Abstract
Antisense-induced exon skipping can restore the open reading frame, and thus correct the dystrophin deficiency that causes Duchenne muscular dystrophy (DMD), a lethal muscle wasting condition. Successful proof-of-principle in preclinical models has led to human clinical trials. However, it is still not known what percentage of dystrophin-positive fibers and what level of expression is necessary for functional improvement. This study directly address these key questions in the mdx mouse model of DMD. To achieve a significant variation in dystrophin expression, we locally administered into tibialis anterior muscles various doses of a phosphorodiamidate morpholino oligomer (PMO) designed to skip the mutated exon 23 from the mRNA of murine dystrophin. We found a highly significant correlation between the number of dystrophin-positive fibers and resistance to contraction-induced injury, with a minimum of 20% of dystrophin-positive fibers required for meaningful improvement. Furthermore, our results also indicate that a relatively low level of dystrophin expression in muscle fibers may have significant clinical benefits. In contrast, improvements in muscle force were not correlated with either the number of positive fibers or total dystrophin levels, which highlight the need to conduct appropriate functional assessments in preclinical testing using the mdx mouse.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder caused primarily by nonsense or frameshift mutations that severely limit the production of dystrophin.1,2 Dystrophin is a 427-KDa protein that localizes to the subsarcolemmal region of muscle fibers and forms part of a large oligomeric complex known as the dystrophin-associated protein complex.3,4,5 The function of the dystrophin-associated protein complex remains unclear, but appears to play a role in protecting the sarcolemma from stresses imposed during muscle contractions, by linking the actin-based cytoskeleton to the extracellular matrix.6,7,8 The absence of dystrophin in DMD patients results in a severe myopathy and progressive muscle wasting, while the milder allelic form of the disease, Becker muscular dystrophy, is usually caused by in-frame deletions, resulting in the expression of shortened and commonly partially functional dystrophin. As a result, Becker muscular dystrophy manifests as a spectrum of phenotypes ranging from asymptomatic to similar to DMD.9,10
A promising therapy for DMD consists of using antisense oligonucleotides (AOs) to induce targeted exon skipping of the dystrophin pre-mRNA in order to correct the reading frame and restore dystrophin protein synthesis. This AO-mediated therapy has been extensively tested on the mdx mouse model of DMD, which harbors a nonsense point mutation in exon 23 of the dystrophin gene and lacks dystrophin expression in muscle tissues.11 Although these dystrophic mice do not reproduce the severity of pathology observed in DMD patients, they remain very useful for assessing the potential of therapeutic interventions. Previous work has demonstrated that intramuscular delivery of a 2′-O-methyl phosphorothioate AO into mdx mice was able to induce the specific removal of exon 23 from the dystrophin transcript and produce apparently functional amounts of dystrophin protein.12 Alternative AO chemistries have been tested to achieve higher exon-skipping efficiencies and reduce potential toxicity. The phosphorodiamidate morpholino oligomer (PMO) is one such compound and has been shown to induce higher levels of exon skipping and dystrophin expression compared with the 2′-O-methyl phosphorothioate AO.13,14,15 Furthermore, systemic delivery of PMOs was able to restore dystrophin expression in multiple muscle groups of mdx mice albeit at low levels compared to normal.16,17
Although data emerging from these preclinical studies are undoubtedly encouraging, there are limited data on the efficacy of PMOs to improve muscle function in mdx mice. This is best illustrated by the fact that to date there has been no assessment of resistance to contraction-induced stress in mdx mice following PMO treatment, which is arguably the most important measure of muscle strength. Furthermore, there are no studies that examine the efficacy and physiological consequences of PMO treatment in aged mdx mice, which would more accurately assess the therapeutic potential of PMOs in muscles with more advanced deterioration and thus a better predicator of therapeutic success in DMD patients. Therefore, we conducted a comprehensive study of muscle function in aged mdx mice following intramuscular delivery of PMO, which included isometric force measurements and most importantly resistance to lengthening contraction-mediated damage. Moreover, by varying the dose and volume administered to the muscles of mdx mice, we were able to assess the relationship between dystrophin expression and improvements in muscle function. Interestingly, we found a highly significant correlation between the number of dystrophin-positive fibers and improved resistance to lengthening contractions, but no correlation with specific force. These findings clearly establish the need to conduct stress-inducing protocols in mdx mice, when assessing the efficacy of potential treatments for DMD and indicate the necessary levels of dystrophin required to improve muscle function in patients with DMD.
