Abstract
Gene therapy provides a promising approach to curing diabetes. However, an effective route for islet-specific targeting has yet to be established. Toward this end, the pancreatic blood circulation system in Balb/c mice was determined by the injection of rhodamine-containing beads. The efficiency of islet targeting was then measured by the injection of adenoviral vectors carrying a green fluorescence gene via the celiac trunk (C.T.). The results showed that >95% of islets and about 60% of β cells within the pancreatic body and tail could be labeled 3 days after surgery. α-Cell labeling was not as efficient, whereas labeling of nonendocrine tissues was barely detectable. For proof of principle, adenoviral vectors carrying a Sirtuin transgene were injected similarly to test the islet protection effect in the streptozotocin (STZ)-induced type 1 diabetic model. The results demonstrated that overexpression of Sirtuin in STZ-treated mice reduced the level of β-cell death and extent of glucose intolerance. This study reports on efficient islet-specific targeting by using adenoviral injection. This procedure could be invaluable to the treatment of diabetes and the study of islet biology.
Introduction
Gene therapy has the potential to cure type 1 diabetes by preventing autoimmunity, or by stimulating β-cell regeneration.1 Several viral vectors including adenovirus, adeno-associated virus, and lentivirus have been used to transfer genes into the pancreas in vivo.2,3,4,5 However, an effective route to achieving islet-specific targeting has not been established. Direct injection of adenoviruses into the pancreatic parenchyma largely led to transduction of the exocrine tissue.6 On the other hand, retrograde pancreatic duct injection limited viruses within the ductal system. A small degree of leakiness of viruses into the interstices, however, could occur when a strong injection force was applied.7,8
It has been shown that pancreatic islets have fenestrated capillaries with pores of 50–80 nm in diameter, whereas the capillaries in other pancreatic tissues have continuous sealed endothelia.9,10 Taking advantage of this unique feature, Ayuso et al. reported an adenoviral injection procedure through the jugular vein (J.V.) which targeted specifically the pancreatic β cells.11 Other tissues such as the glomerulus, lung, spleen, and kidney were also affected to a certain degree.12 However, we failed to reproduce the reported result of a significant islet labeling.11 Therefore, it could be important to develop a local administration route via the celiac trunk (C.T.).
Sirt1 is a histone deacetylase and belongs to the silent information regulator (Sir2) family.13 Recent studies have shown that Sirt1 could inhibit the transcriptional nuclear factor-κB signaling pathway by direct deacetylation of the p65 subunit,14 and could downregulate the levels of inducible form of nitric oxide synthase and nitric oxide production,15 which was an essential component of the inflammatory response that caused β-cell destruction.16 Furthermore, treatment with the β-cell-specific toxin, streptozotocin (STZ), could rapidly increase the nuclear translocation of nuclear factor-κB subunits and the production of inducible form of nitric oxide synthase.17 Activation of Sirt1, therefore, protected β cells from apoptosis in STZ-treated mice.17
The results in this study showed that β-cell-specific labeling could be accomplished with high efficiency by adenoviral vectors injected via the C.T. Furthermore, overexpression of Sirt1 protected β cells from apoptosis in STZ-treated mice. This procedure could be invaluable to the treatment of diabetes and the study of islet biology.
Results
Blood circulation and injection route
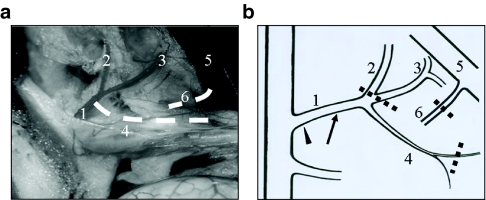
After anesthesia, the abdomen of Balb/c mouse was open through a midline incision. The duodenum and the pancreatic head were flipped over to the left, to expose the underneath circulation system (Figure 1a). To determine the pancreatic blood supply, we injected rhodamine-containing beads into different arteries while occluding the C.T. and portal vein. We found that the pancreatic body and tail were supported by the lieno-pancreatic artery. Due to the complexity of the pancreatic blood circulation, it was difficult to perfuse the whole parenchyma with a single procedure. Therefore, we chose the islet-abundant pancreatic body and tail for testing the efficiency of adenoviral transduction via intra-artery injection.
Figure 1.
Pancreatic blood circulation system and surgical procedure. (a) The visceral contents were externalized after a Balb/c mouse was killed. The photo was taken under a dissecting microscope, and was processed with Photoshop to remove the reflected light. The lieno-pancreatic artery and pancreatic vein (dotted white lines) were invisible under the microscope. (b) Schematic illustration of the pancreatic blood circulation and surgical procedure. The celiac trunk was clamped with a hemostatic forceps (arrowhead) before placing ligations sequentially on illustrated vessels (dotted lines). The arrow indicated the injection site on the celiac trunk. 1, Celiac trunk; 2, lieno-gastric artery; 3, common hepatic artery; 4, lieno-pancreatic artery; 5, hepatic portal vein; 6, pancreatic vein.
