reaching >105 TU/ml by day 3.
Figure 2.
In vitro production and spread of RCR following AdRCR infection. (a) Time course of RCR production from AdRCR-GFP-infected MDA-MB-231 cells. Conditioned medium from cells infected with AdRCR-GFP (MOI = 0.125, 0.25, and 0.5) or RCR (MOI =0.3) was harvested and replenished daily for 6 days, and GFP titers on naive MDA-MB-231 cells were determined in the presence of AZT. Data shown are mean ± SD from experiments performed in triplicate. (b) Replication kinetics of secondary RCR vectors following RCR versus AdRCR infection. MDA-MB-231 cells were inoculated with RCR-GFP versus AdRCR-GFP vector at MOI 0.01 or 0.1. On the days indicated, cells were passaged and analyzed for GFP expression by flow cytometry. Data shown are mean ± SD, experiments performed in triplicate. AdRCR, adenovirus–RCR hybrid vector; GFP, green fluorescent protein; MOI, multiplicity of infection; RCR, replication-competent retrovirus; TU, transducing units.
RCR vector replication following transduction of MDA-MB-231 cells with AdRCR-GFP hybrid vector (first-stage HDAd) versus RCR-GFP was then compared. So that secondary RCR spread from RCR-transduced cells and AdRCR-transduced cells could be directly compared, the initial transduction efficiency on day 1 was adjusted to the same level between RCR and AdRCR (~10% at MOI 0.1, and 1% at MOI 0.01). With either AdRCR or RCR, a classic triphasic pattern (lag phase—log phase—plateau phase) of GFP transmission was evident. Notably, secondary RCR vector spread from AdRCR-infected cells was significantly accelerated compared to cells infected with RCR itself, particularly at lower MOIs. For example, after infection of MDA-MB-231 cells at MOI 0.01, even though both vectors showed similar transduction levels initially, by day 9 the percentage of GFP+ cells was 82.4 ± 5.2% with AdRCR, versus 36.9 ± 3.2% with RCR (Figure 2b). AdRCR also mediated accelerated spread compared to the original RCR vector even after equivalent levels of initial transduction in other cell lines which also show reduced permissivity for the original RCR vector, such as Gli36 and HeLa, confirming that these results were not specific to MDA-MB-231 cells (data not shown).
AdRCR-mediated gene transfer in human xenograft tumors in vivo
We then compared in vivo transduction efficiency of RCR versus AdRCR in subcutaneous MDA-MB-231 xenograft tumors in athymic mice. Tumors were injected with 100 µl of RCR vector (4 × 105 TU total dose) or the same volume of AdRCR vector (2 × 109 TU total dose), and harvested 4 and 10 days later. As expected, initial transduction efficiencies were significantly enhanced with AdRCR, as compared to the original RCR vector itself (%GFP+ tumor cells on day 4: RCR 0.40 ± 0.22% versus AdRCR 17.93 ± 12.42%). Notably, between day 4 and 10 after vector administration, βgal staining in AdRCR-injected tumors diminished from 19.93 ± 12.36 to 8.09 ± 1.77%, whereas GFP expression increased dramatically from 17.93 ± 12.42 to 77.85 ± 8.91% (Figure 3a), indicating successful production and propagation of secondary RCR vectors in situ. Secondary RCR spread after AdRCR transduction was significantly accelerated compared to that after transduction with RCR itself (%GFP+ tumor cells on day 10: RCR 4.63 ± 4.93% versus AdRCR 77.85 ± 8.91%) (P < 0.001, Figure 3b).
Figure 3.