Results
Dystrophin expression in PMO-treated mdx muscle
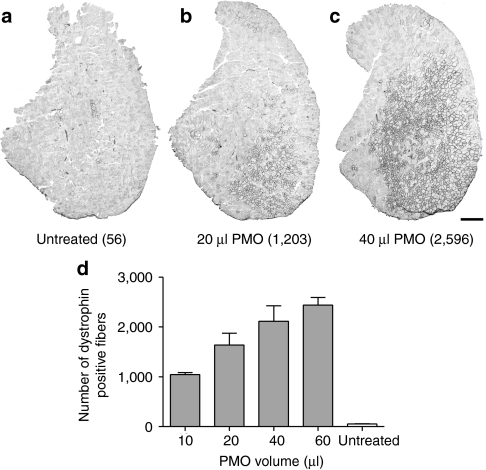
In order to examine the relationship between restored dystrophin expression in aged (10-month-old) mdx mice following PMO treatment and possible functional improvements, it was essential to induce a significant variation in dystrophin expression. This was achieved by injecting a number of different volumes (10, 20, 40, and 60 µl) of PMO (1 µg/µl) into the tibialis anterior (TA) muscles of mdx mice. In addition, the contralateral TA muscle in each animal was injected with an equal volume of saline, which served as the internal control. Four weeks after PMO delivery, dystrophin-positive fibers were identified using immunohistochemistry. Whole-muscle transverse sections exhibited uniform distribution of dystrophin-positive fibers at the site of injection (Figure 1a–c), which importantly remained consistent throughout a large proportion of longitudinal axis of the TA muscle. As expected the total number of dystrophin-positive fibers in treated TA muscles increased with increasing volumes, ranging from 1,046 ± 36.4 (n = 6) with 10 µl, to 2,439 ± 143 (n = 7) with 60 µl (Figure 1d). A commonly used estimate of the number of TA fibers in mdx mice is ~2,200.18,19,20 This was not the case for aged mdx mice used in this study, as in many instances the number of dystrophin-positive fibers following PMO delivery exceeded 2,500. Therefore, to gain a more meaningful estimate of the proportion of dystrophin-positive fibers in the mdx TA muscle, we performed an assessment of TA fiber number using perclecan immunostaining to clearly identify muscle fiber profiles and found that in 10-month-old mdx mice there was an average of 4,967 ± 158 (n = 5) fiber profiles. Representing the number of dystrophin-positive fibers following PMO treatment as a proportion of the total number revealed a range of ~18–65% in individual mdx mice, which could then be correlated with changes in physiological function.
Figure 1.
Dystrophin expression in tibialis anterior (TA) muscles of mdx mice following increasing doses of PMO. Dystrophin immunostaining in TA muscle sections from 10-month-old mdx mice, 4 weeks after an intramuscular injection of (a) saline, (b) 20 µl of PMO, and (c) 40 µl of PMO. In parentheses are the numbers of dystrophin-positive fibers in each of the TA sections. (d) Quantitative evaluation of dystrophin-positive fibers in mdx TA muscles following increasing volumes of PMO. The number of dystrophin-positive fibers in the contralateral muscles from some of the 40 and 60 µl PMO-treated mice (n = 5) show the expected 1–2% dystrophin-positive revertant fibers and no evidence of significant systemic transfer of PMO from the injected side. The error bars represent SEM. Bar = 500 µm. PMO, phosphorodiamidate morpholino oligomer.