To optimize the delivery efficiency, the pancreatic blood-inlet was blocked and, subsequently, the blood-outlet was occluded so that empty space for accommodation of the vectors could be created (Figure 1b). By using rhodamine-containing beads, we found that clamping the C.T. for at least 2 minutes was necessary to empty the pancreatic blood. Ligations of the proximal end of the common hepatic artery and gastro-lienal artery, and distal end of the lieno-pancreatic artery resulted in a fully perfused pancreas by preventing temporarily the beads from going into the liver, stomach, and spleen. In addition, placing a ligation on the pancreatic vein led to a great retention of beads within the pancreas.
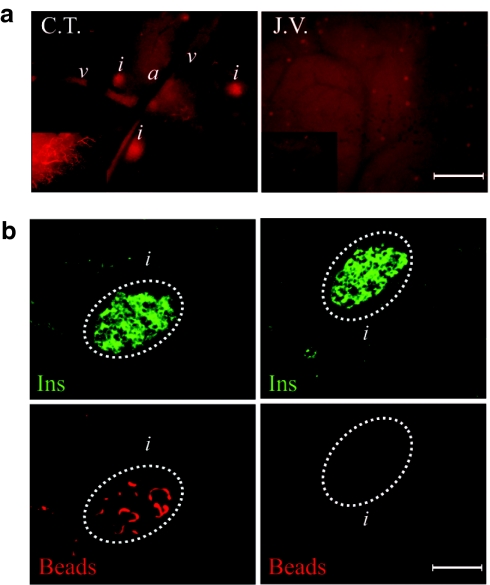
Having established the procedure, we compared the effectiveness of bead delivery into the pancreas via two injection routes: the C.T., established in this study, versus the J.V. reported previously in the literature.11 As shown in Figure 2a, the intensity of rhodamine within the C.T.-pancreas detected directly under a fluorescence microscope was dramatically brighter than that of J.V.-pancreas. In addition, capillaries by the edges of pancreatic lobes were clearly illuminated. Moreover, C.T.-pancreas displayed a few bright red speckles probably revealing the locations of a subset of islets. The preferential retention of beads within islets was verified by immunofluorescence staining (Figure 2b).
Figure 2.
Rhodamine-containing beads injected via C.T. were retained predominantly within islets. Equal amount of rhodamine-containing beads were injected into the pancreases of Balb/c mice according to different procedures (left: C.T.; right: J.V.). The pancreases were harvested immediately after the surgery without releasing the ligations. (a) Direct fluorescence of pancreases examined under a fluorescence microscope (×4 objective). The inserts were micrographs taken with a ×10 objective. (b) Micrographs of insulin staining (upper panel) and direct fluorescence of rhodamine-containing beads (lower panel) on frozen sections of the pancreases. Islets were delineated with a white dotted circle. a, bar = 400 µm; b, bar = 100 µm. a, arteriole; C.T., ciliac trunk; Ins, insulin staining; i, islet; J.V., jugular vein; v, venule.
Transduction efficiency of islet β cells
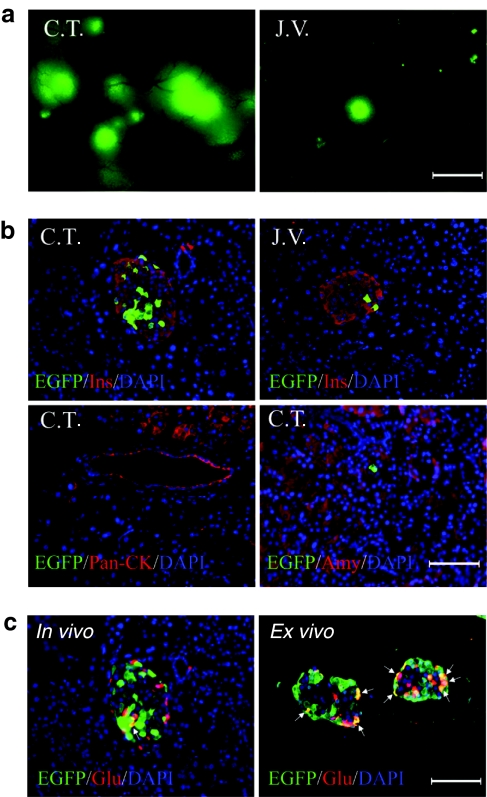
A density of 5 × 109 infection units (IU) of adenoviral vectors carrying a gene for the green fluorescence protein (Ad-EGFP) were then injected via C.T. or J.V. Pancreases were harvested 3 days after operation. Consistent with the result of bead injection, Ad-EGFP injected C.T.-pancreas displayed many big fluorescence clusters comparable to the size of an islet, while J.V.-pancreas showed only a few smaller fluorescence spots (Figure 3a). On the other hand, higher fluorescence intensity could be detected in the liver and spleen of J.V.-mice (Supplementary Figure S1a,b).
Figure 3.
Islet-specific transduction by Ad-EGFP injected via C.T. A density of 5 × 109 IU of Ad-EGFP were injected into the pancreases of Balb/c mice according to different procedures (indicated as C.T. or J.V. on micrographs). The pancreases were harvested 3 days post operation. (a) Direct fluorescence of pancreases examined under a fluorescence microscope (×4 objective). (b) Micrographs of immunofluorescence staining for EGFP/Insulin, EGFP/Pan-CK, or EGFP/Amylase on paraffin sections of the pancreases. (c) α-Cell labeling was examined on paraffin sections of C.T.-pancreas (in vivo) or islets incubated with Ad-EGFP for 24 hours (ex vivo, multiplicity of infection = 100). Arrows indicated EGFP-positive α cells. a, bar = 400 µm; b,c, bar = 100 µm. Amy, amylase staining; C.T., celiac trunk; DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; Glu, glucagon staining; Ins, insulin staining.
