Abstract
Currently, there is no effective clinical treatment to prevent abdominal aortic aneurysm (AAA). To develop a novel therapeutic approach, we modified decoy oligodeoxynucleotide (ODN) against nuclear factor κB (NFκB) and ets, to a ribbon-shaped circular structure without chemical modification, to increase its resistance to endonuclease for systemic administration. Intraperitoneal administration of ribbon-type decoy ODNs (R-ODNs) was performed in an elastase-induced rat AAA model. Fluorescent isothiocyanate (FITC)-labeled R-ODNs could be detected in macrophages migrating into the aneurysm wall, and NFκB and ets activity were simultaneously inhibited by chimeric R-ODN. Treatment with chimeric R-ODN significantly inhibited aortic dilatation, whereas conventional phosphorothioate decoy ODN failed to prevent aneurysm formation. Significant preservation of elastic fibers was observed with chimeric R-ODN, accompanied by a reduction of secretion of several proteases from macrophages. Activation of matrix metalloproteinase (MMP)-9 and MMP-12, but not MMP-2, was suppressed in the aneurysm wall by chimeric R-ODN, whereas recruitment of macrophages was not inhibited. Treatment with chimeric R-ODN also inhibited the secretion of cathepsin B and K from macrophages. Overall, the present study demonstrated that systemic administration of chimeric R-ODNs prevented aneurysm formation in a rat model. Further modification of the decoy strategy would provide a means of less invasive molecular therapy for human AAA.
Introduction
Abdominal aortic aneurysm (AAA) is a common degenerative condition with high mortality in older men. Elective surgical or endovascular repair is performed to prevent rupture of large AAAs. However, survival of patients with a small AAA is not improved by these invasive procedures.1,2 In addition, there is no proven medical therapy to inhibit AAA progression in the clinical setting. Therefore, despite gradual expansion of AAA, patients with a small AAA do not receive effective treatment. With advances in vascular biology, the mechanism of AAA formation has been elucidated at the cellular and molecular levels.3 Based on these basic investigations, we have focused on two important transcription factors, nuclear factor κB (NFκB) and ets, for regulating a set of genes associated with AAA formation. The human aneurysm wall is characterized by chronic inflammation and matrix degradation, and NFκB is well known to control transcription of many genes in inflammatory and immune responses. Similarly, members of the ets family play important roles in regulating gene expression in response to multiple developmental and mitogenic signals, including cell differentiation and apoptosis. Importantly, these transcription factors directly enhance expression of a number of proteases and inhibit the transcription of extracellular matrix protein genes such as elastin and collagen.4,5,6,7,8,9,10,11 Indeed, our previous study showed marked activation of NFκB and ets in the human aneurysm wall, and demonstrated preventive and regressive effects of inhibition of NFκB and/or ets using a decoy strategy in experimental AAA models.12,13
The decoy approach is a new class of antigene strategy that utilizes modulation of endogenous transcriptional regulation.14 The decoy is a synthetic double-stranded cis-element oligodeoxynucleotide (ODN), and chemical modifications such as phosphorothioation are usually utilized to increase the stability. However, local application is required for in vivo usage of conventional phosphorothioate linear-type decoy ODNs (PS-ODNs), because of their rapid degeneration from the 3′-terminus by several nucleases under physiological conditions.15 Therefore, we employed a cellulose-based delivery sheet-containing decoy ODNs for direct application in previous studies.12,13 Although this approach is useful to introduce decoy ODNs into the outer aneurysm wall, low invasive procedures, such as laparoscopy, might be necessary for clinical utility. To overcome this limitation, we further developed a novel decoy ODN with a ribbon-shaped circular structure, to increase the stability without chemical modification (Figure 1). Our basic investigations demonstrated that ribbon-type decoy ODN (R-ODN) was more stable than the conventional PS-ODN after incubation in the presence of exonuclease or serum, without interfering with its binding activity.16,17 Therefore, we hypothesized that R-ODN could be applicable for systemic administration as a less invasive approach to treat AAA. In addition, we have developed a chimeric decoy strategy to regulate multiple transcription factors. Our previous studies demonstrated that chimeric decoy ODN against NFκB and ets was very potent as compared to single transfection of NFκB or ets decoy ODN.12,13 Thus, in this study, the effects of intraperitoneal administration of chimeric R-ODN against NFκB and ets on the formation of AAA were evaluated in an elastase-induced rat AAA model.