AdRCR-mediated gene transfer in vivo in a subcutaneous breast cancer model. (a) Green fluorescent protein (GFP) versus β-galactosidase (βgal) expression in vivo after intratumoral injection of AdRCR-GFP into subcutaneous MDA-MB-231 human breast cancer xenografts on day 0. On day 4 and 10, tumors digested into cell suspensions were immediately analyzed for expression of βgal (bar graph) and GFP (line graph). Data shown are mean ± SD. (b) GFP expression in vivo on day 4 and day 10 after intratumoral injection of AdRCR-GFP (open circles) versus RCR-GFP (solid circles) determined by flow cytometry of disaggregated tumor cells. Data shown are mean ± SD. AdRCR, adenovirus–RCR hybrid vector; RCR, replication-competent retrovirus.
Prodrug activator gene-mediated cell killing following AdRCR transduction in vitro
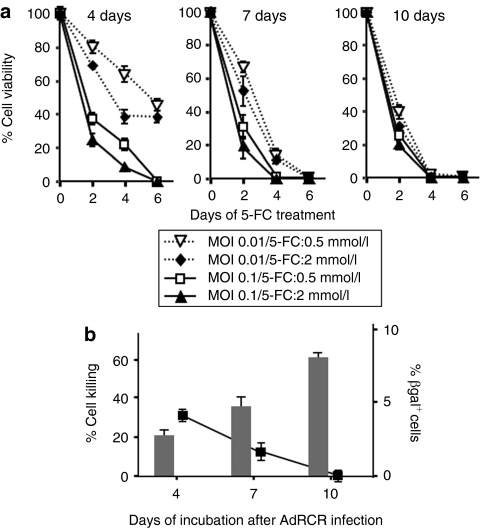
To test whether the highly efficient gene transfer into tumors achieved by AdRCR would translate into enhanced therapeutic efficacy, we constructed AdRCR-CD, in which GFP is replaced with the CD prodrug activator gene (Figure 1a). After testing various concentrations of the prodrug 5-FC in vitro, 2 mmol/l was found to be the maximum tolerated dose in naive uninfected MDA-MB-231 cells. Therefore, after AdRCR-CD first-stage HDAd transduction (MOI 0.01 or 0.1), second-stage RCR propagation was allowed to proceed for 4, 7, or 10 days before adding 5-FC to MDA-MB-231 cells at 0.5 mmol/l (low dose) or 2 mmol/l (high dose) concentrations, and cell viability was monitored 2, 4, and 6 days later (Figure 4a).
Figure 4.
Prodrug activator gene-mediated cell killing effect of RCR following AdRCR infection in vitro. (a) Cell viability of MDA-MB-231 human mammary carcinoma cells after AdRCR-CD transduction (MOI 0.01, 0.1), and secondary RCR vector spread in vitro for 4, 7, and 10 days, as indicated. x axis: days postinitiation of 5-FC prodrug treatment at 0.5 mmol/l or 2 mmol/l, as indicated; y axis: percentage of viable cells by MTS assay. (b) Cell viability versus βgal expression in vitro after AdRCR-CD infection. Left vertical axis: Cell killing (reciprocal of cell viability by MTS assay) of MDA-MB-231 cells by day 4 after initiation of 0.5 mmol/l 5-FC, after prior AdRCR-CD transduction (MOI 0.01) and secondary RCR spread for 4, 7, or 10 days, as indicated by bar graph. Right vertical axis: Percentage of adenovirus-infected cells by X-gal staining at each time point, indicated by line graph. AdRCR, adenovirus–RCR hybrid vector; 5-FC, 5-fluorocytosine; MOI, multiplicity of infection; RCR, replication-competent retrovirus.