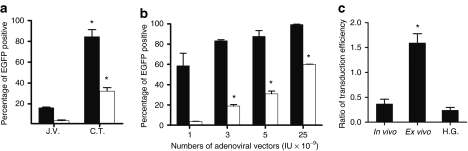
Immunofluorescence analyses were carried out to identify the transduced pancreatic cell types (Figure 3b). In both C.T.- and J.V.-pancreases, the result showed that EGFP-positive cells resided exclusively within islets. Quantification analyses showed that labeling efficiencies for islets and β cells were ~85 and 30%, respectively, in C.T.-pancreases as compared to that of <20 and 5% in J.V.-pancreases (Figure 4a). In contrast, J.V. injection led to a more efficient transduction of liver cells and spleen cells (Supplementary Figure S1c,d). The lack of transduction of acinar and ductal cells was further confirmed by the staining of amylase and Pan-CK, respectively (Figure 3b, <0.1%). In addition, we could not find any EGFP-positive cells on the endothelial lining of the vessels (data not shown). Relative transgene expression levels in organs from C.T.- and J.V.-mice were determined by real-time reverse transcription-PCR (Supplementary Figure S2 and Supplementary Materials and Methods).
Figure 4.
Transduction efficiency of pancreatic cells by Ad-EGFP. (a) Labeling percentage of islet (black bars) and β cell (white bars) in J.V.- or C.T.-pancreases injected with 5 × 109 IU of Ad-EGFP. (b) Labeling percentage of islet (black bars) and β cell (white bars) in C.T.-pancreases injected with different amounts of Ad-EGFP. (c) Ratio of transduction efficiency between β and α cells in C.T.-pancreases (in vivo), isolated islets incubated with Ad-EGFP (ex vivo), or C.T.-pancreases plus glucose injected peritoneally (H.G.). At least a total of 60 islets were evaluated in each assay. (*P < 0.05, as compared to its immediate counterpart to the left; n = 4–5 for each bar). C.T., celiac trunk; EGFP, enhanced green fluorescent protein; H.G., high glucose; IU, infection units; J.V., jugular vein.
We then tested the dose-dependent adenoviral transduction efficiency of pancreatic β cells. The results showed that as low as 1 × 109 IU of viruses were able to label about 60% of islets and 5% of β cells. When 2.5 × 1010 IU of viruses were injected, the transduction efficiency increased to >95 and 60% for islets and β cells, respectively (Figure 4b).
Transduction efficiency of islet α cells
Curiously, we found that α-cell targeting was inefficient as compared to that of β cells (Figure 3c). To test whether this was due to a selective tropism of adenovirus toward β cells, we carried out a suspension culture with isolated islets in the presence of Ad-EGFP. The ex vivo result showed that α cells could be transduced efficiently by adenoviral vectors, indicating the existence of a possible physical barrier between α cells and islet capillaries (Figure 4c). Alternatively, it has been proposed that the opening of capillaries within islets could be controlled by “gates” consisting of endothelial cells in response to plasma glucose levels.18 Therefore, glucose was injected into the peritoneal cavity 30 minutes in advance of the vector injection. However, the ratio of transduction percentage between β and α cells was not correlated to the variation of glucose concentration (Figure 4c).
Expression of Sirt1 protected islet β cells
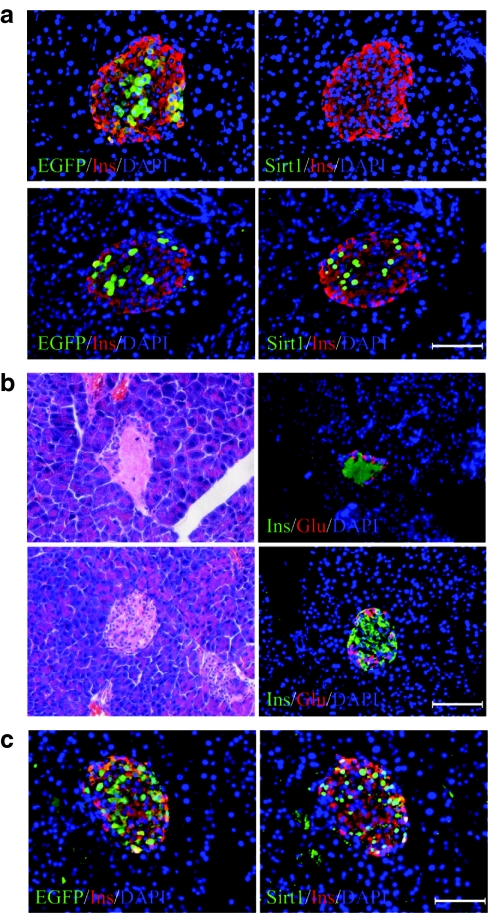
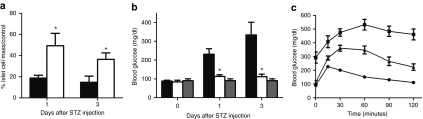
For proof of principle, we tested the islet protective function of Sirt1 in STZ-treated mice. Three days after the injection of Ad-Sirt1 (5 × 109 IU) via C.T., the expression of Sirt1 in islets increased dramatically as compared to that of Ad-EGFP-injected controls (Figure 5a). Consequently, intraperitoneal injection of STZ led to severe β-cell death in Ad-EGFP, but mild in Ad-Sirt1-treated mice (Figure 5b and Supplementary Figure S4 and Supplementary Materials and Methods). Islet cell mass in Ad-EGFP-treated mice was reduced to ~20% of normal mice 3 days after STZ injection. However, about 40% of islet cell mass was maintained in Ad-Sirt1-treated mice (Figure 6a). Furthermore, the residual β cells were mostly EGFP-positive and Sirt1-positive, confirming an essential role of Sirt1 on β survival (Figure 5c). On the contrary, β-cell mass was not preserved in Ad-Sirt1-treated mice when the vector was injected via J.V. (Supplementary Figure S5a).