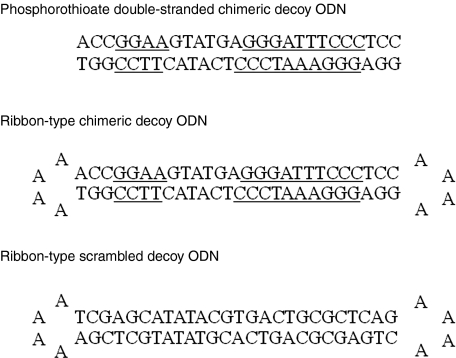
Figure 1.
Construction of decoy ODN. Schema of phosphorothioate linear-type chimeric decoy ODN, nonmodified ribbon-type scrambled and chimeric decoy ODN. GGGATTTCCC and GGAA are the consensus sequences for the NFκB and ets-binding sites, respectively. NFκB, nuclear factor κB; ODN, oligodeoxynucleotide.
Results
Distribution of fluorescent isothiocyanate (FITC)-labeled R-ODN
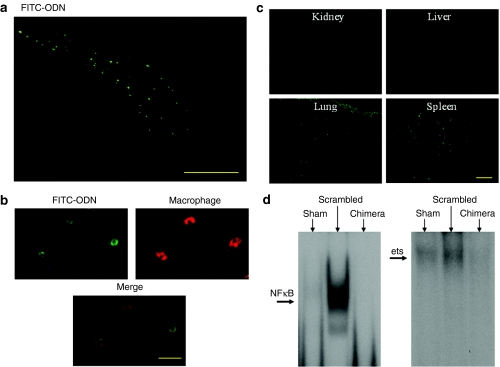
To confirm the successful transfer of R-ODN into the aorta by intraperitoneal administration, we first evaluated the distribution of FITC-labeled R-ODN in a rat AAA model. Histologically, fluorescence could be detected mainly in the outer aortic wall and retroperitoneal space beside the aorta on day 7 after injection of FITC-labeled R-ODNs (Figure 2a). Successfully transfected cells were defined as those with localized fluorescence in the nucleus. Moreover, immunofluorescent staining revealed that FITC-labeled R-ODNs were found in the migrating macrophages (Figure 2b). In addition, we evaluated the distribution of FITC signals in other organs. Fluorescence was also detected in the spleen and lung (Figure 2c).
Figure 2.
Distribution of FITC-labeled R-ODN and inhibition of binding activity of NFκB and ets. (a) Typical photograph of fluorescence in aneurysm wall of rat. Fluorescence could be detected mainly in the outer aortic wall and retroperitoneal space beside the aorta on day 7 after injection of FITC-labeled R-ODNs. (b) Immunofluoresent staining for macrophages at 1 week after intraperitoneal injection of FITC-labeled R-ODN. FITC-labeled R-ODNs were found in the migrating macrophages. (c) Distribution of FITC-labeled R-ODN in untargeted organs. Fluorescence was detected in the spleen and lung. (d) Representative results of electrophoretic mobility shift assay for NFκB and ets-binding sites at 1 week after transfection. Bar in a = 200 µm; in b = 20 µm; in c = 200 µm. chimera, AAA model transfected with chimeric R-ODN; FITC-ODN, fluorescent isothiocyanate-labeled oligodeoxynucleotide; NFκB, nuclear factor κB; scrambled, AAA model transfected with scrambled R-ODN.
Binding activity of NFκB and ets
The inhibitory effect of R-ODN on transcriptional activation of NFκB and ets in the aneurysm wall was confirmed by electrophoretic mobility shift assay. Activity of these transcription factors was significantly increased at 1 week after incubation with elastase. In contrast, treatment with chimeric R-ODN significantly inhibited the activation of both NFκB and ets (Figure 2d).
Preventive effect of chimeric R-ODN on AAA formation
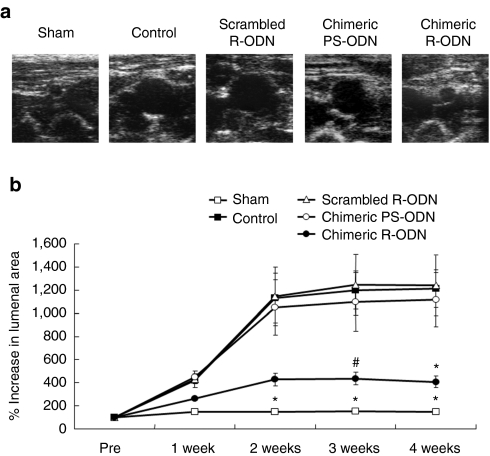
Ultrasound analysis demonstrated that treatment with chimeric R-ODN significantly inhibited the progression of elastase-induced aortic dilatation. In contrast, scrambled R-ODN failed to prevent aortic dilatation, and there was no significant difference in the progression of AAA between control (untransfected animals) and scrambled R-ODN-treated animals. Importantly, linear-type chimeric PS-ODN did not inhibit the progression of experimental AAA (Figure 3a,b, Supplementary Table S1). These studies clearly demonstrated that chimeric R-ODN was more potent than conventional chimeric PS-ODN.