Correlating the number dystrophin-positive fibers with muscle force in mdx mice
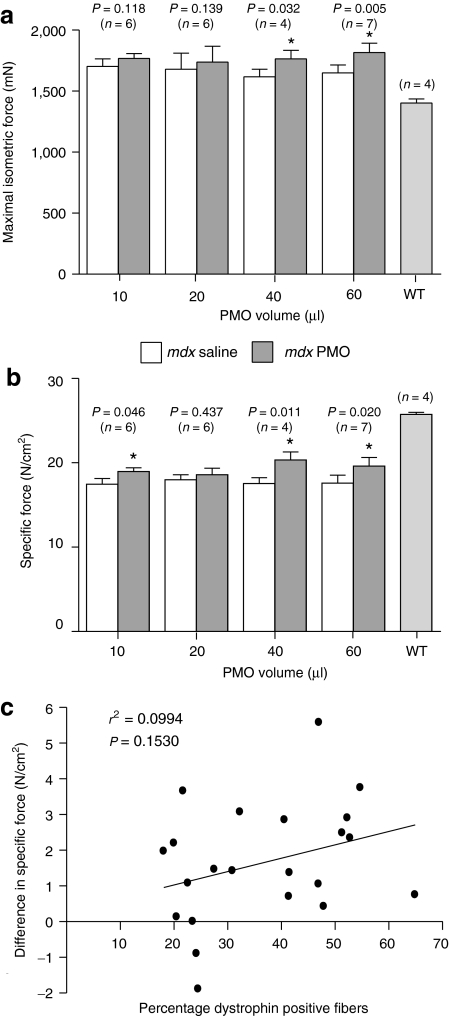
To examine whether PMO-induced dystrophin expression in muscles of mdx mice resulted in an improvement in physiological function, we measured the isometric force production of PMO-treated TA muscles compared with the saline-treated contralateral TA muscles. In addition, the force production of TA muscles from age-matched wild-type (C57/BL10) mice was also measured, which served as a reference for normal muscle function. Consistent with previous reports,21,22 the maximum isometric force of untreated mdx TA muscles was ~20% greater than that of wild-type mice (Figure 2a) which is likely to be a result of their greater muscle weights (Supplementary Figure S1). Four weeks after PMO treatment, TA muscles of mdx mice injected with 40 and 60 µl of PMO generated greater forces than the untreated contralateral control muscles. However, this improvement was not found following delivery of 10 and 20 µl of PMO (Figure 2a). When values of maximal isometric force were normalized for cross-sectional area of the TA, the specific force was ~29% lower in untreated mdx muscle compared to wild-type controls (Figure 2b). Injecting a number of different volumes of PMO (10, 40, and 60 µl) significantly improved the specific force generation of TA muscles compared to their contralateral control muscles, although these forces were distinctly lower than the TA muscles of age-matched wild-type mice (Figure 2b). Next, we examined whether there was a relationship between the percentage of dystrophin-positive fibers induced by PMO delivery and improvements in specific force generation. Linear regression analysis revealed that there was no significant correlation between the percentage of dystrophin-positive fibers and improvements in specific force, 4 weeks following intramuscular delivery of PMO (Figure 2c).
Figure 2.
The effect of increasing doses of PMO on force generation in aged mdx mice. The left tibialis anterior (TA) muscles of aged mdx mice (9 months old) were injected with either: 10, 20, 40, or 60 µl of PMO (1 µg/µl), and the right TA injected with matched volumes of saline, which served as the contralateral control. One month following treatment the isometric tetanic forces of both treated and untreated TA muscles were examined in vivo. (a) Absolute isometric muscle forces following increasing volumes of PMO. Force generation of TA muscles from age-matched wild-type (WT) mice are also shown. (b) Normalized TA forces (N/cm2) in both treated and untreated TA muscles. *Significant difference in isometric force generation between PMO-treated TA muscles and the contralateral control (P < 0.05). All error bars represent SEM. (c) Linear regression analysis showed no significant correlation between percentage of dystrophin-positive fibers and differences between treated and contralateral controls in specific force generation in TA muscles of mdx mice. PMO, phosphorodiamidate morpholino oligomer.