Figure 5.
Islet-specific expression of Sirt1-protected β cells from STZ-induced apoptosis. (a,b) A density of 5 × 109 IU of Ad-EGFP (upper panel) or Ad-Sirt1 (lower panel) were injected via C.T. into the pancreases. (a) Micrographs show the staining of EGFP/Insulin (left), or Sirt1/Insulin (right) on consecutive paraffin sections of the pancreases. (b) Two days after viral injection, STZ (195 mg/kg body weight) was injected peritoneally. Micrographs show the staining of hematoxylin and eosin (left), or Insulin/Glucagon (right) on paraffin sections of the pancreases 24 hours after STZ injection. (c) Pancreases of the Ad-Sirt1/STZ mice in b were stained for EGFP/Insulin (left), or for Sirt1/Insulin (right). Bar = 100 µm. EGFP, enhanced green fluorescent protein; DAPI, 4′,6-diamidino-2-phenylindole; Glu, glucagon staining; Ins, insulin staining.
Figure 6.
Functional analysis of islets in STZ-treated mice. Ad-EGFP or Ad-Sirt1 (5 × 109 infection units) was injected via C.T. into the pancreases. Two days after surgery, STZ (195 mg/kg body weight) was injected peritoneally. Normal mice without STZ treatment were used as controls. (a) Percentage of islet cell mass of pancreases harvested 1 and 3 days after STZ injection (black bars: Ad-EGFP/STZ; white bars: Ad-Sirt1/STZ). (b) Fasting plasma glucose levels measured at indicated time points (black bars: Ad-EGFP/STZ; white bars: Ad-Sirt1/STZ; gray bars: normal controls). (c) Glucose tolerance test performed 3 days after STZ injection. (circles: normal controls; squares: Ad-EGFP/STZ; triangles: Ad-Sirt1/STZ) (*P < 0.05, as compared to untreated mice in a and b; in c, P < 0.05 for controls versus Ad-Sirt1/STZ, and Ad-Sirt1/STZ versus Ad-EGFP/STZ at 15–120 minutes; n = 5). STZ, streptozotocin.
Finally, we tested the function of islets in Ad-Sirt1-treated mice. The fasting glucose levels were significantly increased in Ad-EGFP mice 24 hours after STZ treatment. On the contrary, Ad-Sirt1-treated mice via C.T., but not via J.V., maintained euglycemia before and after STZ injection (Figure 6b and Supplementary Figure S6a). However, the administration of STZ apparently led to mild glucose intolerance in Ad-Sirt1-treated C.T.-mice although neither as severe as that in Ad-EGFP-treated mice (Figure 6c), nor as that in Ad-Sirt1-treated J.V.-mice (Supplementary Figure S6b). The protective effect of Sirt1 in C.T.-mice was further confirmed by results from measurement of insulin transcripts in the pancreas and insulin protein in the circulation (Supplementary Figure S5b,c and Supplementary Materials and Methods).
Discussion
An effective route to achieving islet-specific targeting in vivo is key to the success of gene therapy for diabetes. Systematic administration from the J.V. reportedly transduced >20% of islet cells with 5 × 108 plaque-forming unit of adenoviral vectors.11 However, the result was not repeated in this study. We noticed that the adenovirus of serotype 2, instead of serotype 5, was used in the reported study.11 However, adenoviral vector of serotype 5 has been shown to transduce a broad spectrum of cell types including islet β cells.19 Nevertheless, we reasoned that local delivery could be more favorable as systemically injected viral particles might be neutralized by the complement, or opsonized by the macrophage. In addition, high loads of viral particles in the circulation inevitably would provoke a strong immune response.20
Bead injection via C.T. showed a characteristic speckle pattern within the pancreas, probably revealing the locations of a subset of islets (Figure 2b). This is consistent with the disproportional distribution of blood supply to pancreatic islets, which constitute <2% of the pancreatic cell mass and yet consume ~20% of the arterial blood volume.21 Furthermore, a previous study using electromicroscopy showed that islets, especially interlobular ones, could be easily distinguished from the exocrine tissue due to the presence of prominent vessels.22 However, we did notice that the speckles were not evenly distributed in the pancreas. We are not sure presently whether this reflects different perfusion features of individual islets, or whether our procedure needs further improvement.
