Abstract
The endothelium imposes a structural barrier to the extravasation of systemically delivered oncolytic adenovirus (Ad). Here, we introduced a transendothelial route of delivery in order to increase tumor accumulation of virus particles (vp) beyond that resulting from convection-dependent extravasation alone. This was achieved by engineering an Ad encoding a syncytium-forming protein, gibbon ape leukemia virus (GALV) fusogenic membrane glycoprotein (FMG). The expression of GALV was regulated by a hybrid viral enhancer-human promoter construct comprising the human cytomegalovirus (CMV) immediate-early enhancer and the minimal human endothelial receptor tyrosine kinase promoter (“eTie1”). Endothelial cell-selectivity of the resulting Ad-eTie1-GALV vector was demonstrated by measuring GALV mRNA transcript levels. Furthermore, Ad-eTie1-GALV selectively induced fusion between infected endothelial cells and uninfected epithelial cells in vitro and in vivo, allowing transendothelial virus penetration. Heterofusion of infected endothelium to human embryonic kidney 293 (HEK 293) cells, in mixed in vitro cultures or in murine xenograft models, permitted fusion-dependent transactivation of the replication-deficient Ad-eTie1-GALV, due to enabled access to viral E1 proteins derived from the HEK 293 cytoplasm. These data provide evidence to support our proposed use of GALV to promote Ad penetration through tumor-associated vasculature, an approach that may substantially improve the efficiency of systemic delivery of oncolytic viruses to disseminated tumors.
Introduction
The ability of intravenously (i.v) administered adenoviruses (Ads) to infect tumor cells within cancer deposits is confounded by many obstacles, notably the vascular endothelium separating the blood circulation from the tumor parenchyma. Only a small fraction of the input dose of virus particles (vp) achieves extravasation within solid tumors and is able to infect cancer cells.1 In contrast, circulating vp interact readily with the tumor-associated endothelium, which is directly accessible from the blood stream. Because tumor-associated endothelial cells express a range of receptors that allow natural or retargeted infection by various viruses, including Ad and vaccinia,2,3 targeted infection of tumor-associated endothelial cells may allow efficient delivery of vp to disseminated tumors. One of the most promising cancer gene therapy approaches makes use of oncolytic viruses, however oncolytic Ads are generally designed so that their replication is triggered by tumor-associated molecular changes.4 Accordingly they cannot achieve lytic self-amplification within noncancerous, and therefore nonpermissive, endothelial cells. This turns the tumor endothelium from a potentially useful target into a significant structural barrier to oncolytic agents, which must gain infection of target tumor cells in order to fulfill their therapeutic potential.5 This impediment could be partially overcome by conditioning the tumor endothelium with VEGF165 to transiently support the replication of oncolytic agents such as reovirus and vesicular stomatitis virus,6 or by the use of an oncolytic agent such as vaccinia virus which naturally targets tumor-associated endothelium.3 However, the activity of most oncolytic agents remain limited by their inability to penetrate through the endothelium. In the present study, we investigate a new approach to achieving transendothelial delivery by exploiting endothelial-specific production of a viral fusogenic membrane glycoprotein (FMG), thereby utilizing endothelial–epithelial fusion as a means of creating an access route for therapeutic viruses from the blood circulation into the tumor parenchyma.
The use of FMGs to enhance cell-to-cell spread of oncolytic agents has been demonstrated in previous studies.7,8,9,10 FMGs such as gibbon ape leukemia virus (GALV) have been explored as therapeutic transgenes to arm oncolytic viruses, inducing the formation of multinucleated syncytia which may facilitate viral spread from infected to adjacent uninfected cells.7,8,9,10 The ability of FMGs to synergize with virotherapy and enhance their antitumor efficacy has been demonstrated in previous studies,7,11,12,13,14 raising the possibility that FMGs may function as effective cytotoxics as well as transduction agents to complement virotherapy. Here, we report the GALV-driven formation of heterocellular syncytia between tumor-associated endothelial cells and tumor cells as a means of promoting Ad penetration from the bloodstream into the tumor. This was accomplished through transcriptionally directing GALV expression to activated, proliferating endothelium using a construct containing a 460 base pair enhancer from the human cytomegalovirus (CMV) and a 880 base pair promoter of human endothelial receptor tyrosine kinase (Tie1).15 Tie1 is expressed almost exclusively on endothelial cells,16 mainly during embryonic vasculogenesis but also in adults during both physiological and tumor angiogenesis.17,18,19,20,21,22 We engineered an Ad vector encompassing this fusogenic system which expressed high levels of the transgene exclusively in endothelial cells, and mediated endothelial-tumor cell fusion in vitro and in vivo. Formation of heterocellular syncytia following infection of endothelial cells resulted in fusion-dependent infection of human embryonic kidney 293 (HEK 293) cells in vitro and HEK 293 xenografts in vivo, resulting in dramatically increased viral titers. We have therefore provided the first demonstration of using heterocellular syncytium formation to assist viral penetration through the tumor vasculature, ultimately allowing target cell transduction. This strategy may be applicable to oncolytic Ads, e.g., AdEHE2F, vKH6, and CV706,4 which are reliant on gain of function mutations in tumor cells, whereby cancer-specific factors could then become imported into endothelial nuclei-containing virus genomes to transactivate replication.