The results indicate that AdRCR-mediated gene transfer of the CD prodrug activator gene is vector dose- and time-dependent, with a smaller effect of prodrug concentration. Cells exposed to the higher dose of AdRCR-CD vector showed accelerated cell killing regardless of prodrug dose, with the differences being most apparent when prodrug exposure was initiated early, allowing less time for secondary RCR vector spread. Cultures exposed to prodrug 4 days after transduction with AdRCR-CD at MOI 0.1 showed complete cell killing by day 6 after initiation of prodrug, whereas parallel cultures infected at MOI 0.01 showed only ~50% cell killing during the same interval (Figure 4a, left). At lower MOI and earlier time points, cell killing was slightly more potent with 2 mmol/l 5-FC as compared to 0.5 mmol/l, but this effect disappeared by day 6. Cultures exposed to prodrug 7 days after transduction showed vector dose–dependent kinetics of cell killing, with complete cell killing by day 4 after initiation of prodrug with AdRCR-CD at MOI 0.1, but not until day 6 with AdRCR-CD at MOI 0.01, regardless of prodrug concentration (Figure 4a, middle). When cultures were incubated for 10 days before initiation of 5-FC, complete cell killing was achieved by day 4 regardless of initial vector dose or prodrug concentration (Figure 4a, right), indicating that the longer interval of time allowed secondary RCR spread to a majority of the cell population. This was consistent with the previous results showing that secondary RCR-GFP could spread to ~60–95% of MDA-MB-231 cells by day 8 following AdRCR-GFP transduction at MOI 0.01–0.1 (Figure 2b). In contrast to the progressive increase in percentage of cells killed (i.e., reciprocal value of cell viability) with increasing time interval between initial AdRCR-CD transduction to starting 5-FC treatment, the percentage of adenovirus-transduced cells progressively decreased over time as confirmed by βgal staining from the AdRCR-CD backbone (Figure 4b), again indicating that the progressive spread of second-stage RCR-CD was responsible for therapeutic efficacy following first-stage HDAd transduction at low MOI.
AdRCR-mediated prodrug activator gene therapy in vivo
For in vivo analysis of AdRCR-mediated prodrug activator gene therapy, we prepared MDA-MB-231 cells pretransduced with a replication-defective lentiviral vector expressing firefly luciferase, which allows noninvasive estimates of tumor burden by bioluminescence imaging.7 Quantitative correlation between cell number and luminescent signal intensity was confirmed by imaging in vitro (data not shown).
Subcutaneous MDA-MB-231 tumors established in athymic nude mice were injected with phosphate-buffered saline (PBS) (100 µl) for controls, or AdRCR-CD (2 × 109 TU/100 µl). Eight days later, daily 5-FC treatment was initiated, and in vivo imaging was performed on day 8 (before 5-FC), 14, and 18. Bioluminescent signal intensities in all control mice increased over time, indicating tumor progression. However, signal intensities in AdRCR-CD/5-FC-treated mice were significantly decreased at later time points (Figure 5a), demonstrating decreased levels of viable tumor.
Figure 5.
In vivo antitumor effect of AdRCR-CD in human breast cancer xenograft model. (a) Bioluminescence imaging of AdRCR-mediated prodrug activator gene therapy in a human breast cancer xenograft model. Subcutaneous MDA-MB-231-luc tumors were injected intratumorally with PBS vehicle or AdRCR-CD on day 0, followed by intraperitoneal administration of 5-FC from day 8 to 18, and monitored by optical bioluminescence imaging as indicated. Images of representative mice from both groups are shown. (b) Subcutaneous MDA-MB-231 tumors established in nude mice were injected intratumorally with PBS vehicle control, RCR-CD, or AdRCR-CD on day 0, followed by intraperitoneal administration of 5-FC or PBS from day 8 until the end of the experiment (n = 5/group). Tumor volumes were measured every other day, and data shown are mean ± SD. AdRCR, adenovirus–RCR hybrid vector; CD, cytosine deaminase; 5-FC, 5-fluorocytosine; MOI, multiplicity of infection; PBS, phosphate-buffered saline; RCR, replication-competent retrovirus.