Not surprisingly, Ad-EGFP injection via C.T. showed a much brighter speckle pattern; though, the highly specific transduction of islet cells was unexpected (Figure 3b). In contrast to the sealed and continuous endothelium in the exocrine tissue, intraislet capillaries were lined by fenestrated endothelial cells, which had a pore size in the range of 50–80 nm in diameter.9,10 Adenovirus of serotype 5 with a particle size of ~80 nm probably has the capability to emigrate from the pore with the help of blood pressure or the pressure created during viral injection.
Additional specificity might be provided by the basement membrane that has been found to function as a physical barrier for the islets.23 Viral particles, apparently coming out off capillaries, were trapped within islet capsules consisting of many components of the extracellular matrix. The possibility of a selective tropism of the adenoviral vectors could be ruled out. Studies from our lab and others had demonstrated that acinar and ductal cells could be labeled efficiently when adenoviral vectors were injected into the duct or directly into the parenchyma.7,8 However, a physical nature probably was not accountable for the lack of targeting of the vessel endothelial cells, suggesting a longer duration in the lumen might be necessary, or that endothelial cells were intrinsically inert to adenoviral transduction in vivo.24
Here, the study also found a bias against α-cell targeting. The result from the ex vivo study clearly pointed to a physical explanation (Figure 3c). Each islet was vascularized by 1–5 arterioles that eventually branched into a spherical structure similar to a glomerulus.25 In rodents, β cells usually resided in the center, whereas α cells located primarily around the periphery of islets.26 Higher β-cell specificity, therefore, was consistent with the hypothesis that β cells were perfused first.27 Alternatively, β cells might stimulate a more active vasogenesis as β cells expressed more angiogenic factors than α cells.28 Finally, the result in this study argued against the existence of “capillary gates” controlling blood perfusion to subsets of islet cells according to plasma glucose levels (Figure 4c).
Nevertheless, the results demonstrated that β-cell targeting could be accomplished with high specificity and high efficiency by a local adenoviral injection procedure (Figure 4b). As low as 1 × 109 IU of viruses were able to label about 60% of islets and 5% of β cells. This number increased to >95 and 60%, respectively, when 2.5 × 1010 IU of viruses were injected. Of note, the titration method used in this study might result in a much superficially higher IU than that of plaque-forming unit (AdEasy manual, Stratagene, La Jolla, CA). It was also noteworthy that minimal cytotoxicity and inflammation were detected within islets.
Although a local arterial perfusion is difficult to perform in small animal models, the development of a straightforward approach in the mouse is very important as most of the resources are available in this species. Our procedure appeared quite invasive; however, around 70% of mice survived and looked healthy. When the animals were killed 3 days after surgery, the pancreas, liver, spleen, and stomach were in normal shape and color (Supplementary Figure S1a,b). Intra-arterial techniques have been published for the treatment of muscle disease, liver cancer, and brain tumor.29,30,31 In these studies, small molecule drug, naked plasmid DNA, recombinant adeno-associated virus, and even stem cells were able to diffuse or migrate into the targeted organs.29,30,31,32,33 Intra-arterial injection of DNA-containing liposomes has also been reported to transfect endothelial cells, and occasionally acinar cells, but not endocrine cells in the pancreas.34 In bigger animal, Tal et al. have successfully performed surgeries in nonhuman primates, in which the β-cell-specific STZ was injected via a percutaneous access of the common femoral artery.35 Therefore, the technique established in this study might have great potentials for the treatment of diabetes and other pancreatic diseases.
Finally, to prove this concept, we tested the β-cell protection effect of Sirt1 in STZ-treated mice. Sirt1 is a multifunctional protein deacetylase capable of stimulating insulin secretion by repressing the expression of the uncoupling protein 2, and reducing β-cell death by downregulating the proapoptotic nuclear factor-κB signaling, which could be induced by the treatment of STZ or inflammatory cytokines (Supplementary Figure S3 and Supplementary Materials and Methods).13,17 Ad-Sirt1 injection via C.T. dramatically increased its expression levels in islets and, therefore, protected a portion of β cells from STZ-induced apoptosis (Figures 5 and 6a; Supplementary Figure S4). Apparently, the residual β cells in Ad-Sirt1 mice were able to maintain euglycemia under fasting condition, but were not enough to preserve normal glucose kinetics when a large amount of glucose was injected (Figure 6b,c). Transduction of hepatocytes with Ad-Sirt1 probably had no effect on glucose metabolism (Supplementary Figure S6c). We are currently testing the islet targeting via C.T. using lentiviral vectors that may provide a long-term protection effect.
In summary, we described an efficient intra-arterial technique to target specifically islet β cells by adenoviral injection via the C.T.. This approach could be important to the treatment of diabetes as well as to the study of islet biology in the future.
Materials and Methods
Animals and materials. Eight- to 12-week-old Balb/c mice were purchased from the Shanghai Laboratory Animal Company (Shanghai, China) and raised in our specific pathogen-free and air-conditioned animal facility. Mice were fed ad libitum with a standard diet (Shanghai Laboratory Animal Company) and kept under a light–dark cycle of 12 hours. Animal care and experimental procedures were in compliance with the guidelines for the use and care of laboratory animals established by Shantou University.