Results
Transcriptional targeting of GALV expression to angiogenic endothelium
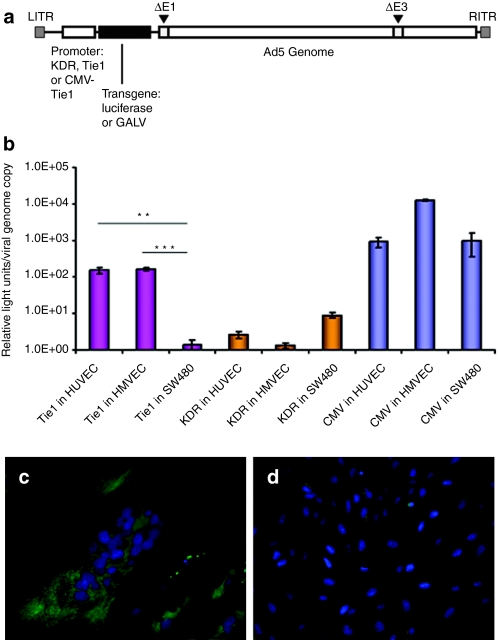
The use of Ad to achieve the fusion of tumor-associated endothelium to tumor cells requires tight transcriptional regulation to minimize the risk of off-target toxicity. One means of achieving such regulation is through the use of tissue-specific promoters. To select the optimum endothelial-selective promoter to drive GALV expression, the minimal promoter sequences of Tie115 and the kinase insert domain receptor (KDR) of vascular endothelial growth factor23,24 were isolated from primary human umbilical vein endothelial cells (HUVECs), and tested in the context of luciferase-expressing reporter Ads based on the AdEasy system (Qbiogene, MP Biomedicals, Montreal, Quebec, Canada). Replication-deficient vectors were constructed with each promoter cloned upstream of luciferase or GALV (Figure 1a), allowing quantitative comparisons of promoter strength and cell-type selectivity (Figure 1b). Tie1 or KDR-driven luciferase-expressing reporter viruses, Ad-KDR-Luc and Ad-Tie1-Luc, were used to infect HUVECs, human microvascular endothelial cells or SW480 (human colorectal cancer) cells. Activity of Ad-KDR-Luc was close to background levels in all cells tested; in contrast, Ad-Tie1-Luc exhibited high endothelial specificity, with a 100-fold greater luciferase expression per genome copy in HUVECs (P < 0.01) and human microvascular endothelial cells (P < 0.001) than in SW480 cells. In contrast the CMV promotor driven Ad-Luc control virus showed no selectivity for endothelial cells (P > 0.05) (Figure 1b).
Figure 1.
Construct organization and promoter optimization. (a) Construction of reporter and GALV-expressing viruses: genome organization of E1/E3-deleted adenovirus serotype 5 vectors-expressing luciferase or GALV under KDR or Tie1 promoter control (Ad-KDR-Luc, Ad-Tie1-Luc, Ad-KDR-GALV, Ad-Tie1-GALV); and a GALV-expressing vector driven by a hybrid CMV enhancer-human Tie1 promoter system (Ad-eTie1-GALV). (b) Comparison of the activity and endothelial-selectivity of Tie1, KDR, and CMV promoters. Luciferase expression from reporter viruses was assayed at 48 hours postinfection with Ad-KDR-Luc, Ad-Tie1-Luc, or Ad-Luc at 1,000 vp/cell; viral DNA was extracted from cell lysates for real-time quantitative PCR (QPCR) analysis. Data represent mean values (±SE of the mean), n = 4. Statistical analyses were performed by one-way ANOVA Tukey's multiple comparison test for each vector in the three different cell lines, or for comparing the performance of the three vectors in each cell line; **P < 0.01; ***P < 0.001. (c,d) Demonstration of Ad-eTie1-GALV fusogenicity. Cytoskeleton and nuclei of HUVECs infected with (c) Ad-eTie1-GALV or (d) Ad-Luc control were fluorescently labeled using an anti-α-tubulin antibody and the nuclear dye DAPI. Ad, adenovirus; ANOVA, analysis of variance; CMV, cytomegalovirus; GALV, gibbon ape leukemia virus; HUVEC, human umbilical vein endothelial cell; KDR, kinase insert domain receptor.
However, the corresponding GALV-expressing viruses (Ad-Tie1-GALV and Ad-KDR-GALV) did not drive adequate levels of GALV expression in HUVECs to permit fusion (data not shown). We therefore sought to increase the functional output of the tightly endothelial-selective Tie1 promoter by introducing the CMV immediate-early enhancer element, producing transcriptional control by a hybrid CMV enhancer-Tie1 promoter (Ad-eTie1-GALV). This virus induced extensive syncytium formation in cultured HUVECs, leading to loss of cell morphology and clustering of nuclei as shown by DAPI staining; tubulin organization in syncytia was shown by staining with an anti-α tubulin-fluorescein isothiocyanate antibody (Figure 1c). These effects were not observed for the control Ad-Luc vector (Figure 1d).
Ad-eTie1-GALV mediates endothelial-specific cell–cell fusion
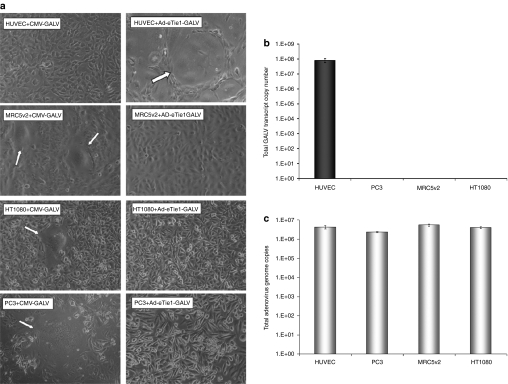
The cell-type specificity of the CMV enhancer-Tie1 promoter transcriptional activation system of Ad-eTie1-GALV was tested in a range of human cell lines including those of epithelial (PC-3, prostate cancer), mesenchymal (MRC5v2, immortalized fibroblast and HT1080, fibrosarcoma) or endothelial (HUVEC) origin. As GALV-mediated fusion is dependent on the recognition of the cellular transmembrane receptor Pit-1,25 the ability of each of the nonendothelial cell lines to undergo GALV-induced homocellular fusion was tested by transfection with a control CMV-driven GALV-expressing plasmid (CMV-GALV) (left panel images, Figure 2a). In each of these cell lines, the CMV-GALV plasmid induced extensive syncytium formation with the exception of HUVECs (where the absence of syncytia reflects low transfection efficiency, as confirmed using a CMV-GFP plasmid, data not shown). In contrast, following infection with Ad-eTie1-GALV syncytium formation was observed exclusively in HUVECs with no observable syncytia in any of the nonendothelial cell lines tested (right panel images, Figure 2a). In order to provide quantitative analysis of fusion activity in different cell lines, a fusion index was calculated for each experimental condition as described in the methods section. The average fusion indexes for MRC5v2, HT1080, and PC-3 cells following transfection with the CMV-driven, GALV-expressing plasmid were 0.22, 0.09, and 0.13, respectively (Figure 2a). The average fusion index for HUVECs following infection with Ad-eTie1-GALV was 0.10 (Figure 2a), whereas in MRC5v2, HT1080, and PC-3 cells it was 0.
Figure 2.