In parallel experiments, subcutaneous MDA-MB-231 tumors established in nude mice were injected intratumorally with the same volume (100 µl) of PBS, undiluted AdRCR-CD (total dose 2 × 109 TU/100 µl), or control vector RCR-CD (total dose 4 × 105 TU/100 µl, a typical dosage level achievable with retrovirus). Eight days after vector injection, daily treatment with PBS or 5-FC was initiated, and tumor volumes were measured every 3 days. As expected, control groups treated with RCR-CD or AdRCR-CD followed by PBS (instead of prodrug) showed no significant difference in tumor growth compared to untreated controls. Injection of RCR-CD at this dosage, followed by 5-FC, induced significant growth inhibition during the prodrug treatment interval. However, subsequent regrowth of tumors was observed (Figure 5b), indicating that, consistent with the results from marker gene studies above (Figure 3b), RCR alone at this dosage was not able to achieve enough replicative spread in these less permissive MDA-MB-231 cells in vivo, and thus could not achieve enough prodrug activator gene transduction and consequent cell killing to prevent regrowth of residual tumor. On the other hand, injection of AdRCR-CD at the higher titer characteristic of the first-stage adenovirus, followed by 5-FC treatment, induced much more potent growth suppression, and no tumor regrowth was observed (Figure 5b). Again, this result would not be expected to be due simply to higher initial transduction levels by HDAd itself, but is consistent with transduction levels achieved by secondary RCR vectors produced in situ from AdRCR, based on the results from marker gene studies above (Figure 3b).
Discussion
Adenovirus can be grown at high titer, and they release DNA into the nucleus of the host cells within 1 hour after binding to cell-surface receptor,29 and expression of functional transgene products from adenoviral vectors can be observed as early as 3 hours after infection.30 Consistent with adenovirus biology, we found that first-stage HDAd transduction mediated robust RCR production at an earlier stage after initial infection compared to that after transduction with RCR itself. Furthermore, the AdRCR hybrid vector system enabled not only a significant enhancement in initial transduction efficiency, but also a significant acceleration of the kinetics of subsequent second-stage RCR vector spread, particularly in vivo after intratumoral injection of the high titers readily achievable by adenovirus, as compared to retrovirus in an equivalent volume. These combined characteristics of enhanced transduction and accelerated kinetics of RCR spread, as directly demonstrated with AdRCR vectors delivering the GFP marker gene, were found to translate into a vector dose–dependent improvement in therapeutic benefit with the use of AdRCR vectors for delivery of the CD prodrug activator gene in vivo.
It was notable that, under the experimental conditions employed, intratumoral injection of RCR vector in MDA-MB-231 cell–derived tumors, which are poorly permissive for retrovirus replication, resulted only in transient tumor growth inhibition for the duration of prodrug treatment. This indicates that killing of the ~5% of tumor mass transduced by RCR-CD vector (as indicated by AdRCR-GFP studies) within the 10-day time frame allowed for spread, along with any bystander effect of CD/5-FC prodrug activator gene therapy, achieved at best only an equilibrium with the on-going proliferation of uninfected tumor cells.
In contrast, use of AdRCR-CD hybrid vector resulted in complete tumor regression upon prodrug treatment. Although the higher titers afforded by the first-stage adenovirus allowed administration of ~1,000-fold higher vector dosage as compared to the same volume of RCR supernatant, it is unlikely that the increase in initial transduction efficiency solely accounts for this degree of improved therapeutic efficacy. Even with direct intratumoral injection of high-titer adenovirus, it has been shown in clinical studies that transduction is limited to the area immediately surrounding the injection track.31 Consistent with such observations, AdRCR-GFP injected at the same dosage under equivalent conditions in the same tumor model showed that transduction levels on day 8 after vector injection (the time point at which prodrug treatment was initiated) would only be expected to be 20% at most (according to β-gal staining for HDAd transduction level on day 4) and more likely closer to 8% (HDAd transduction level on day 10). Rather, these results indicate that the higher initial transduction level combined with subsequent RCR vector spread, which reached almost 80% of the tumor by day 10 as indicated by AdRCR-GFP studies, was necessary to achieve complete regression.