Figure 3.
Prevention of aortic expansion by chimeric R-ODN. (a) Representative ultrasound images of aortic dilatation at 4 weeks after transfection (short axis view). (b) Time course of aortic size assessed by ultrasound (n = 9 per group). Treatment with chimeric R-ODN significantly inhibited aortic dilatation as compared to control, scrambled R-ODN and chimeric PS-ODN. The bars represent mean ± SEM. *P < 0.05 versus control, scrambled R-ODN and chimeric PS-ODN, #P < 0.05 versus control and scrambled R-ODN analyzed by Tukey–Kramer test. control, AAA model treated with saline; PS-ODN, phosphorothioate linear-type decoy oligodeoxynucleotide; R-ODN, ribbon-type decoy oligodeoxynucleotide.
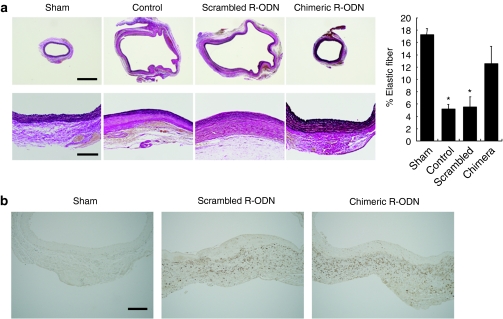
Elastic fibers maintain the structure of the vascular wall against hemodynamic stress, resulting in the prevention of aortic dilatation. Thus, the effect of R-ODN on the destruction of elastic fibers in the aneurysm wall was examined by histological study. Elastic van Gieson's staining demonstrated that treatment with chimeric R-ODN significantly inhibited proteolysis of elastin as compared to control and scrambled R-ODN (Figure 4a).
Figure 4.
Preservation of elastic fibers by chimeric R-ODN without suppression of macrophage accumulation. (a) Histological sections of rat aorta stained with EVG stain at 4 weeks after transfection, and percentage of area positive for elastic fibers (n = 6). (b) Immunohistochemical staining of macrophages at 1 week after operation. The bars represent mean ± SEM. *P < 0.05 versus sham and chimeric R-ODN analyzed by Tukey–Kramer test. Bar in a upper panels = 1,000 µm; lower panels = 200 µm; in b = 200 µm. chimera, AAA model transfected with chimeric R-ODN; control, AAA model treated with saline; EVG, elastic van Gieson's; PS-ODN, phosphorothioate linear-type decoy oligodeoxynucleotide; R-ODN, ribbon-type decoy oligodeoxynucleotide; scrambled, AAA model transfected with scrambled R-ODN.
To clarify the mechanisms of preservation of the medial layer by chimeric R-ODN, we next examined infiltration of monocytic leukocytes into the aortic wall by immunohistochemical study, because inflammation is a major pathologic feature of the human aneurysm wall. Interestingly, many macrophages migrated into the outer aorta transfected with chimeric R-ODN as well as scrambled R-ODN (Figure 4b). Unexpectedly, treatment with chimeric R-ODN did not affect macrophage recruitment in the process of AAA formation.