Ad-eTie1-GALV mediates extensive syncytium formation in endothelial cells but not in cells of epithelial or mesenchymal origin. (a) HUVEC, MRC5v2, HT1080, and PC-3 cells at 70% confluency were transfected with a CMV-driven GALV-expressing plasmid (left panel images) or infected with Ad-eTie1-GALV at 5,000 vp/cell (right panel images). Phase-contrast images were taken at 24 hours postinfection. Typical multinucleated syncytial structures are indicated by arrows. (b) GALV mRNA production was restricted to endothelial cells infected with Ad-eTie1-GALV. A range of cell lines including those of endothelial, epithelial, and mesenchymal origin were infected, total cellular RNA from each sample was recovered and the levels of GALV transcript were quantified by RT-QPCR. (c) Ad infectivity in each cell line was measured by harvesting cells at 24 hours postinfection with Ad-eTie1-GALV at 5,000 vp/cell, followed by DNA extraction and QPCR to quantify adenoviral genome copy numbers. Results are presented as an average of triplicates ± SD. Statistical analysis was performed using one-way ANOVA Tukey's multiple comparison test for all experimental groups. Ad, adenovirus; ANOVA, analysis of variance; CMV, cytomegalovirus; GALV, gibbon ape leukemia virus; HUVEC, human umbilical vein endothelial cell; KDR, kinase insert domain receptor; RT-QPCR, reverse-transcription real-time quantitative PCR.
To quantify differential expression of GALV, mRNA levels were determined by reverse-transcription real-time quantitative PCR (RT-QPCR) (Figure 2b). At 24 hours postinfection ~8.2 × 107 GALV transcripts were obtained from infection of 10,000 HUVECs with Ad-eTie1-GALV at 5,000 vp/cell, whereas GALV mRNA was undetectable in all other cell lines. This equates to ~19 GALV mRNA molecules from each virus genome, and ~8,200 transcripts per HUVEC. Statistical analysis confirms significant differences between transcript levels found in HUVECs versus all epithelial cell lines (P < 0.001 for all groups). Ad-Luc infection of each cell line served as negative controls for the design of GALV-specific primers and probe; no GALV mRNA was detected in these controls. To account for possible differences in virus infectivity in different cell lines, viral genome copy numbers were determined at 24 hours postinfection by real-time QPCR. No significant difference in the levels of Ad-eTie1-GALV associated with each cell line was detected (Figure 2c). These results demonstrate the strict endothelial specificity of the CMV enhancer-Tie1 promoter system.
Heterocellular endothelial/epithelial syncytium formation induced by endothelial infection with Ad-eTie1-GALV in vitro
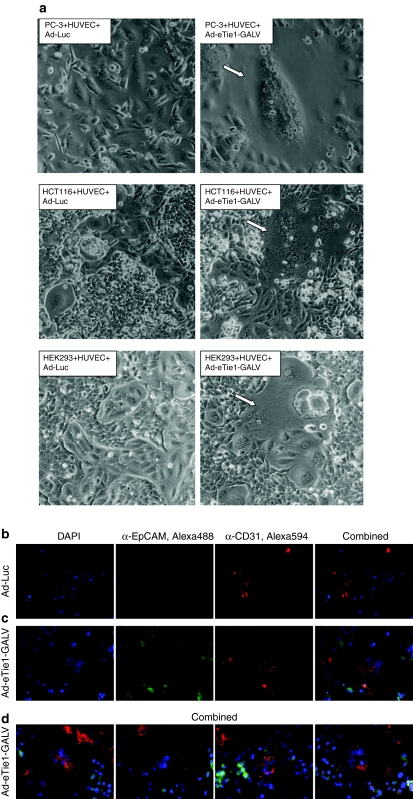
Coculturing experiments were performed to test whether Ad-eTie1-GALV could induce fusion between two cell types. Coculturing of Ad-Luc infected HUVECs with each nonendothelial cell line resulted in the maintenance of morphologically distinct mixed cell populations (left panel images, Figure 3a). In contrast, widespread heterocellular syncytium formation was observed within 12 hours of the addition of uninfected PC-3, HCT116, or HEK 293 cells to HUVECs that had been preinfected with Ad-eTie1-GALV (right panel images in Figure 3a). The average fusion indexes (F) for PC-3, HCT116, and HEK 293 cells cocultured with HUVECs preinfected with Ad-eTie1-GALV were 0.59, 0.38, and 0.21, respectively. For Ad-Luc the F value was 0.
Figure 3.
Ad-eTie1-GALV induces heterocellular endothelial–epithelial syncytium formation in vitro. Subconfluent HUVECs were infected at 5,000 vp/cell with Ad-Luc or Ad-eTie1-GALV and subsequently cocultured with uninfected PC-3, HCT116, or HEK 293 cells. (a) Phase-contrast images of syncytia (indicated by arrows) formed between HUVECs and each epithelial cell line in the presence of endothelial-specific GALV expression. (b) Fluorescence microscopy of PC-3 cells cocultured with HUVECs preinfected with Ad-Luc. (c,d) Fluorescently labeled heterocellular syncytia obtained from cocultures of PC-3 cells with HUVECs preinfected with Ad-eTie1-GALV. An anti-CD31 primary antibody was used as an endothelial-specific marker, in combination with an Alexa Fluor 594-conjugated secondary antibody. An anti-EpCAM primary antibody was used as an epithelial-specific marker, in combination with an Alexa Fluor 488-conjugated secondary antibody (see Materials and Methods section). Ad, adenovirus; CMV, cytomegalovirus; GALV, gibbon ape leukemia virus; HEK 293, human embryonic kidney 293; HUVEC, human umbilical vein endothelial cell; KDR, kinase insert domain receptor; RT-QPCR, reverse-transcription real-time quantitative PCR.
In order to characterize the composition of the syncytia formed under such mixed culture conditions PC-3 cells were visualized using an antibody against epithelial cell adhesion molecule (EpCAM, in green), and HUVECs preinfected with Ad-eTie1-GALV were visualized using an antibody against CD31 (in red). When the Ad-Luc control was used only single cells stained either red or green were observed (Figure 3b). However, abundant syncytial structures were apparent when HUVECs were preinfected with Ad-eTie1-GALV (Figure 3c). An image gallery of these structures is shown in Figure 3d, they share the features of multinucleation (as shown by DAPI) and are comprised of both HUVEC endothelial (red) and PC-3 epithelial cells (green). Single stained PC-3 and HUVEC which have yet to be incorporated into the syncytia are also apparent at the margins of these images. These data confirmed the ability of GALV expressing HUVECs to form heterocellular syncytia with a range of tumor cell types.