On the other hand, it is conceivable that similar results could be achieved if RCR vectors could be produced in large-scale quantities and concentrated to high titers. Indeed, this is the “good manufacturing practice” production strategy now being implemented for the initial clinical trials of RCR vector–mediated cancer gene therapy. However, as noted above, even at the same MOI, AdRCR-derived secondary RCR vector production and spread was more robust than the equivalent RCR-derived progeny. This suggests that, in less permissive human cell lines, one cause of slower RCR replication kinetics may be lower strength of the natural retrovirus promoter in the U3 region of the 5′ long terminal repeat, which is replaced by the cytomegalovirus promoter in the RCR provirus sequence contained within the HDAd vector. It is also possible that, although introduced at the same MOI into the primary target cells, secondary RCR transcripts are expressed from higher episomal copy numbers of AdRCR vectors as compared to fewer integrated copies of RCR vectors.
In this study, we employed a human xenograft tumor model established in immunodeficient athymic mice, in order to more precisely demonstrate the intrinsic contribution to enhanced transduction efficiency and therapeutic efficacy achieved by the hybrid vector system, in the absence of potentially confounding immunological responses to either the first-stage adenovirus or second-stage RCR vectors. Certainly, in future studies, it will be of considerable interest to determine how well this hybrid vector system will perform in syngeneic tumor models in immunocompetent animals, and although beyond the scope of this report, these studies are currently on-going.
In summary, AdRCR hybrid vectors exhibited significantly higher initial transduction and higher levels of second-stage RCR production in situ, subsequently leading to accelerated RCR vector spread, and thereby achieving enhanced therapeutic efficacy of prodrug activator gene therapy for cancer. AdRCR vectors may also have additional advantages for combined tumor targeting via fiber modification of the first-stage adenovirus and transcriptional regulation of the second-stage RCR vector. It has already been well established that the target cell binding tropism of adenovirus vectors, including high-capacity adenovirus vectors, can be altered by modifications to the fiber knob.32,33,34 To date, such physical targeting by modification of viral envelope proteins has rarely been achievable at realistic efficiencies for retrovirus vectors. Thus, hybrid vectors based on helper-dependent adenoviruses directing the in situ production of RCR vectors could represent an ideal combination, with the potential for high titer production, low immunogenicity, and targeting of binding tropism via the first-stage adenovirus, with permanent transgene integration and amplification of initial input titer by propagation strictly restricted to actively dividing tumor cells via the second-stage RCR, whose production from the adenovirus genome in infected cells could further be regulated by the use of tissue-specific or inducible promoters.2,35,36
Materials and Methods
Cell lines. Human cell lines, including 293 (ref. 37), Gli36 (ref. 38), MDA-MB-231 breast cancer, and HeLa cervical carcinoma, were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. All cell lines were obtained from American Type Culture Collection (Manassas, VA), and maintained in a humidified atmosphere with 5% CO2.
Vector plasmids and virus production. To construct AdRCR vectors (AdRCR-GFP and AdRCR-CD), the complete 11-kb provirus and linked transgene cassette from RCR vector ACE-GFP (RCR-GFP) or ACE-CD (RCR-CD)4,5,6,7 was inserted into an HDAd plasmid, pSKlacZ,22 which was derived from the parental plasmid pSTK120 (refs. 39,40,41) as previously described. HDAd virus was prepared using the FLPe/FRT and improved CRE/LoxP-based helper virus systems.25,26,27,28 The RCR virus preparations were produced by transient transfection of 293T cells with RCR vector–encoding plasmids, also as described previously.3
Analysis of RCR replicative kinetics following RCR and AdRCR infections in vitro. Target cells were transduced with AdRCR-GFP or RCR-GFP at various MOIs in triplicate, and culture media were sampled daily and titered on naive cells by flow cytometry for GFP expression in the presence of 3′-azido-3′-deoxythymidine (50 µmol/l; Sigma, St Louis, MO) to prevent further virus spread. To examine replicative kinetics of RCR vectors produced from RCR versus and AdRCR, transduced target cells were maintained in the absence of 3′-azido-3′-deoxythymidine.