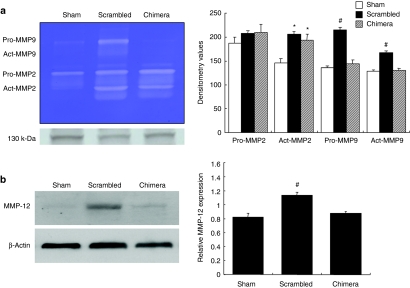
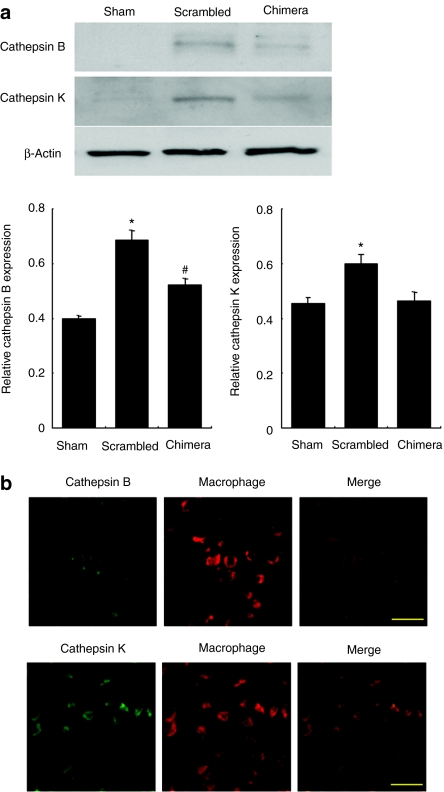
Finally, we investigated the effects of chimeric R-ODN on protease secretion in the aneurysm wall. Both matrix metalloproteinase (MMP)-2 and MMP-9 activity were significantly increased at 1 week after incubation with elastase. However, transfection of chimeric R-ODN significantly reduced the activation of MMP-9, but not MMP-2, as compared to scrambled R-ODN (Figure 5a). Similarly, the expression of MMP-12 was also inhibited by treatment with chimeric R-ODN (Figure 5b). As cathepsins are the other major proteases associated with the degradation of extracellular matrix protein, we examined the expression of cathepsins in the aneurysm wall by western blotting. Treatment with chimeric R-ODN significantly inhibited cathepsin B and K expression as compared to scrambled R-ODN (Figure 6a). In addition, double immunofluorescent staining using tissue sections from scrambled R-ODN-treated rats demonstrated an increase in cathepsin B- and K-positive cells in the outer aneurysm wall, and these cathepsins were detected in migrating macrophages (Figure 6b).
Figure 5.
Inhibition of MMP expression in aneurysm wall by chimeric R-ODN. (a) Representative gelatin zymography and silver staining as a loading control (lower panel) at 1 week after transfection, and densitometric evaluation of gelatin zymography (n = 5). (b) Inhibition of MMP-12 expression by chimeric R-ODN in aneurysm wall at 1 week after transfection assessed by western blotting (n = 5). β-Actin was used as the internal control for normalization. The bars represent mean ± SEM. *P < 0.05 versus sham, #P < 0.05 versus sham and chimera analyzed by Tukey–Kramer test. chimera, AAA model transfected with chimeric R-ODN; R-ODN, ribbon-type decoy oligodeoxynucleotide; MMP, matrix metalloproteinase; scrambled, AAA model transfected with scrambled R-ODN.
Figure 6.
Inhibition of cathepsin B and K secretion from migrating macrophages by chimeric R-ODN. (a) Inhibition of cathepsin B and K expression by chimeric R-ODN in aneurysm wall at 1 week after transfection assessed by western blotting (n = 5). β-Actin was used as the internal control for normalization. (b) Double immunofluorescent staining for cathepsins and macrophages in the tissue sections from scrambled R-ODN-treated rats. The bars represent mean ± SEM. *P < 0.05 versus sham and chimera, #P < 0.05 versus sham analyzed by Tukey–Kramer test. Bar in b = 20 µm. chimera, AAA model transfected with chimeric R-ODN; R-ODN, ribbon-type decoy oligodeoxynucleotide; scrambled, AAA model transfected with scrambled R-ODN.
Discussion
Several chemical modifications of ODN have been introduced to improve the efficacy of ODN-based therapeutics. The circular structure of double-stranded termini of decoy ODN possesses increased nuclease resistance and structural stability, leading to enhanced target affinity and biological potency.16,17,18 Utilizing this advantage, we examined the possibility of less invasive therapy, via systemic administration, for inoperable AAA. First, we investigated whether R-ODN could be introduced into target cells by intraperitoneal administration. After injection of FITC-labeled R-ODN, fluorescence could be detected in macrophages migrating into the aneurysm wall. Since ODN internalization is thought to occur by some form of endocytosis, high endocytotic activity of macrophages might become an advantage for the introduction of decoy ODN.19 In addition, R-ODN is reported to enhance cellular uptake, and our previous in vitro study showed successful transfection of R-ODN into macrophages without the use of a delivery system.17,20 In contrast, we could not detect fluorescence of FITC-labeled ODN in vascular smooth muscle cells. As vascular smooth muscle cells have relatively low-level endocytotic activity, the injected dose of decoy ODN was not sufficient to introduce decoy ODN into vascular smooth muscle cells. These data suggest that R-ODN injected into the peritoneal cavity mainly acts on migrating macrophages. Consistent with this distribution study, intraperitoneal administration of chimeric R-ODN simultaneously inhibited the binding activity of both NFκB and ets in the aneurysm wall, whereas these transcription factors were markedly activated at the sites of AAA formation treated with saline or scrambled R-ODN.