Transactivation of virus replication upon heterocellular syncytium formation in vitro
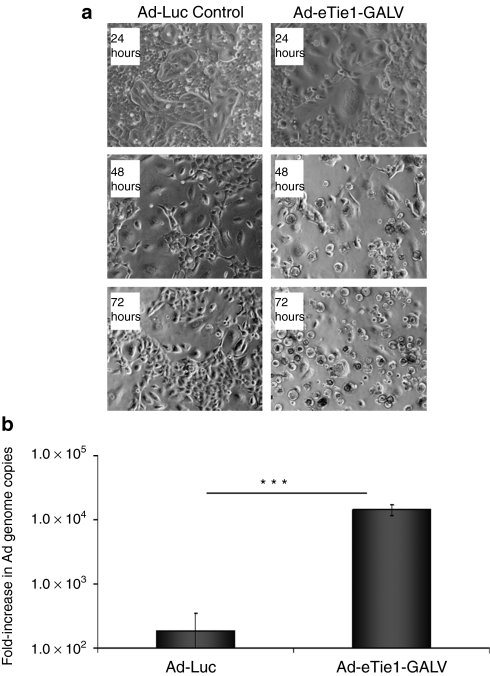
The activation of an oncolytic Ad within the endothelial nucleus of a heterocellular syncytium would generally require the conversion of the nucleus into a site for virus production by the nuclear import of tumor-associated cytoplasmic factors. To assess the feasibility of this approach, HUVECs were infected with the E1/E3-deleted Ad-eTie1-GALV or Ad-Luc virus before coculture with HEK 293 cells, which stably express adenoviral E1 gene products and should be capable of transcomplementing missing viral functions in these replication-deficient vectors. Mixed cultures containing HUVECs preinfected with the control Ad-Luc virus did not give observable cytopathic effect (Figure 4a) and distinct HUVEC and HEK 293 populations were still observable after 72 hours. In contrast complete cytopathic effect was achieved 72 hours after mixing Ad-eTie1-GALV-infected HUVECs with uninfected HEK 293 cells (Figure 4a), where the disappearance of syncytia at 48 and 72 hours is linked to the raised cytotoxic replication (Figure 4b). QPCR analysis of the number of Ad genome copies in the culture supernatant revealed an ~10,000-fold increase in Ad genomes between 24 and 72 hours when using Ad-eTie1-GALV, whereas Ad-Luc gave only a 100-fold rise over the same period (Figure 4b). This statistically significant difference (P < 0.001) in genome copy numbers obtained from cocultures with or without GALV demonstrate the successful transactivation of the replication-deficient virus in heterocellular syncytia.
Figure 4.
Transactivation of virus replication upon heterocellular syncytium formation permits fusion-dependent transduction of HEK 293 cells and destruction of cell culture monolayer. HUVECs (50% confluency) were preinfected with either Ad-Luc control or Ad-eTie1-GALV at 5,000 vp/cell and washed thoroughly at the completion of the 2-hour infection. Uninfected HEK 293 cells were added to the endothelial cell cultures at 24 hours after HUVEC infection to give a 1:1 ratio of HUVEC:HEK 293. Culture supernatant was removed and replaced with fresh media at 24 hours after the addition of HEK 293 cells. (a) Phase-contrast images taken at 24, 48, and 72 hours after mixing different cell populations. (b) Samples of culture supernatant were taken at 24 and 72 hours after mixing the two cell populations for the determination of viral genome copy numbers by QPCR. Results are expressed as fold-increase in genome copies from the 24 to the 72-hour time point. All data points are presented as averages of triplicates ± SD. Statistical analyses were performed by t-tests (n = 3); ***P < 0.001. Ad, adenovirus; HEK 293, human embryonic kidney 293; HUVEC, human umbilical vein endothelial cell; QPCR, quantitative PCR.
Endothelial-directed GALV expression leads to target cell infection and enhanced virus replication in vivo
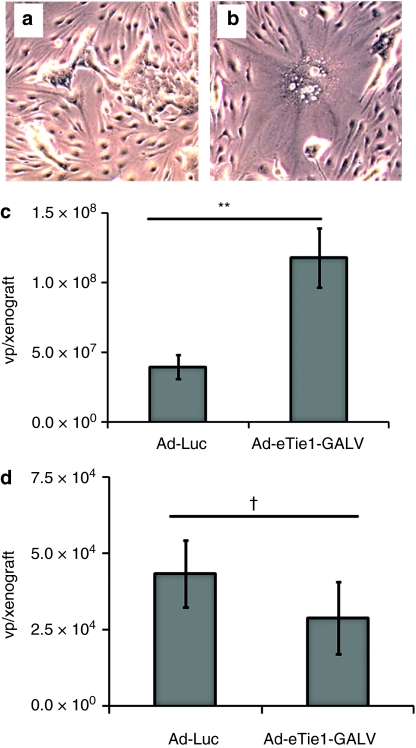
The feasibility of testing the Ad-eTie1-GALV vector in a murine model was demonstrated by the successful induction of murine endothelial (bEnd.3) fusion to human epithelial (HEK 293) cells in vitro. When Ad-Luc-infected bEnd.3 cells were mixed with uninfected HEK 293 cells no multinucleated syncytia were obtained (Figure 5a). However, when uninfected HEK 293 cells were mixed with bEnd.3 cells which had been preinfected with Ad-eTie1-GALV syncytia were obtained (Figure 5b), demonstrating murine (i.e., Pit-1 negative) endothelial cells producing GALV could undergo fusion with human HEK 293 (Pit-1 positive) cells.
Figure 5.