Cytotoxicity assay in vitro. Target cells in triplicate 96-well plates (2 × 103 cells/well) were transduced with AdRCR at different MOIs and exposed to various concentrations of 5-FC prodrug (Sigma). Cell viability was determined using a tetrazolium dye conversion assay (CellTiter Pro 96 MTS assay; Promega, Madison, WI), and normalized to control cells without 5-FC.
In vivo experiments. For marker gene experiments, 1 × 106 MDA-MB-231 cells were subcutaneously injected in nude mice (Charles River Laboratories, Wilmington, MA) to establish tumors, which were then injected with RCR-GFP or AdRCR-GFP (n = 5/group) upon reaching 5 mm in diameter. Tumors were harvested 4 or 10 days later, and immediately digested with collagenase into cell suspensions for analysis of βgal and GFP expression.
For prodrug activator gene experiments, subcutaneous tumors were established with 1 × 106 MDA-MB-231 cells in nude mice to establish tumors, which were then injected with 4 × 105 TU/100 µl RCR-CD, 2 × 109 TU/100 µl AdRCR-CD, or 100 µl PBS vehicle, followed by intraperitoneal administration of PBS or 5-FC (500 mg/kg) daily, starting from day 8 after vector injection. Tumors were measured three times per week, and tumor volumes calculated as a × b2 × 0.5 (a: large diameter, b: small diameter).
Optical imaging analysis. MDA-MB-231 cells engineered to express firefly luciferase by lentiviral transduction (MDA-MB-231-luc) were examined by bioluminescence optical imaging using a cooled CCD system (Xenogen IVIS) 2 minutes after addition of 𝒹-luciferin (150 µg/well), as previously described.7
For in vivo imaging, MDA-MB-231-luc cells were grown subcutaneously in nude mice to a diameter of ~5 mm. Mice were injected intratumorally with PBS (control) or AdRCR-CD (2 × 109 TU) on day 0, followed by intraperitoneal administration of 5-FC (500 mg/kg/day) from day 8 to 18. Optical bioluminescence imaging was performed using the Xenogen system with a 30-second acquisition time on day 8 after vector injection (just before 5-FC treatment), day 14 (7 days after 5-FC treatment), and day 18 (11 days after 5-FC treatment).
Statistical analysis. Statistical analyses were done with Student's t-test or one-way analysis of variance (Kruskal–Wallis test) performed with Prism 5 statistical software (GraphPad Software, San Diego, CA), and P values of <0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL Data. Analysis of RCR replication kinetics in different cell lines.
Acknowledgments
We thank Pedro Lowenstein for the FLPe/FRT system; Stefan Kochanek for the STK plasmids. This work was supported in part by NIH R01 # CA93709 (N.K.); R01 CA105171 (N.K.); R21 DK54280 (N.K.); a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S.K.); and Susan G. Komen Breast Cancer Foundation post-doctoral fellowship PDF0503958 (K.H.).
Supplementary Material
Analysis of RCR replication kinetics in different cell lines.