The present study demonstrated that treatment with chimeric R-ODN via intraperitoneal administration significantly prevented aortic dilatation in a rat AAA model. However, chimeric PS-ODN without a ribbon structure failed to show in vivo efficacy to inhibit AAA formation, despite chemical modification. In our previous studies, local application of PS-ODN showed a potent therapeutic effect on AAA formation.12,13 Therefore, these results clearly indicate that increased structural stability and nuclear resistance are important to enhance the pharmacological effects of systemically administrated decoy ODN. In addition, although phosphorothioate ODN are relatively nuclease resistant, these molecules bind numerous serum proteins, thus inducing cellular toxicity and side effects.21 Therefore, it might be important to induce a specific inhibitory effect on aneurysm development by nonmodified R-ODN.
The therapeutic effect of chimeric R-ODN was also confirmed to show preservation of the medial layer. The normal aortic wall exhibits strict regulation of the turnover of extracellular matrix protein. However, increased expression of a variety of proteases causes destruction of the medial layer, leading to aneurysm growth and rupture. Activated macrophages are thought to be the main cells secreting proteases.22 Therefore, the inhibition of inflammatory cell accumulation has emerged as a therapeutic target for the treatment of AAA.23 Unexpectedly, intraperitoneal injection of chimeric R-ODN did not inhibit the recruitment of macrophages in the aneurysm wall. Migration of macrophages is mainly due to the expression of adhesion molecules and chemotactants in residual vascular cells. Although NFκB controls the expression of intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1, it might be not enough to introduce decoy ODN into residual cells and reduce the expression of these factors by intraperitoneal administration.
Among a number of proteases, MMPs are considered to be the predominant proteases in the process of destruction of the aortic wall.24,25 MMP-9 activation and MMP-12 expression were inhibited in the aneurysm wall as a result of transfection of chimeric R-ODN into macrophages. MMP gene expression is primarily regulated at the transcriptional level. NFκB and ets-binding sites have been found in almost all inducible MMP promoters, including MMP-9 and MMP-12.4,5,6,9,10,11,26 Therefore, these therapeutic effects were associated with direct inhibition of MMP gene expression driven by either the NFκB or ets-binding site. Importantly, enhanced expression of MMP-9 and MMP-12 is observed in the human aneurysm wall, and MMP-9 and MMP-12 knockout mice were resistant to AAA development in experimental studies.24,25,27,28,29,30 Moreover, the importance of MMP-9 secretion from macrophages is supported by the report that AAA was reconstituted in MMP-9 knockout mice by intravenous injection of wild-type macrophages.30 In contrast, chimeric R-ODN transfection did not inhibit MMP-2 activity, although MMP-2 expression is also regulated by NFκB and ets.6,9 It has been reported that MMP-2 was mainly secreted from vascular smooth muscle cells, whereas the main cells that secreted MMP-9 and MMP-12 were activated macrophages.22,30,31 The difference in cell types might explain these findings.
Recently, cysteine proteases have received attention in the process of AAA formation. Cathepsins are cysteine proteases and express potent elastolytic and collagenolytic activity. In human studies, the activity of cathepsin B, D, H, K, L, and S was increased, and an endogenous inhibitor of cathepsin, cystein C, was decreased in aneurysm lesions as compared with the wall in aortic occlusive disease or normal aorta.32,33 In addition, cysteine proteases, such as cathepsin B, can activate cytokines and other proteases including MMPs.34,35 The present study demonstrated that transfection of chimeric R-ODN inhibited cathepsin B and K expression in migrating macrophages. Several basic studies demonstrated that macrophages are capable of releasing active cathepsins, and the expression of these cathepsins is controlled by NFκB and ets.36,37,38,39 These findings suggest that treatment with chimeric R-ODN suppressed not only MMP expression, but also expression of cysteine proteases, leading to potent therapeutic effects on the degeneration of extracellular matrix protein during AAA formation.
Here, the present study provided a novel strategy to treat AAA by simultaneous inhibition of NFκB and ets in migrating macrophages via intraperitoneal administration of R-ODN in a rat model. The therapeutic effects of chimeric R-ODN were mainly due to inhibition of protease secretion from macrophages, but not migration, through direct inhibition of gene expression of several proteases. Excess accumulation and/or inappropriately controlled activation of macrophages can cause extensive damage to the host in several inflammatory diseases, including AAA and atherosclerosis. However, monocytes/macrophages play an important role in response to various inflammatory and immune stimuli for host defense and tissue repair. Therefore, regulation of inappropriate macrophage function is though to be a potent therapeutic target for inflammatory diseases. Taken together, these results indicate that the ribbon-type decoy strategy could provide a novel therapeutic approach for treating human AAA, as less invasive molecular therapy.