Correlating the number of dystrophin-positive fibers with resistance to contraction-induced injury in mdx mice
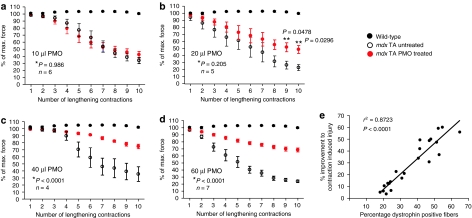
A primary functional weakness of muscles in mdx mice is an increased susceptibility to contraction-induced stress, which manifests as progressive deficits in force generation over successive stress-inducing lengthening contractions. This functional assay is widely regarded as the most meaningful method to evaluate the efficacy of a potential DMD therapy,21,22,23,24 but surprisingly has not been used to examine the functional benefits of PMO treatment in mdx mice. Therefore, we investigated the ability of PMOs to improve the resistance of mdx TA muscles to lengthening contractions and moreover, the relationship between the levels of dystrophin expression and the extent of functional improvement. We established that TA muscles of aged mdx mice undergo a progressive loss of force following a series of lengthening contractions, resulting in an overall force deficit of ~75% when normalized to the initial maximum contraction force (Figure 3a–d). In contrast, the TA muscles of wild-type mice were completely resistant to this level of contraction-induced stress. Treating mdx TA muscles with 10 or 20 µl of PMO did not result in a significant amelioration of force deficits over the complete series of lengthening contractions compared to the contralateral control muscles (Figure 3a,b; NS, *GLM repeated measures analysis of variance), although an improvement was observed after the 9th and 10th lengthening contraction cycle following delivery of 20 µl of PMO (Figure 3b; P < 0.05, **Student's paired t-test). Treatment with either 40 or 60 µl of PMO provided a substantial improvement in the resistance to lengthening contraction–induced injury, resulting in the treated mdx TA muscles maintaining ~75% of the their maximum force capacity compared to only ~25% in the contralateral control muscles (Figure 3c,d; P < 0.05, GLM repeated measures analysis of variance). Further analysis of these data revealed that there was a highly significant correlation between the percentage of dystrophin-positive fibers in PMO-treated mdx TA muscles and the improvement in resistance to lengthening contractions (Figure 3e).
Figure 3.
The effect of increasing doses of PMO on resistance to contraction-induced injury in aged mdx mice. Four weeks after intramuscular delivery of PMO, both the treated and the contralateral control TA muscles of mdx mice underwent a lengthening contraction protocol to determine their susceptibility to mechanical stress. The extent of force drop over successive lengthening contractions was measured and is presented as the percentage of the initial maximum force. Both (a) 10 µl and (b) 20 µl intramuscular injections failed to significantly improve the muscles resistance over 10 eccentric contractions (*GLM repeated measures analysis of variance). However, following delivery of 20 µl of PMO, there was significant protection following the 9th and 10th lengthening contraction (**Student's t-test). Following delivery of (c) 40 µl and (d) 60 µl of PMO, there was a sustained and highly significant improvement in resistance to mechanical stress. (a–d) As a reference, all graphs show that TA muscles from wild-type were completely resistant to lengthening contractions. (e) Linear regression analysis showed a highly significant correlation between percentage of dystrophin-positive fibers and percentage improvement to contraction-induced injury. Percentage improvement to contraction-induced injury was determined by measuring the effect of 10 lengthening contractions on force generation (expressed as a percentage of initial maximum) in the treated TA muscles compared to the contralateral control TA muscles. PMO, phosphorodiamidate morpholino oligomer; TA, tibialis anterior.
Correlating total levels of dystrophin protein with functional improvements in mdx mice
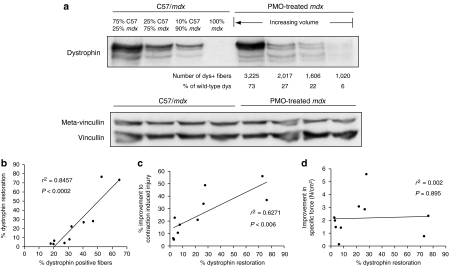
We also examined the levels of total dystrophin protein in mdx TA muscles following PMO delivery, to gain an insight into whether the proportion of dystrophin-positive fibers or the total levels of dystrophin protein are a more reliable predictor of functional improvement. Figure 4a shows a representative western blot for dystrophin expression in TA muscles of both wild-type and treated mdx mice. We ran a series of wild-type control samples at a number of different percentages (10–75%), so that we could quantify as accurately as possible the dystrophin levels in PMO-treated muscle. Quantification of the dystrophin band intensity against wild-type controls revealed a large range of dystrophin protein levels from 6% up to 75% of normal levels (Figure 4a), which reflects the large range of dystrophin-positive fibers present in PMO-treated TA muscles. A number of dystrophin bands were detected in both wild-type and treated mdx muscle samples, which are possibly due to sample preparation.25,26 As expected there was a significant positive correlation between total dystrophin expression and the percentage of dystrophin-positive fibers in PMO-treated mdx TA muscles (Figure 4b). Further regression analysis revealed that there was also a significant correlation between levels of total dystrophin protein and improvements in resistance to contraction-induced injury (Figure 4c). In contrast, there was no correlation with improvement in specific force (Figure 4d). Although these correlations are in agreement with those comparing the percentage of dystrophin-positive fibers with functional improvements, assessing the correlation data collectively, it appears that the percentage of dystrophin-positive fibers is a better predictor of improvements in muscle strength.