Heterocellular syncytium formation provides viral access to target cells and elicits transactivation of virus replication in vivo. Murine endothelial to human epithelial fusion was tested in vitro by the mixing of uninfected HEK 293 cells with bEnd.3 cells which had been preinfected with (a) Ad-Luc or (b) Ad-eTie1-GALV (5,000 particles per cell, 2 hours). Phase-contrast images taken at 24 hours after mixing different cell populations. For in vivo studies, female SCID mice (Charles River) were implanted subcutaneously with 2.5 × 106 HEK 293 or PC-3 cells; xenografts were allowed to develop to ~4 mm in diameter before i.v. administration of either the control Ad-Luc virus or Ad-eTie1-GALV at 3 × 1010 vp/animal. Xenografts were harvested at 48 hours after virus injection; tissues were homogenized and genomic DNA was extracted. Ad genomes present in (c) HEK 293 xenografts or (d) control PC-3 tumors were quantified by QPCR. Data represent mean values ± SE of the mean; statistical analyses were performed by t-tests (n = 7, except for Ad-eTie1-GALV in PC-3 where n = 6); **P < 0.01, †P > 0.05. Ad, adenovirus; GALV, gibbon ape leukemia virus; HEK 293, human embryonic kidney 293; HUVEC, human umbilical vein endothelial cell; KDR, kinase insert domain receptor; QPCR, quantitative PCR.
The demonstration of fusion-mediated viral transduction of noninfected cells in vitro encouraged the testing of this strategy in vivo to provide a more relevant, three-dimensional model. Ad-Luc or Ad-eTie1-GALV was given i.v. at 3 × 1010 vp/animal to mice-bearing subcutaneous HEK 293 xenografts, or control PC-3 tumors which do not express E1 gene products and therefore cannot provide transcomplementation. Xenografts and tumors were harvested after 48 hours. Measurement of Ad genomes copies in the HEK 293 xenografts revealed a threefold higher level of Ad-eTie1-GALV (1.2 × 108/xenograft) than Ad-Luc (4.0 × 107vp/xenograft) (P = 0.008), providing evidence for enhanced access of the replication-deficient virus to HEK 293 cells in the presence of GALV expression (Figure 5c). This likely to be a conservative measure of the extent to which endothelial GALV expression has increased virus access to xenografted cells, as plaque assays revealed that the titer of the control Ad-Luc used here was approximately tenfold higher than that of Ad-eTie1-GALV. In contrast to the significantly higher levels of Ad-eTiel-GALV compared to Ad-Luc found in HEK 293 xenografts, there was no statistically significant difference between the quantities of the viruses in the control E1-deficient PC-3 tumors (P = 0.38) (Figure 5d), demonstrating that the higher levels of Ad-eTie1-GALV was a result of increased virus access to and replication in HEK 293 xenografts. Since GALV expression is endothelial-selective, our data suggest that virus infection of tumor-associated endothelial cells has enabled GALV-mediated spread of the virus to adjacent xenografted epithelial cells.
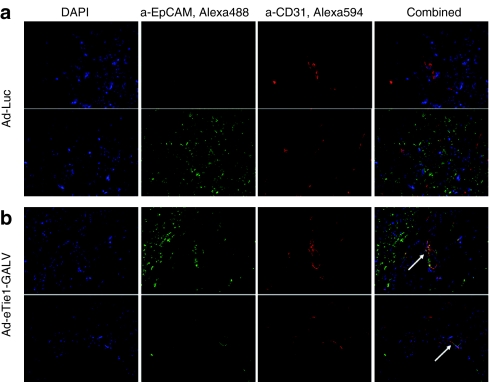
In order to confirm the in vivo formation of heterocellular syncytia between murine endothelium and HEK 293 cells, immunohistochemistry of tissue cross-sections was performed. Distinct endothelial (red) and epithelial (green) cells were observed in xenograft cross-sections from Ad-Luc treated animals (Figure 6a) with no evidence of colocalized staining. However, dual-stained, multinucleated structures were observed in sections obtained from animals treated with Ad-eTie1-GALV (Figure 6b). Heterocellular structures were rare; examination of 50 nonconsecutive tissue sections afforded observations of four dual-stained syncytia. The areas of intense yellow staining (see arrows) are indicative EpCAM and CD31 colocalization made possible by the GALV-mediated fusion of epithelial cells to endothelial cells. These data confirm the GALV-mediated formation of mouse endothelial-human epithelial heterocellular syncytia.
Figure 6.
Detection of heterocellular syncytia formed between murine vascular endothelium and HEK 293 cells. Xenografts harvested at 48 hours post-treatment with Ad-Luc or Ad-eTie1-GALV were snap-frozen and cryosections were prepared. Subsequent immunofluorescence labeling of murine endothelium and HEK 293 cells was carried out on multiple nonconsecutive tissue cross-sections, providing observations of murine vasculature in HEK 293 xenografts following i.v. injection of (a) Ad-Luc as well as dual-stained, multinucleated structures unique to xenografts harvested from animals receiving (b) Ad-eTie1-GALV. The murine endothelium was stained red and human epithelial cells were stained green using conditions described in materials and methods. Ad, adenovirus; GALV, gibbon ape leukemia virus; HEK 293, human embryonic kidney 293.
Discussion
The tumor-associated vasculature presents a highly accessible target for vp circulating in the bloodstream,3,26 enabling active targeting approaches.27 In the present study, we explored a strategy to enhance viral vascular penetration in order to increase the therapeutic index of a systemically delivered Ad. We tested the hypothesis that vascular transcriptional targeting and subsequent induction of site-specific syncytium formation can be combined to turn a major structural obstacle imposed by the tumor endothelium into an entry route toward tumor cells. A hybrid viral enhancer/human promoter system encompassing the CMV enhancer and the Tie1 promoter was used to mediate conditional activation of GALV expression upon Ad infection of proliferating endothelium. Using this vector (Ad-eTie1-GALV) we achieved tight endothelial-selective transcriptional activity and targeted induction of endothelial–epithelial fusion in vitro and in vivo, presenting the first demonstration of exploiting a viral FMG to induce heterocellular syncytium formation in order to create access to target tumor cells.