Materials and Methods
Synthesis of ODN and selection of target sequences. The sequences of chimeric PS-ODN, scrambled R-ODN, and chimeric R-ODN are shown in Figure 1. To develop R-ODN, we designed two single strands of ODN (30-mer) and ligated their extremities. Then, 3 µl T4 DNA ligase (Takara, Otsu, Japan) was added to a mixture of 5′-phosphorylated ODN and buffer (Takara), followed by incubation for 24 hours at 16 °C to generate covalently ligated R-ODN.
Experimental animal models and intraperitoneal administration of decoy ODN. Male Sprague Dawley rats (250–350 g; Charles River Laboratories, Yokohama, Japan) were used in this experiment as described previously.12 Briefly, the abdominal aorta was isolated from the level of the left renal vein to the bifurcation. The right femoral artery was exposed, and a PE-10 polyethylene tube (Becton Dickinson, Franklin Lakes, NJ) was introduced through the femoral artery into the distal aorta. The aorta was clamped above the level of the tip of the polyethylene tube and ligated with a silk suture near the aortic bifurcation, followed by perfusion with 1 ml saline containing 50 U type I porcine pancreatic elastase (Sigma, St Louis, MO) for 1 hour at 100 mm Hg. After incubation with elastase, the retroperitoneum was tightly closed with 6-0 silk sutures. The elastase-induced AAA model is very popular, and is considered to be very suitable for analysis of the mechanisms of pathogenesis of human AAA. Indeed, numerous previous reports have shown that elastase activity plays an important role in the progression of AAA.40,41
Before abdominal cavity closure, transfection of decoy ODN was performed without the use of viral vectors or other delivery techniques. Then, 2 ml phosphate-buffered saline containing 50-nmol naked decoy ODN was injected into the peritoneal cavity. Then, rats were also administered naked decoy ODN (50 nmol/week) intraperitoneally for 1 week using an osmotic mini-pump (Alzet, model 2001; Durect, Cupertino, CA). Animals were divided into five groups (n = 9 each): sham, control, chimeric PS-ODN, scrambled R-ODN, and chimeric R-ODN treatment.
This study was performed under the supervision of the Animal Research Committee in accordance with the Guidelines on Animal Experiments of Osaka University Medical School and the Japanese Government Animal Protection and Management Law (No. 105).
Measurement of size of AAA by ultrasound. Ultrasonography was used to assess dilatation of the abdominal aorta.12 A cardiovascular ultrasound system (Power Vision 6000; Toshiba, Tokyo, Japan) and linear transducer (15 MHz) were used to image the abdominal aorta noninvasively in anesthetized rats (n = 9 each). Rats were scanned transversely and longitudinally to obtain images for measurement of the luminal diameter and the area of the lumen of the aneurysm in the segment with maximum diameter. The aortic size was measured before and after operation once a week up to 4 weeks.
Electrophoretic mobility shift assay. Electrophoretic mobility shift assay was performed to analyze the expression of NFκB and ets in nuclear extracts (10 µg) of the rat aneurysm wall at 1 week after the operation, with a gel shift assay system (Promega, Madison, WI), according to the manufacturer's specifications. Double-stranded ODNs containing the NFκB binding site (5′-CCTTGAAGGGATTTCCCTCC-3′ only sense strand is shown) or ets-binding site (5′-GTGCCGGGGTAGGAAGTGGGCTG GG-3′ only sense strand is shown) were used as primers.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis zymography. MMP-2 and MMP-9 activity in the aneurysm wall were measured in each group (n = 5) as previously described.13 Rats were sacrificed at 1 week after operation, and total protein extracts of the aneurysm wall (20 µg) were examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis zymography. Areas of protease activity appeared as unstained bands against a blue background. After electrophoresis, the upper portion of the gel was divided and stained with silver stain to confirm equal amounts of loaded protein in each lane. The density of each band was measured by densitometry.
Histological and immunohistochemical studies. Animals were sacrificed at 4 weeks after operation. The aorta was carefully dissected to remove adherent tissue, fixed in 10% neutral-buffered formalin, and processed for routine paraffin embedding. Aortic tissue cross-sections (6 µm) were stained with both hematoxylin and eosin and elastic van Gieson's stain in a standard manner. To evaluate residual mature elastin, the area of elastic fibers in elastic van Gieson's-stained sections was calculated by quantitative morphometric analysis with a computerized sketching program (n = 6). Results were expressed as a percentage of the total fractional area of the cross-section.