Figure 4.
Quantification of total dystrophin protein in PMO-treated mdx tibialis anterior (TA) muscles and correlations with functional characteristics. (a) Western blot analysis of total dystrophin protein in TA muscles of mdx mice, 4 weeks after intramuscular injections of various volumes of PMO. The number of dystrophin-positive fibers in the treated mdx TA muscle is also given, as well as the corresponding levels of dystrophin relative to wild-type (C57/BL10). A series of wild-type controls were run in parallel with the treated mdx TA muscles in which a mixture of mdx and wild-type muscle was used to maintain equal loading of protein. Western blots were simultaneously probed with a monoclonal antibody recognizing metavincullin (upper band) and vincullin (lower band), which was used as a loading control. (b) Linear regression analysis between the percentage of dystrophin-positive fibers and the levels of total dystrophin protein in PMO-treated mdx TA muscle. (c) Linear regression analysis between the levels of dystrophin protein and percentage improvement in resistance to lengthening contractions of treated mdx TA muscles. (d) Linear regression analysis between the levels of dystrophin protein and improvement in specific force generation of treated mdx TA muscles. dys, dystrophin; PMO, phosphorodiamidate morpholino oligomer.
Discussion
Numerous preclinical investigations have provided convincing evidence for the therapeutic potential of AO-mediated exon-skipping therapy for DMD. Studies in mdx mice have shown that PMO administration is able to induce the specific removal of exon 23 and restore apparently functional levels of dystrophin expression.12,16,17 Two independent clinical trials in the Netherlands and the United Kingdom have also recently demonstrated safety and efficiency of this therapeutic approach after intramuscular injections of 2′OMePS (PRO051) and PMO (AVI-4658) AOs in DMD patients.27,28 In the Dutch trial, which used the 2'OMePS chemistry, treated patients produced levels of dystrophin in biopsy samples that were up to 35% of healthy control muscle.27 In the UK trial using the PMO AO, similar results were achieved with a number of patients expressing dystrophin up to 42% of normal levels of dystrophin-positive fibers.28 Both groups have recently undertaken systemic delivery trials in DMD patients. In preclinical models, the efficiency of systemic delivery with PMO AOs can be enhanced by the addition of cell-penetrating peptides or additional modifications.26,29,30 However, these modified PMOs have a poorly understood toxicity31 and have yet to be used in man, so we conducted our study with the standard unmodified PMO used in clinical trials to date.
An important question that is often raised by these studies is how much dystrophin is necessary to protect the muscle of DMD patients from further degeneration and to obtain clinical efficacy. This question can be further broken down into what percentage of dystrophin-positive fibers in a given dystrophic muscle is necessary for functional improvement and within those fibers, is there a threshold level of dystrophin expression required for membrane stability. This study directly addresses these key questions in the mdx mouse model of DMD. Moreover, we conducted these experiments in aged mdx mice, which display a more extensive dystrophic phenotype than observed in young mdx mice, as previously demonstrated by an increase in the proportion of fibers with abnormal morphology and a much greater vulnerability to contraction-induced injury.23,32 Indeed, consistent with these findings, our study showed that the number of fiber profiles was considerably greater than studies using younger mdx mice12,19,20 and is most likely the consequence of extensive fiber splitting, as previously recorded in the mdx23,32,33 rather than any true hyperplasia. In order to achieve a widespread variation in dystrophin levels, we injected the TA muscles of mdx mice with different volumes of PMO. We found that the proportion of dystrophin-positive fibers in TA muscles of mdx mice ranged from 18 to 65%. Importantly, following intramuscular delivery of 10, 20, 40, and 60 µl of PMO, the restored dystrophin was present throughout the longitudinal plane of the muscle and not purely localized at the site of injection. Furthermore, the larger the volume injected, the greater the spread in the transverse plane (Figure 1b,c). These observations were also supported by analysis of total protein in treated muscles, which revealed a strong correlation between the proportion of dystrophin-positive fibers and the percentage of dystrophin protein compared to wild-type controls (Figure 4b). The number of dystrophin-positive fibers in the contralateral muscles from some of the 40 and 60 µl PMO-treated mice (n = 5) showed the expected 1–2% dystrophin-positive revertant fibers typical of the mdx mouse;34 thus there was no evidence of significant systemic transfer of PMO from the injected side, as expected given the relatively low efficiency of systemic delivery of naked PMO (Figure 1d). Functional analysis demonstrated a highly significant correlation between the number of dystrophin-positive fibers and the resistance to lengthening contractions, suggesting that sufficient levels of dystrophin are expressed to restore sarcolemmal