Upon endothelial infection and the induction of heterofusion, the ability of a GALV-expressing Ad to successfully replicate in heterocellular syncytia is crucial for subsequent vp dissemination. As there is no internuclear spread of Ad DNA upon GALV-induced syncytium formation,28 the ability of transactivating factors to be imported into nuclei-containing Ad genomes would be essential for triggering replication. Tumor-associated endothelial cells are noncancerous and should therefore be unable to support the replication of conditionally replicating Ad; therefore, the capacity of heterocellular syncytia to support virus transactivation following fusion of a virus-infected, nonpermissive cell type with a permissive cell type was investigated. The initiation of Ad replication upon fusion between endothelial cells infected with the E1, E3-deleted vector Ad-eTie1-GALV and cocultured permissive HEK 293 cells demonstrated the successful transactivation of the replication-defective virus in vitro. Dramatically increased (~2-log) levels of virus were recovered from these cocultures compared to those infected with a luciferase-expressing control vector, correlating with more rapid development of cytopathic effect. This is a novel demonstration that the cytoplasmic factors of an epithelial cell can be shared by the nuclei of an Ad-infected endothelial cell in a heterocellular syncytium, thereby driving virus replication, spread, and cell killing. These data therefore support the hypothesis that GALV-induced heterofusion may promote target cell transduction and subsequent vp dissemination. The in vitro data encouraged further testing of the hypothesis that heterofusion may aid the penetration of systemically administered adenoviral vectors through tumor-associated endothelium in an in vivo setting. Levels of Ad-eTie1-GALV found in HEK 293 xenografts were significantly higher than levels of the control Ad-Luc virus at 48 hours post-i.v. administration, whereas similar amounts of the two viruses were recovered from corresponding control PC-3 tumors, suggesting enhanced access of the replication-defective vector to HEK 293 xenografts resulting from conditionally activated GALV expression. Identification of costained heterocellular syncytia in cross-sections of xenografts harvested from HEK 293-bearing animals treated with Ad-eTie1-GALV confirmed that the endothelial–epithelial fusion evidenced in vitro can also be achieved in vivo. The complete absence of such syncytial structures in Ad-Luc-treated animals indicated that the raised levels of Ad-eTie1-GALV in HEK 293 xenografts can be accounted for by such fusion. Our data therefore suggests that heterofusion assists target cell transduction in vivo and results in enhanced virus replication. It is worth noting that the process of heterofusion may be suboptimal in the present in vivo model, as evidenced by the fact that the occurrence of dual-stained, multinucleated heterocellular syncytia was infrequent. As the Pit-1 receptor required for GALV-mediated fusion is absent on murine cells,10 any host stromal cells such as pericytes and fibroblasts within the xenograft will create a barrier to limit the interaction of infected endothelial cells with the HEK 293 cells. Another possible factor limiting the efficiency of this model system is the abortive or poor replication efficiency of human Ads in mouse cells29,30,31 due to repression of viral E1A enhancer activity32 and defects in the synthesis of structural proteins.33 Such repression could still occur to some extent despite the E1 gene products being provided in trans by the HEK 293 component of the syncytium. Despite the limitations of the present model system, we have demonstrated that heterocellular syncytium formation following infection of endothelial cells rendered fusion-dependent transduction of HEK 293 cells in vitro and HEK 293 xenografts in vivo, enabling transactivation of virus replication and resulting in significantly increased virus production in both settings. We have therefore provided the first demonstration of exploiting heterocellular fusion as a means to aid viral vascular penetration, leading ultimately to enhanced target cell transduction. This presents a novel therapeutic approach to overcome a major physical barrier to the systemic delivery of oncolytic viruses. This strategy may enable conditional activation of FMG expression under tumor-specific conditions and maximize the therapeutic potential of the bioavailable fraction of a circulating oncolytic agent.
Materials and Methods
Cell lines and culture. HUVECs (Lonza, Basel, Switzerland) and human adult dermal microvascular endothelial cells (HMVECs; Cambrex, Walkersville, MD) were cultured in EGM-2 endothelial cell growth medium supplemented with EGM-2 bullet kit (Lonza). Human embryonic kidney epithelial 293 cells (HEK 293; American Type Culture Collection, Manassas, VA), PC-3 (American Type Culture Collection), SW480 (kindly provided by Prof Richard Iggo, University of St Andrews, St Andrews, UK), MRC5V2 (kindly provided by Dr Richard Wade-Martins, University of Oxford, Oxford, UK) and HT1080 (kindly provided by Prof Eric Bernhard, University of Oxford), were cultured in high-glucose Dulbecco's modified Eagle's medium (PAA Laboratories, UK), supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria), and 1% penicillin (10,000 units/ml)-streptomycin (10 mg/ml) (pen/strep) solution (Sigma, Dorset, UK). All cells were cultured at 37 °C in a humidified, 5% CO2 environment. Routine maintenance of cells and preparation for experiments involving cell cultures were performed using aseptic techniques.
Molecular engineering and virus production. The human KDR23,24 and Tie115 promoters were isolated by PCR from genomic DNA extracted from HUVECs. Oligonucleotide primer sequences used for PCR are: For KDR—5′ GTC GAC AAC AAA GTT GTT GCT CTG GGA TGT TCT C 3′ and 5′ GGG AGC CGG TTC TTT CTC 3′ for Tie1—5′ GTC GAC CGG CAA AAT GAA TGA CAC CTG GCA GAC 3 and 5' TAC TCC AGA GGC CGA CCC AG 3′. Resultant PCR products were inserted into TOPO-XL cloning vectors (Invitrogen, Paisley, UK).
Endothelial promoter (Tie1/KDR) driven luciferase or GALV-expressing Ads were generated based on the AdEasy System (MP Biomedicals, Solon, OH). Luciferase (firefly Photinus pyralis luciferase; Promega, Madison, WI) and GALV (a kind gift from Prof Richard Vile, Mayo Clinic, Rochester, MN) were ligated downstream of each promoter in TOPO-XL. The resultant four constructs containing KDR/Tie1 upstream of luciferase or GALV were ligated into the multiple cloning site of the pShuttle transfer vector (AdEasy recombinant Ad production kit; MP Biomedicals). The CMV major immediate-early enhancer was PCR-amplified from the pShuttle-CMV vector (AdEasy recombinant Ad production kit; MP Biomedicals) and subcloned upstream of Tie1-GALV in pShuttle. The resultant pShuttle vectors containing the five (enhancer)-promoter-transgene constructs were subjected to recombination with the AdEasy-1 plasmid, which contains the E1 and E3-deleted human Ad type 5 genome. Correct sequences of all constructs were confirmed by DNA sequencing. Successful recombinants were linearized by digestion with PacI and transfected into E1-expressing HEK 293 cells using a calcium-phosphate transfection protocol. Resultant viruses were double-purified by cesium chloride gradient centrifugation. Virus DNA concentrations were determined by PicoGreen assay (PicoGreen, dsDNA quantitation reagent; Invitrogen) and virus titers determined by plaque assay, values of 3.3 × 1010 Ad-Luc and 1.7 × 1010 Ad-eTie1-GALV plaque forming units/ml.