Biochemical reagents and antibodies were purchased from the companies indicated below: 4′,6-diamidino-2-phenylindole (Dojindo, Kumamoto, Japan); rabbit anti-EGFP (Beyotime, Shanghai, China); rabbit anti-Sirt1, mouse anti-Pan-CK, mouse anti-amylase, and goat anti-insulin (Santa Cruz, Santa Cruz, CA); mouse anti-glucagon (Boster, Wuhan, China); FITC-conjugated goat anti-rabbit IgG (Boster); Cy3-conjugated sheep anti-mouse IgG (Sigma, St Louis, MO); mounting fluid (Applygen, Beijing, China); collagenase (type IV), and STZ (Sigma); rhodamine-containing beads with a size of 100–200 µm were kindly provided by Yucui Xiong.36 All other reagents were of analytical grade.
Recombinant adenoviral vectors. The E1-deleted adenovirus (serotype 5) carrying the cytomegalovirus promoter/EGFP hybrid gene was purchased from Vector Gene Technology.(Beijing, China). For amplification of the Ad-EGFP viruses, 1 × 108 IU of viruses were added into a 10-cm dish preseeded with 1 × 106 Ad293 cells (Stratagene, La Jolla, CA) overnight. After incubation for 30–48 hours, the cells were harvested by scrapping and centrifugation at 3,000 r.p.m. for 10 minutes, while the supernatant was saved for the next round of virus amplification. The harvested cells underwent four freeze/thaw cycles and were spun at 12,000g for 10 minutes to obtain cell lysates. Serial dilutions of the supernatant and cell lysates were used to transduce Ad293 cells in a 96-well plate preseeded with 5,000 cells overnight. The viral titers (IU) were determined by counting EGFP-positive cells under a fluorescence microscope after 30-hour cultivation. The construct containing the Sirt1 gene was kindly provided by Qiwei Zhai.37 Sirt1 was subcloned into the shuttle vector pAdTrack (which carries an EGFP reporter under the control of a cytomegalovirus promoter; Stratagene) for the generation of Ad-Sirt1 according to the procedure provided by the company. The amplification and titration of Ad-Sirt1 was the same as described above.
Surgical procedures. Balb/c mice were fasted for 12 hours before being anesthetized by an intraperitoneal injection of sodium barbital (10 mg/kg; Sigma). Laparotomy was performed through a midline abdominal incision. The duodenum and the head of the pancreas were flipped over to the left, to expose the underneath circulation system. Rhodamine-containing beads, or adenoviral vectors, in 50 µl of saline solution were injected according to one of the following two procedures.
C.T.: The proximal end of the C.T. was clamped with a hemostatic forceps for 2 minutes. After that, three ligations were placed sequentially on the proximal end of the common hepatic artery and lieno-gastric artery, distal end of lieno-pancreatic artery, and pancreatic vein with 5-0 sutures. The beads or adenoviral vectors were then injected with a 31G insulin needle (BD, Franklin Lakes, NJ) via the C.T. The ligation at the common hepatic artery and lieno-gastric artery was then removed. The bleeding was stopped by pressing down a cotton swap on the site of injection for 5 minutes after releasing the forceps. The ligations at the distal end of the lieno-pancreatic artery and pancreatic vein were maintained for another 15 minutes before the abdominal wall was closed with sutures.
J.V.: Adenoviral vectors were injected via the J.V. with a hepatic ligation according to a previously reported procedure.11 Animals were killed 3 days after the administration of Ad-EGFP. The pancreas, liver, and spleen were harvested and examined directly under a fluorescence microscope (Supplementary Figure S1). The pancreatic tails were embedded in paraffin and processed for immunostaining analysis. For animals injected with beads, the pancreases were visualized directly under a fluorescence microscope without removal of the ligations. The pancreatic body and tail were then embedded in OCT (Sakura Finetek, Torrance, CA) for frozen sectioning.
Islet purification and viral infection. The procedure for islet isolation has been described previously.8 Briefly, 2 ml of 0.5% type IV collagenase was injected into the pancreas via the pancreatic duct. The pancreas was then harvested and chopped into small pieces before being digested in a 37 °C water bath for about 10 minutes. The enzyme was inactivated with Dulbecco's modified Eagle's medium containing 2% fetal bovine serum. After being washed three times, the pancreas was suspended in Dulbecco's modified Eagle's medium and placed into a 6-well plate. Islets were then handpicked under a microscope and infected with Ad-EGFP in a 6-well plate for 2 hours in serum-free medium (multiplicity of infection = 100, each islet was estimated to have 1,000 cells on average). Then, viruses were removed and the medium was replaced with fresh Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. After 24-hour incubation, the islets were harvested and encased with 200 µl of 1% agar in a 50 °C water bath. Islets were then processed as paraffin sections for immunostaining.
Immunofluorescence staining. For paraffin sections (4 µm), the immunostaining procedure has been described.8 Briefly, after antigen retrieval with sodium citric solution (0.01 mol/l, pH 6.0) at 95 °C for 20 minutes, primary antibody (predetermined dilution plus 1% bovine serum albumin) incubation was carried out at 4 °C overnight. Staining for the secondary antibody was done at room temperature for 1 hour. After incubation, samples were washed three times with phosphate-buffered saline (5 minutes each wash). A volume of 10 µl of 4′,6-diamidino-2-phenylindole (1 µg/ml) was added to each sample after the last wash. One drop of mounting fluid was added to the sample before it was laid over with a cover slide and sealed with nail polish. Images were taken under a fluorescence microscope equipped with a CCD camera (Eclipse TE 2000; Nikon, Tokyo, Japan). For frozen sections (10 µm), antibody staining was performed as stated above without antigen retrieval.