integrity. Furthermore, these results indicate that >1,000 dystrophin-positive fibers (~20% of total number) are required to generate a meaningful improvement in protection against contraction-induced injury. Surprisingly, we did not find a positive correlation between dystrophin-positive fibers and improvements in specific muscle force in the absence of contraction-induced injury (Figure 2c). One possibility for this disparity is that force measurements in mdx mice are a relatively insensitive method for evaluating functional changes associated with restored dystrophin expression. This is likely to be due to the robust regenerative capacity of mdx mice, which results in extensive hypertrophy, an increase in force-generating capacity, and only a 20–30% decrease in specific force (Figure 2a,b).21 Expressing dystrophin in muscle fibers of aged mdx mice is, therefore, unlikely to have a large impact on force generation, as a significant correction of muscle force is largely dependent on preventing recurrent phases of degeneration/regeneration, which will significantly reduce the hypertrophic response and stabilize muscle weights. Together, our functional data suggest that the most robust predictor of therapeutic efficacy is protection from contraction-induced injury. Furthermore, our study demonstrates that the de novo dystrophin produced following AO-mediated exon skipping in mdx mice is able improve resistance to mechanical stress in proportion to the percentage of dystrophin-positive fibers.
Functional improvements were also associated with the levels of dystrophin as determined by western blot analysis (Figure 4c). The strength of this association, however, was not as significant as the correlation between percentage of dystrophin-positive fibers and functional protection (Figure 3e). Indeed, in some cases, a relatively low level of dystrophin expression was able to achieve much greater protection than would be predicted. As a result, we can speculate that the therapeutic threshold of dystrophin expression in individual fibers is relatively low and thus inducing restoration in a large number of fibers but at relatively low levels should provide therapeutic benefit. This contention is supported by previous observations showing that 20–30% of endogenous dystrophin levels in transgenic mdx mice was able to dramatically reduce the dystrophic pathology35,36 and similar dystrophin concentrations in human patients was sufficient to avoid muscle weakness.37 Indeed, even lower levels of near full-length dystrophin expression in all fibers showed some functional benefit in the mdx3cv mouse.38 This study furthers these therapeutic implications because it clearly demonstrates that restoring dystrophin expression in muscle that has an established dystrophic state (i.e., aged mdx mice) is still able to achieve significant functional improvements even at relatively low expression levels.
Intravenous treatment has the potential to deliver a more uniform expression of dystrophin after antisense treatment, especially with the peptide linked PMOs (P-PMO). Therefore, an interesting avenue of further investigation would be to examine the physiological improvements over a range of systemic doses. However, intravenous delivery with naked PMOs yields patchy expression of dystrophin, with only 10–20% of normal levels found in TA muscles after 7 weekly injections16 and thus may not add additional insight to the existing data. We will be assessing the physiological effects of P-PMOs, as they have been shown to yield higher dystrophin levels that are more likely to have a significant functional impact.19,26,29,30
This study underscores the importance of assessing the extent of dystrophin expression following AO treatment in terms of the percentage of dystrophin-positive fibers and the levels of expression within fibers. The use of techniques such as quantitative imaging39 will be important for the latter assessment. These data also demonstrate that changes in muscle force are not a reliable indicator of the protection against exercise-associated damage achieved by treatment and this needs to be considered when setting outcome measures in DMD clinical trials.
Materials and Methods
Animals and intramuscular injection of PMO. All animal experiments were carried under license from the Home Office (UK) in accordance with The Animals (Scientific Procedures) Act 1986 and Imperial College guidelines. Aged female mdx mice (9 months old) were used in this study. Mdx mice were maintained as homozygous/hemizygous colonies (where all X-chromosomes carry the gene mutation and thus all females and males are affected). Under fentanyl/fluanisone (Hypnorm; Vetapharma, Leeds, UK) and midazolam (Hypnovel; Roche, Welwyn Garden City, UK) anesthesia, a single TA muscle from each experimental mdx mouse was injected with PMO (1 µg/µl) at various volumes (10, 20, 40, and 60 µl) using a 29-gauge needle (Becton Dickinson, Oxford, UK). The contralateral TA muscle was injected with sterile saline, which served as an internal control. The PMO (M23D +7–18) anneals to the last 7 nucleotides of mouse dystrophin exon 23 and the first 18 nucleotides of intron 23 and has been described previously.13 The PMO was supplied by Gene Tools (Philomath, OR) and suspended in sterile normal saline for intramuscular administration.