REFERENCES
- Liu TC., and, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- Logg CR, Logg A, Matusik RJ, Bochner BH., and, Kasahara N. Tissue-specific transcriptional targeting of a replication-competent retroviral vector. J Virol. 2002;76:12783–12791. doi: 10.1128/JVI.76.24.12783-12791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logg CR., and, Kasahara N. Retrovirus-mediated gene transfer to tumors: utilizing the replicative power of viruses to achieve highly efficient tumor transduction in vivo. Methods Mol Biol. 2004;246:499–525. doi: 10.1385/1-59259-650-9:499. [DOI] [PubMed] [Google Scholar]
- Tai CK, Wang WJ, Chen TC., and, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12:842–851. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WJ, Tai CK, Kasahara N., and, Chen TC. Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum Gene Ther. 2003;14:117–127. doi: 10.1089/104303403321070810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Kimura T, Logg CR., and, Kasahara N. Tumor-selective gene expression in a hepatic metastasis model after locoregional delivery of a replication-competent retrovirus vector. Clin Cancer Res. 2006;12:7108–7116. doi: 10.1158/1078-0432.CCR-06-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007;67:5345–5353. doi: 10.1158/0008-5472.CAN-06-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, et al. Highly efficient gene delivery for bladder cancers by intravesically administered replication-competent retroviral vectors. Clin Cancer Res. 2007;13 15 Pt 1:4511–4518. doi: 10.1158/1078-0432.CCR-07-0151. [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Menendez S, Ozu C, Ohori M, Cordon-Cardo C, Logg CR, et al. Delivery of replication-competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther. 2007;14:279–286. doi: 10.1038/sj.cgt.7701013. [DOI] [PubMed] [Google Scholar]
- Bachrach E, Duch M, Pelegrin M, Dreja H, Pedersen FS., and, Piechaczyk M. In vivo infection of mice by replication-competent MLV-based retroviral vectors. Methods Mol Med. 2003;76:343–352. doi: 10.1385/1-59259-304-6:343. [DOI] [PubMed] [Google Scholar]
- Bachrach E, Pelegrin M, Piechaczyk M, Pedersen FS., and, Duch M. Efficient gene transfer into spleen cells of newborn mice by a replication-competent retroviral vector. Virology. 2002;293:328–334. doi: 10.1006/viro.2001.1284. [DOI] [PubMed] [Google Scholar]
- Metzl C, Mischek D, Salmons B, Günzburg WH, Renner M., and, Portsmouth D. Tissue- and tumor-specific targeting of murine leukemia virus-based replication-competent retroviral vectors. J Virol. 2006;80:7070–7078. doi: 10.1128/JVI.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solly SK, Trajcevski S, Frisén C, Holzer GW, Nelson E, Clerc B, et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003;10:30–39. doi: 10.1038/sj.cgt.7700521. [DOI] [PubMed] [Google Scholar]
- Trajcevski S, Solly SK, Frisén C, Trenado A, Cosset FL., and, Klatzmann D. Characterization of a semi-replicative gene delivery system allowing propagation of complementary defective retroviral vectors. J Gene Med. 2005;7:276–287. doi: 10.1002/jgm.663. [DOI] [PubMed] [Google Scholar]
- Qiao J, Moreno J, Sanchez-Perez L, Kottke T, Thompson J, Caruso M, et al. VSV-G pseudotyped, MuLV-based, semi-replication-competent retrovirus for cancer treatment. Gene Ther. 2006;13:1457–1470. doi: 10.1038/sj.gt.3302782. [DOI] [PubMed] [Google Scholar]
- Dalba C, Bellier B, Kasahara N., and, Klatzmann D. Replication-competent vectors and empty virus-like particles: new retroviral vector designs for cancer gene therapy or vaccines. Mol Ther. 2007;15:457–466. doi: 10.1038/sj.mt.6300054. [DOI] [PubMed] [Google Scholar]