Quantification of adenoviral transduction efficiency. To determine the transduction efficiency, the pancreatic body and tail were harvested and immunostaining was performed on five paraffin sections separated by 150 µm. Islets were considered positive when at least one of the islet cells expressed EGFP. A total of ~60 islets were evaluated for each animal. To determine the area on photomicrographs, the islet was circulated manually with Image J (NIH, Bethesda, MD). The cell number within an islet was calculated by dividing the islet area by the average area of a single islet cell. The EGFP-positive cells were determined according to the 4′,6-diamidino-2-phenylindole staining and the number was counted manually in each islet. The percentage of transduction for α and β cells was calculated only for positive islets. Islets with a size <30 µm were not counted.
Administration of STZ. A density of 5 × 109 IU of Ad-EGFP or Ad-Sirt1 in 50 µl of saline solution was injected into the pancreases of Balb/c mice according to the procedure described above (C.T.). Mice were injected via peritoneum with STZ (195 mg/kg of body weight) dissolved in 0.1 mol/l sodium citrate buffer (pH 4.5) 48 hours postsurgery. Mice were analyzed for fasting plasma glucose or glucose tolerance before or after the administration of STZ. The pancreases were then harvested for determination of the relative β-cell mass.
Analysis of the relative islet cell mass. The relative islet cell mass of the pancreatic body and tail was estimated by hematoxylin and eosin staining. Briefly, each paraffin block of the pancreas was sectioned consecutively. A total of 10 slides (separated by 80 µm) were analyzed. To determine the area on photomicrographs, the islet was circulated manually with Image J (NIH). The relative islet cell mass was calculated as the sum of total islet areas.
Glucose homeostasis. After a 12-hour fast, blood sample from the tail vein was measured using a handheld glucometer (OneTouch Ultra; LifeScan, Milpitas, CA). For glucose tolerance test, mice were injected intraperitoneally with 2 g/kg of glucose after fasting. Blood was sequentially sampled at different time points from the tail vein and analyzed as above.
Statistical analysis. All data were presented as the mean value ± SD of each group. The Student's t-test was carried out by using GraphPad Prizm 5. P values <0.05 were considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Transduction of the liver and spleen. Figure S2. Transcript levels of transgenes in the pancreas, liver and spleen. Figure S3. Nuclear translocation of NF-κB. Figure S4. Measurement of beta-cell apoptosis. Figure S5. Measurement of beta-cell survival. Figure S6. Measurement of beta-cell function. Materials and Methods.
Acknowledgments
This research was supported by the Li Ka Shing Foundation, the Natural Science Foundation of China (Grant No. 30971665) and the Guangdong Provincial Natural Science Foundation (Grant No. 8151503101000014). We also thank Qiwei Zhai (Institute for Nutritional Sciences, China) for providing the valuable Sirt1 construct and Yucui Xiong (Shantou University, China) for proving the rhodamine-containing beads. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Supplementary Material
Transduction of the liver and spleen.
Transcript levels of transgenes in the pancreas, liver and spleen.
Nuclear translocation of NF-κB.
Measurement of beta-cell apoptosis.
Measurement of beta-cell survival.
Measurement of beta-cell function.
REFERENCES
- Tamada K, Wang XP., and, Brunicardi FC. Molecular targeting of pancreatic disorders. World J Surg. 2005;29:325–333. doi: 10.1007/s00268-004-7821-6. [DOI] [PubMed] [Google Scholar]
- Wang AY, Peng PD, Ehrhardt A, Storm TA., and, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15:405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- Shifrin AL, Auricchio A, Yu QC, Wilson J., and, Raper SE. Adenoviral vector-mediated insulin gene transfer in the mouse pancreas corrects streptozotocin-induced hyperglycemia. Gene Ther. 2001;8:1480–1489. doi: 10.1038/sj.gt.3301544. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54:1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J., and, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokui Y, Kozawa J, Yamagata K, Zhang J, Ohmoto H, Tochino Y, et al. Neogenesis and proliferation of beta-cells induced by human betacellulin gene transduction via retrograde pancreatic duct injection of an adenovirus vector. Biochem Biophys Res Commun. 2006;350:987–993. doi: 10.1016/j.bbrc.2006.09.154. [DOI] [PubMed] [Google Scholar]
- Peng SW, Zhu LY, Chen M, Zhang M, Li DZ, Fu YC, et al. Heterogeneity in mitotic activity and telomere length implies an important role of young islets in the maintenance of islet mass in the adult pancreas. Endocrinology. 2009;150:3058–3066. doi: 10.1210/en.2008-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Ayuso E, Chillón M, Agudo J, Haurigot V, Bosch A, Carretero A, et al. In vivo gene transfer to pancreatic beta cells by systemic delivery of adenoviral vectors. Hum Gene Ther. 2004;15:805–812. doi: 10.1089/1043034041648426. [DOI] [PubMed] [Google Scholar]