Calculating fusion indexes for various syncytia and heterocellular syncytia. In order to provide quantitative analysis of fusion activity in different cell lines or mixed endothelial/epithelial cultures after transfection with a CMV-driven GALV-expressing plasmid or infection with Ad-eTie1-GALV, a fusion index was calculated by counting the number of cells and nuclei present in a microscopic field according to the equation: F (fusion index) = 1 − (C/N), where C is the number of cells and N is the number of nuclei in a field after fusion.34 Hence where each cell contains one nucleus the F value equals 0. As syncytia formation causes an increase in the number of nuclei per cell the F value tends toward 1. For each experimental condition, the fusion index was determined by taking the average of three F values obtained from three different microscopic views.
Real-time QPCR. Real-time QPCR was used to detect and quantify adenoviral DNA in extracted DNA samples using an ABI 7000 Sequence Detection System and software. Amplification of an 84 base pair fragment of the Ad fiber gene was carried out using primers, probes, and conditions as outlined by Green et al.26.
RT-QPCR. RT-QPCR was performed where it was necessary to quantify levels of the GALV transcript. Total cellular RNA was harvested from HUVEC, PC-3, MRC5V2, and HT1080 cells infected with either Ad-eTie1-GALV or the control Ad-Luc at 24 hours postinfection, using the mirVana miRNA isolation kit (Applied Biosystems, Warrington, UK). One-step RT QPCR was performed using the TaqMan RNA-to-CT 1-step kit (Applied Biosystems) and an ABI 7000 Sequence Detection System and software according to the manufacturer's instructions. GALV-specific primers: 5′ CAA GCC TCC AGA TCG CCA TA 3′ and 5′ ACC TCG GAC AGG GAA GTC AGT 3′, and probe: 5′ CCC TCC AAG ACT CAG 3′ were employed. An identical set of conditions were prepared for extraction of total viral genomic DNA at 24 hours postinfection. A standard curve was constructed from samples of known GALV DNA concentrations and used to derive transcript copy numbers assuming 100% reverse transcription efficiency; this data therefore represents minimum GALV mRNA levels accounted for by RT-QPCR. All conditions were tested in triplicate and results expressed as the number of GALV mRNA molecules per virus genome detected.
In vitro luciferase assay. HUVEC, human microvascular endothelial cell, and SW480 cells were seeded at 6,000 cells/well in 96-well plates 24 hours before infection with Ad-KDR-Luc, Ad-Tie1-Luc, or Ad-Luc at 1,000 vp/cell. Cells were lysed in luciferase cell culture lysis reagent (Luciferase Assay System; Promega) and freeze-thawed at 48 hours postinfection. Relative promoter activities as reflected by luciferase expression and light emission upon addition of luciferase assay substrate (Luciferase Assay System; Promega) were measured using a Lumat LB 9507 tube luminometer (Berthold Technologies, Harpenden, UK). Adenoviral DNA was extracted for each sample and quantified by QPCR. Luciferase readings were standardized according to viral genome copy numbers in each sample to take into account the differences in infectivity and virus uptake by each cell line. All conditions were tested in quadruplicate and results expressed as relative light units/genome copy.
Immunocytochemistry. HUVECs cultured in glass-bottom dishes (Electron Microscopy Sciences, Hatfield, PA) were infected with either Ad-eTie1-GALV or the control Ad-Luc (10% confluency at time of infection to ensure maximal cell growth) 24 hours before addition of uninfected cells types (HUVEC or PC-3) to generate syncytia in the presence of GALV. Cells and syncytia were subsequently dehydrated and fixed (Cell-Fixx Spray fixative; Thermo Fisher Scientific, Loughborough, UK) for immunocytochemical manipulations. Fluorescent labeling of the cytoskeleton (α-tubulin) was achieved using a fluorescein isothiocyanate-conjugated anti-α-tubulin antibody (Sigma). For characterization of heterocellular syncytia, cell type-specific primary antibodies and fluorophore-conjugated secondary antibodies were employed. An anti-CD31 primary antibody (mouse antihuman CD31, isotype IgG1; Abcam, Cambridge, UK) was used as an endothelial specific marker, in combination with an Alexa Fluor 594-conjugated goat anti-mouse IgG1 secondary antibody (Invitrogen, UK). An anti-Epcam primary antibody (rabbit antihuman epithelial cell adhesion molecule, isotype IgG; Abcam) was used as an epithelial-specific marker, in combination with an Alexa Fluor® 488-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen). Slides were prepared for microscopic examination using Vectashield mounting media containing DAPI (Vector Laboratories Ltd, UKK).
Immunohistochemistry. HEK 293 xenografts were harvested from Ad-Luc or Ad-eTie1-GALV treated animals at 96 hours post-i.v. injection; sections were prepared from snap-frozen tissues and rehydrated prior to immunolabeling procedures. Mouse endothelium was stained using a rat anti-mouse CD34 primary antibody (Abcam) coupled with an Alexa Fluor® 568 goat anti-rat IgG secondary antibody (Invitrogen, UK). Human epithelial tumour cells were stained using conditions described for immunocytochemistry. Processed slides were stabilized with Vectashield mounting media containing DAPI.
In vivo studies. All animal experimentation was performed in accordance with the terms of UK Home Office guidelines and the UKCCCR Guidelines for the Welfare of Animals in Experimental Neoplasia. For studies of viral transactivation using HEK 293 xenografts, female severe combined immune deficiency beige mice (Charles River, Margate, UK) were injected subcutaneously with 2.5 × 106 HEK 293 or E1-negative control PC-3 cells. Xenografts were allowed to develop to ~4 mm in diameter. HEK 293 or PC-3 xenograft-bearing animals were pretreated with clodronate liposomes (100 µl of original formulation per animal; ClodronateLiposomes.org) 24 hours before i.v. injection with 3 × 1010 particles of either Ad-eTie1-GALV or Ad-Luc. All i.v. injections of viruses were performed via the tail vein in a total volume of 100 µl. Xenografts were harvested at 48 hours after virus injection. All tissues were homogenized in lysis buffer (Luciferase Assay System; Promega) using a motorized homogenizer (Ultra Turrax IKA T18 Basic; Fisher Scientific) to obtain a 300 mg (wet weight)/ml homogenate, from which 180 µl was taken for DNA extraction. A standard curve was prepared from serial dilutions of Ad-eTie1-GALV spiked into a 300 mg/ml mouse tissue homogenate. QPCR analyses were performed as described previously.