Mouse monoclonal antibodies against rat CD68 (1:300; Serotec, Oxford, UK) were used to analyze macrophage recruitment in the aortic wall at 1 week after operation. For negative control experiments, the primary antibody was omitted. Immunohistochemical staining was performed using the immunoperoxidase avidin–biotin complex system, and slides were counterstained with hematoxylin. Double immunofluorescent staining was also performed to identify the expression of cathepsin B and K in macrophages in aneurysm tissues from scrambled R-ODN-treated rats. Monoclonal mouse antibodies against CD68, rabbit polyclonal antibodies against cathepsin B (10 µg/ml; Upstate, Lake Placid, NY) and goat polyclonal antibodies against cathepsin K (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) were used. Protein reacting with primary antibodies was visualized with fluorescence-conjugated anti-mouse immunoglobulin G (Alexa 568; Invitrogen, Carlsbad, CA) for macrophages, anti-rabbit immunoglobulin G (Alexa 488) for cathepsin B, and anti-goat immunoglobulin G (Alexa 488) for cathepsin K. The images were visualized using fluorescence microscopy.
Western blot analysis. Protein extracts from the aneurysm wall (20 µg) were examined by western blot using polyclonal antibodies against cathepsin B (4 µg/ml), cathepsin K (1:200), MMP-12 (1:200; Santa Cruz Biotechnology) and β-actin (1:10,000; Sigma) at 1 week after operation (n = 5). The density of each band was measured by densitometry, and expression of MMP-12, cathepsin B and K was determined relative to the expression of β-actin.
Statistical analysis. All values are expressed as mean ± SEM. One-way analysis of variance and Tukey–Kramer multiple range test were used for comparisons among multiple groups. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Table S1. Time course of aortic size.
Acknowledgments
This work was partially supported by a Grant-in-Aid from the Organization for Pharmaceutical Safety and Research, a Grant-in-Aid from The Ministry of Public Health and Welfare, a Grant-in-Aid from Japan Promotion of Science, and through Special Coordination Funds of the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government. R.M. has AnGes MG stocks and serves as a Board Member of AnGes MG, which developed the decoy ODN.
Supplementary Material
Time course of aortic size.
REFERENCES
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. [PubMed] [Google Scholar]
- Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–1444. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- Shah PK. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 1997;96:2115–2117. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- Bond M, Baker AH., and, Newby AC. Nuclear factor κB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- Takeshita H, Yoshizaki T, Miller WE, Sato H, Furukawa M, Pagano JS, et al. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J Virol. 1999;73:5548–5555. doi: 10.1128/jvi.73.7.5548-5555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., and, Koh G. Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-κB-dependent pathway. Biochem Biophys Res Commun. 2000;269:401–405. doi: 10.1006/bbrc.2000.2308. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Berk JL, Rishikof DC, Foster JA, Humphries DE, Ricupero DA, et al. NF-κB induced by IL-1β inhibits elastin transcription and myofibroblast phenotype. Am J Physiol, Cell Physiol. 2002;283:C58–C65. doi: 10.1152/ajpcell.00314.2001. [DOI] [PubMed] [Google Scholar]
- Kouba DJ, Chung KY, Nishiyama T, Vindevoghel L, Kon A, Klement JF, et al. Nuclear factor-κ B mediates TNF-α inhibitory effect on α 2(I) collagen (COL1A2) gene transcription in human dermal fibroblasts. J Immunol. 1999;162:4226–4234. [PubMed] [Google Scholar]
- Vandenbunder B, Wernert N, Queva C, Desbiens X., and, Stehelin D. Does the transcription factor c-ets1 take part in the regulation of angiogenesis and tumor invasion. Folia Biol (Praha) 1994;40:301–313. [PubMed] [Google Scholar]
- Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, et al. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, et al. The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer. 1998;77:128–137. doi: 10.1002/(sici)1097-0215(19980703)77:1<128::aid-ijc20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Aoki M, Miyake T, Kawasaki T, Iwai M, Jo N, et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor κB and ets transcription factors. Circulation. 2004;109:132–138. doi: 10.1161/01.CIR.0000105725.61763.A2. [DOI] [PubMed] [Google Scholar]
- Miyake T, Aoki M, Masaki H, Kawasaki T, Oishi M, Kataoka K, et al. Regression of abdominal aortic aneurysms by simultaneous inhibition of nuclear factor κB and ets in a rabbit model. Circ Res. 2007;101:1175–1184. doi: 10.1161/CIRCRESAHA.107.148668. [DOI] [PubMed] [Google Scholar]