- Paar M, Schwab S, Rosenfellner D, Salmons B, Günzburg WH, Renner M, et al. Effects of viral strain, transgene position, and target cell type on replication kinetics, genomic stability, and transgene expression of replication-competent murine leukemia virus-based vectors. J Virol. 2007;81:6973–6983. doi: 10.1128/JVI.02470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tai CK, Kershaw AD, Solly SK, Klatzmann D, Kasahara N, et al. Use of replication-competent retroviral vectors in an immunocompetent intracranial glioma model. Neurosurg Focus. 2006;20:E25. doi: 10.3171/foc.2006.20.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logg CR, Tai CK, Logg A, Anderson WF., and, Kasahara N. A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors. Hum Gene Ther. 2001;12:921–932. doi: 10.1089/104303401750195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Kataoka M, Tateno C, Yoshizato K, Kawasaki Y, Kimura T, et al. In vivo stable transduction of humanized liver tissue in chimeric mice via high-capacity adenovirus-lentivirus hybrid vector. Hum Gene Ther. 2010;21:40–50. doi: 10.1089/hum.2009.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S., and, Mitani K. A new hybrid system capable of efficient lentiviral vector production and stable gene transfer mediated by a single helper-dependent adenoviral vector. J Virol. 2003;77:2964–2971. doi: 10.1128/JVI.77.5.2964-2971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Seleme MC, Soifer HS, Perez JL, Moran JV, Kazazian HH, Jr, et al. L1 retrotransposition in nondividing and primary human somatic cells. Proc Natl Acad Sci USA. 2006;103:8036–8041. doi: 10.1073/pnas.0601954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soifer H, Higo C, Kazazian HH, Jr, Moran JV, Mitani K., and, Kasahara N. Stable integration of transgenes delivered by a retrotransposon-adenovirus hybrid vector. Hum Gene Ther. 2001;12:1417–1428. doi: 10.1089/104303401750298571. [DOI] [PubMed] [Google Scholar]
- Soifer H, Higo C, Logg CR, Jih LJ, Shichinohe T, Harboe-Schmidt E, et al. A novel, helper-dependent, adenovirus-retrovirus hybrid vector: stable transduction by a two-stage mechanism. Mol Ther. 2002;5 5 Pt 1:599–608. doi: 10.1006/mthe.2002.0586. [DOI] [PubMed] [Google Scholar]
- Ng P, Beauchamp C, Evelegh C, Parks R., and, Graham FL. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol Ther. 2001;3 5 Pt 1:809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- Palmer D., and, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Palmer DJ., and, Ng P. Methods for the production of helper-dependent adenoviral vectors. Methods Mol Biol. 2008;433:33–53. doi: 10.1007/978-1-59745-237-3_3. [DOI] [PubMed] [Google Scholar]
- Umaña P, Gerdes CA, Stone D, Davis JR, Ward D, Castro MG, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat Biotechnol. 2001;19:582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- Greber UF, Webster P, Weber J., and, Helenius A. The role of the adenovirus protease on virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Kiwaki K, Awata H, Katoh H, Kanegae Y, Saito I, et al. In vivo correction with recombinant adenovirus of 4-hydroxyphenylpyruvic acid dioxygenase deficiencies in strain III mice. Hum Gene Ther. 1997;8:65–71. doi: 10.1089/hum.1997.8.1-65. [DOI] [PubMed] [Google Scholar]
- Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- Campos SK., and, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007;7:189–204. doi: 10.2174/156652307780859062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow JN, Bauerschmitz GJ, Curiel DT., and, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- Volpers C., and, Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med. 2004;6 Suppl 1:S164–S171. doi: 10.1002/jgm.496. [DOI] [PubMed] [Google Scholar]
- Dalba C, Klatzmann D, Logg CR., and, Kasahara N. Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors. Curr Gene Ther. 2005;5:655–667. doi: 10.2174/156652305774964659. [DOI] [PubMed] [Google Scholar]
- Tai CK., and, Kasahara N. Replication-competent retrovirus vectors for cancer gene therapy. Front Biosci. 2008;13:3083–3095. doi: 10.2741/2910. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC., and, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Sena-Esteves M, Saeki Y, Camp SM, Chiocca EA., and, Breakefield XO. Single-step conversion of cells to retrovirus vector producers with herpes simplex virus-Epstein-Barr virus hybrid amplicons. J Virol. 1999;73:10426–10439. doi: 10.1128/jvi.73.12.10426-10439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S., and, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandig V, Youil R, Bett AJ, Franlin LL, Oshima M, Maione D, et al. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc Natl Acad Sci USA. 2000;97:1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of RCR replication kinetics in different cell lines.