- Ye X, Jerebtsova M., and, Ray PE. Liver bypass significantly increases the transduction efficiency of recombinant adenoviral vectors in the lung, intestine, and kidney. Hum Gene Ther. 2000;11:621–627. doi: 10.1089/10430340050015806. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SR, Wright J, Bauter M, Seweryniak K, Kode A., and, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmeier MR, Scarim AL., and, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272:13697–13704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- Lv N, Song MY, Lee YR, Choi HN, Kwon KB, Park JW, et al. Dihydroavenanthramide D protects pancreatic beta-cells from cytokine and streptozotocin toxicity. Biochem Biophys Res Commun. 2009;387:97–102. doi: 10.1016/j.bbrc.2009.06.133. [DOI] [PubMed] [Google Scholar]
- Moldovan S., and, Brunicardi FC. Endocrine pancreas: summary of observations generated by surgical fellows. World J Surg. 2001;25:468–473. doi: 10.1007/s002680020339. [DOI] [PubMed] [Google Scholar]
- Narushima M, Okitsu T, Miki A, Yong C, Kobayashi K, Yonekawa Y, et al. Adenovirus mediated gene transduction of primarily isolated mouse islets. ASAIO J. 2004;50:586–590. doi: 10.1097/01.mat.0000142877.18621.bc. [DOI] [PubMed] [Google Scholar]
- Nguyen TH., and, Ferry N. Liver gene therapy: advances and hurdles. Gene Ther. 2004;11 Suppl 1:S76–S84. doi: 10.1038/sj.gt.3302373. [DOI] [PubMed] [Google Scholar]
- Jansson L. Glucose stimulation of pancreatic islet blood flow by redistribution of the blood flow within the whole pancreatic gland. Pancreas. 1988;3:409–412. doi: 10.1097/00006676-198808000-00007. [DOI] [PubMed] [Google Scholar]
- Murakami T, Hitomi S, Ohtsuka A, Taguchi T., and, Fujita T. Pancreatic insulo-acinar portal systems in humans, rats, and some other mammals: scanning electron microscopy of vascular casts. Microsc Res Tech. 1997;37:478–488. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<478::AID-JEMT10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, et al. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51:1680–1688. doi: 10.1007/s00125-008-1085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM, et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther. 2005;16:235–247. doi: 10.1089/hum.2005.16.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballian N., and, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- Orci L. The microanatomy of the islets of Langerhans. Metab Clin Exp. 1976;25 11 Suppl 1:1303–1313. doi: 10.1016/s0026-0495(76)80129-1. [DOI] [PubMed] [Google Scholar]
- Brunicardi FC, Stagner J, Bonner-Weir S, Wayland H, Kleinman R, Livingston E, et al. Microcirculation of the islets of Langerhans. Long Beach Veterans Administration Regional Medical Education Center Symposium. Diabetes. 1996;45:385–392. doi: 10.2337/diab.45.4.385. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Gonin P, Arandel L, Van Wittenberghe L, Marais T, Perez N., and, Danos O. Femoral intra-arterial injection: a tool to deliver and assess recombinant AAV constructs in rodents whole hind limb. J Gene Med. 2005;7:782–791. doi: 10.1002/jgm.716. [DOI] [PubMed] [Google Scholar]
- Barnett FH, Scharer-Schuksz M, Wood M, Yu X, Wagner TE., and, Friedlander M. Intra-arterial delivery of endostatin gene to brain tumors prolongs survival and alters tumor vessel ultrastructure. Gene Ther. 2004;11:1283–1289. doi: 10.1038/sj.gt.3302287. [DOI] [PubMed] [Google Scholar]
- Geschwind JF, Ko YH, Torbenson MS, Magee C., and, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909–3913. [PubMed] [Google Scholar]
- Liang KW, Nishikawa M, Liu F, Sun B, Ye Q., and, Huang L. Restoration of dystrophin expression in mdx mice by intravascular injection of naked DNA containing full-length dystrophin cDNA. Gene Ther. 2004;11:901–908. doi: 10.1038/sj.gt.3302239. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, et al. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid RM, Weidenbach H, Yamagushi H, Lührs H, Liptay S., and, Adler G. Direct gene transfer into the rat pancreas using DNA-liposomes. Eur J Clin Invest. 1998;28:220–226. doi: 10.1046/j.1365-2362.1998.00269.x. [DOI] [PubMed] [Google Scholar]
- Tal MG, Hirshberg B, Neeman Z, Bunnell D, Soleimanpour S, Bacher J, et al. Induction of diabetes in nonhuman primates by means of temporary arterial embolization and selective arterial injection of streptozotocin. Radiology. 2004;230:163–168. doi: 10.1148/radiol.2301021413. [DOI] [PubMed] [Google Scholar]
- Yao YC, Zhan XY, Zhang J, Zou XH, Wang ZH, Xiong YC, et al. A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials. 2008;29:4823–4830. doi: 10.1016/j.biomaterials.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Jin Q, Yan T, Ge X, Sun C, Shi X., and, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transduction of the liver and spleen.
Transcript levels of transgenes in the pancreas, liver and spleen.
Nuclear translocation of NF-κB.
Measurement of beta-cell apoptosis.
Measurement of beta-cell survival.
Measurement of beta-cell function.