- Morishita R, Higaki J, Tomita N., and, Ogihara T. Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res. 1998;82:1023–1028. doi: 10.1161/01.res.82.10.1023. [DOI] [PubMed] [Google Scholar]
- Eder PS, DeVine RJ, Dagle JM., and, Walder JA. Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3' exonuclease in plasma. Antisense Res Dev. 1991;1:141–151. doi: 10.1089/ard.1991.1.141. [DOI] [PubMed] [Google Scholar]
- Osako MK, Tomita N, Nakagami H, Kunugiza Y, Yoshino M, Yuyama K, et al. Increase in nuclease resistance and incorporation of NF-κB decoy oligodeoxynucleotides by modification of the 3'-terminus. J Gene Med. 2007;9:812–819. doi: 10.1002/jgm.1077. [DOI] [PubMed] [Google Scholar]
- Kunugiza Y, Tomita T, Tomita N, Morishita R., and, Yoshikawa H. Inhibitory effect of ribbon-type NF-κB decoy oligodeoxynucleotides on osteoclast induction and activity in vitro and in vivo. Arthritis Res Ther. 2006;8:R103. doi: 10.1186/ar1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JD, Morishita R, Kaneda Y, Kim HS, Chang YC, Lee KU, et al. Novel E2F decoy oligodeoxynucleotides inhibit in vitro vascular smooth muscle cell proliferation and in vivo neointimal hyperplasia. Gene Ther. 2002;9:1682–1692. doi: 10.1038/sj.gt.3301849. [DOI] [PubMed] [Google Scholar]
- Juliano R, Alam MR, Dixit V., and, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Takai K, Nakada S, Yokota T., and, Takaku H. Specific inhibition of influenza virus RNA polymerase and nucleoprotein gene expression by circular dumbbell RNA/DNA chimeric oligonucleotides containing antisense phosphodiester oligonucleotides. FEBS Lett. 1998;425:91–96. doi: 10.1016/s0014-5793(98)00206-3. [DOI] [PubMed] [Google Scholar]
- Guvakova MA, Yakubov LA, Vlodavsky I, Tonkinson JL., and, Stein CA. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth factor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995;270:2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraya S, Miyake T, Aoki M, Yoshikazu F, Ohgi S, Nishimura M, et al. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis. 2009;202:34–40. doi: 10.1016/j.atherosclerosis.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Thompson RW., and, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- Elmore JR, Keister BF, Franklin DP, Youkey JR., and, Carey DJ. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg. 1998;12:221–228. doi: 10.1007/s100169900144. [DOI] [PubMed] [Google Scholar]
- Westermarck J., and, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Curci JA, Liao S, Huffman MD, Shapiro SD., and, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, et al. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005;137:457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N., and, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis V, Persidskaia R, Baca-Regen L, Itoh Y, Nagase H, Persidsky Y, et al. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- Abisi S, Burnand KG, Waltham M, Humphries J, Taylor PR., and, Smith A. Cysteine protease activity in the wall of abdominal aortic aneurysms. J Vasc Surg. 2007;46:1260–1266. doi: 10.1016/j.jvs.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Lindholt JS, Erlandsen EJ., and, Henneberg EW. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br J Surg. 2001;88:1472–1475. doi: 10.1046/j.0007-1323.2001.01911.x. [DOI] [PubMed] [Google Scholar]
- Kostoulas G, Lang A, Nagase H., and, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases. FEBS Lett. 1999;455:286–290. doi: 10.1016/s0014-5793(99)00897-2. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Solaroglu I, Yatsushige H, Cahill J, Yata K., and, Zhang JH. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Zhang QY., and, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien S, Ritter CA, Gratz M, Sperker B, Sonnemann J, Beck JF, et al. Nuclear factor-κB mediates up-regulation of cathepsin B by doxorubicin in tumor cells. Mol Pharmacol. 2004;65:1092–1102. doi: 10.1124/mol.65.5.1092. [DOI] [PubMed] [Google Scholar]
- Kamolmatyakul S, Chen W, Yang S, Abe Y, Moroi R, Ashique AM, et al. IL-1α stimulates cathepsin K expression in osteoclasts via the tyrosine kinase-NF-κB pathway. J Dent Res. 2004;83:791–796. doi: 10.1177/154405910408301011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Berquin IM, Troen BR., and, Sloane BF. Transcription of human cathepsin B is mediated by Sp1 and Ets family factors in glioma. DNA Cell Biol. 2000;19:79–91. doi: 10.1089/104454900314591. [DOI] [PubMed] [Google Scholar]
- Busuttil RW, Rinderbriecht H, Flesher A., and, Carmack C. Elastase activity: the role of elastase in aortic aneurysm formation. J Surg Res. 1982;32:214–217. doi: 10.1016/0022-4804(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Mandell C, Margolis I, Chang J., and, Wise L. Altered aortic protease and antiprotease activity in patients with ruptured abdominal aortic aneurysms. Surg Gynecol Obstet. 1987;164:355–358. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course of aortic size.