Acknowledgments
We are grateful to Prof Richard Vile (Mayo Clinic, Rochester, MN) for the gift of the GALV cDNA and Dr Peter Hewett (Birmingham University, UK) for assistance with the isolation of the human Tie1 promoter. Clodronate liposomes were kindly provided by Dr Nico van Rooijen at ClodronateLiposomes.org. We are thankful to the New Zealand Tertiary Education Commission for providing the funding for this project.
REFERENCES
- Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, Bazan-Peregrino M, et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol Ther. 2006;14:118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Suominen E, Carrick F, Sauter B, Kähäri VM, Lieber A, et al. Efficient infection of tumor endothelial cells by a capsid-modified adenovirus. Gene Ther. 2006;13:52–59. doi: 10.1038/sj.gt.3302598. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–266. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120:1551–1560. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Jevremovic D, Suzuki K, Kottke T, Thompson J, Emery S, et al. Intratumoral expression of a fusogenic membrane glycoprotein enhances the efficacy of replicating adenovirus therapy. Gene Ther. 2003;10:1663–1671. doi: 10.1038/sj.gt.3302064. [DOI] [PubMed] [Google Scholar]
- Allen C, McDonald C, Giannini C, Peng KW, Rosales G, Russell SJ, et al. Adenoviral vectors expressing fusogenic membrane glycoproteins activated via matrix metalloproteinase cleavable linkers have significant antitumor potential in the gene therapy of gliomas. J Gene Med. 2004;6:1216–1227. doi: 10.1002/jgm.616. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- Diaz RM, Bateman A, Emiliusen L, Fielding A, Trono D, Russell SJ, et al. A lentiviral vector expressing a fusogenic glycoprotein for cancer gene therapy. Gene Ther. 2000;7:1656–1663. doi: 10.1038/sj.gt.3301277. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Bangen JM, Bayer W., and, Wildner O. Synergy between expression of fusogenic membrane proteins, chemotherapy and facultative virotherapy in colorectal cancer. Gene Ther. 2006;13:1534–1544. doi: 10.1038/sj.gt.3302806. [DOI] [PubMed] [Google Scholar]
- Li H, Haviv YS, Derdeyn CA, Lam J, Coolidge C, Hunter E, et al. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum Gene Ther. 2001;12:2155–2165. doi: 10.1089/10430340152710504. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Han Z, Liu B, Wang Y, Campbell G., and, Coffin RS. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- Fu X, Tao L, Jin A, Vile R, Brenner MK., and, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol Ther. 2003;7:748–754. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Korhonen J, Lahtinen I, Halmekytö M, Alhonen L, Jänne J, Dumont D, et al. Endothelial-specific gene expression directed by the tie gene promoter in vivo. Blood. 1995;86:1828–1835. [PubMed] [Google Scholar]
- Partanen J, Armstrong E, Mäkelä TP, Korhonen J, Sandberg M, Renkonen R, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K., and, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Korhonen J, Partanen J, Armstrong E, Vaahtokari A, Elenius K, Jalkanen M, et al. Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood. 1992;80:2548–2555. [PubMed] [Google Scholar]
- Korhonen J, Polvi A, Partanen J., and, Alitalo K. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene. 1994;9:395–403. [PubMed] [Google Scholar]
- Kaipainen A, Vlaykova T, Hatva E, Böhling T, Jekunen A, Pyrhönen S, et al. Enhanced expression of the tie receptor tyrosine kinase mesenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res. 1994;54:6571–6577. [PubMed] [Google Scholar]
- Puri MC., and, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA. 2003;100:12753–12758. doi: 10.1073/pnas.2133552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Borgstrom P, Maynard J, Koziol J, Hu Z, Garen A, et al. Mapping of angiogenic markers for targeting of vectors to tumor vascular endothelial cells. Cancer Gene Ther. 2007;14:346–353. doi: 10.1038/sj.cgt.7701030. [DOI] [PubMed] [Google Scholar]
- Modlich U, Pugh CW., and, Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- Jaggar RT, Chan HY, Harris AL., and, Bicknell R. Endothelial cell-specific expression of tumor necrosis factor-alpha from the KDR or E-selectin promoters following retroviral delivery. Hum Gene Ther. 1997;8:2239–2247. doi: 10.1089/hum.1997.8.18-2239. [DOI] [PubMed] [Google Scholar]
- O'Hara B, Johann SV, Klinger HP, Blair DG, Rubinson H, Dunn KJ, et al. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990;1:119–127. [PubMed] [Google Scholar]
- Herz J., and, Gerard RD. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci USA. 1993;90:2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan-Peregrino M, Seymour LW., and, Harris AL. Gene therapy targeting to tumor endothelium. Cancer Gene Ther. 2007;14:117–127. doi: 10.1038/sj.cgt.7701001. [DOI] [PubMed] [Google Scholar]
- Guedan S, Gros A, Cascallo M, Vile R, Mercade E., and, Alemany R. Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication. Gene Ther. 2008;15:1240–1245. doi: 10.1038/gt.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci USA. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oualikene W, Gonin P., and, Eloit M. Short and long term dissemination of deletion mutants of adenovirus in permissive (cotton rat) and non-permissive (mouse) species. J Gen Virol. 1994;75 (Pt 10):2765–2768. doi: 10.1099/0022-1317-75-10-2765. [DOI] [PubMed] [Google Scholar]
- Fognani C, Della Valle G., and, Babiss LE. Repression of adenovirus E1A enhancer activity by a novel zinc finger-containing DNA-binding protein related to the GLI-Kruppel protein. EMBO J. 1993;12:4985–4992. doi: 10.1002/j.1460-2075.1993.tb06192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggerding FA., and, Pierce WC. Molecular biology of adenovirus type 2 semipermissive infections. I. Viral growth and expression of viral replicative functions during restricted adenovirus infection. Virology. 1986;148:97–113. doi: 10.1016/0042-6822(86)90406-x. [DOI] [PubMed] [Google Scholar]
- White J, Matlin K., and, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]