In vivo force measurements. This procedure was adapted from standard protocols24,40 and has been previously described.41 Muscle physiology recordings were conducted 4–6 weeks after PMO delivery. Mice were deeply anesthetized as described above and were carefully monitored throughout the experiment to ensure that there was no reflex response to toe pinch. The distal tendon of the TA muscle was dissected from surrounding tissue and tied with 4.0 braided surgical silk (Interfocus, Cambridge, UK). The sciatic nerve was exposed and superfluous branches axotomized, leaving the TA motor innervation via the common peroneal nerve intact. The mouse was placed on a thermopad (Harvard Apparatus, Edenbridge, UK) to maintain body temperature at 37 °C. The foot was secured to a platform and the ankle and knee immobilized using stainless steel pins. The TA tendon was attached to the lever arm of a 305B dual-mode servomotor transducer (Aurora Scientific, Aurora, Ontario, Canada) via a custom made steel s-hook.
TA muscle contractions were elicited by stimulating the distal part of common peroneal nerve via bipolar platinum electrodes, using supramaximal square-wave pulses of 0.02 ms (701A stimulator; Aurora Scientific). Data acquisition and control of the servomotors were conducted using a Lab-View-based DMC program (Dynamic muscle control and Data Acquisition; Aurora Scientific). Optimal muscle length (Lo) was determined by incrementally stretching the muscle using micromanipulators until the maximum isometric twitch force was achieved. Maximum isometric tetanic force (Po) was determined from the plateau of the force–frequency relationship following a series of stimulations at 10, 30, 40, 50, 80, 100, 120, and 150 Hz. A 1-minute rest period was allowed between each tetanic contraction. Muscle length was measured using digital calipers based on well-defined anatomical landmarks near the knee and the ankle. The specific force (N/cm2) was calculated by dividing Po by TA muscle cross-sectional area. Overall cross-sectional area was estimated using the following formula: muscle weight (g)/[TA fiber length (Lf; cm) × 1.06 (g/cm3)].
Lengthening contraction protocol. After establishing the force–frequency relationship, the susceptibility of TA muscles to contraction-induced injury was assessed. This consisted of stimulating the muscle at 150 Hz for 700 ms. After 500 ms of stimulation the muscle was lengthened by 10% of Lo at a velocity of 0.5 Lo s/1. At the end of the stimulation, the muscle was returned to Lo at a rate of −0.5 Lo s/1. The stimulation–stretch cycle was repeated every 3 minutes for a total of 10 cycles. The rest time between each cycle limited the potential for developing muscle fatigue. Maximum isometric force was measured after each lengthening contraction and expressed as a percentage of the initial maximum isometric force. At the end of the experiment, the muscles were excised, weighed, and prepared for histological analysis.
Immunohistochemistry. Muscle samples were snap-frozen in isopentane cooled in liquid nitrogen and stored at −70 °C. Transverse muscle sections (10 µm) were cut from the entire length of the TA muscle, with serial sections mounted onto glass slides at 300-µm intervals, and the intervening sections collected for western blot analysis. Unfixed muscle sections were immunostained for dystrophin (1:500; DysC3750)42 and in some cases perlecan (1:1,000, MAB1948; Chemicon, Chandlers Ford, UK). Before incubation with the primary antibodies, the slides were air dried, endogenous biotin blocked, using an avidin/biotin blocking kit (Vector Laboratories, Peterborough, UK) and to reduce nonspecific binding of the primary antibody sections were treated in a 3% nonfat milk solution diluted in phosphate-buffered saline/0.05% Tween-20. Sections were incubated for 1 hour in the primary antibody solution followed by a 2-hour incubation in an appropriate biotinylated secondary antibody (Dako, Ely, UK) and visualized using peroxidase detection (Vectastain ABC kit; Vector Laboratories). Sections were dehydrated in graded alcohols, cleared in xylene, and coverslipped with DPX (VWR, Lutterworth, UK). Muscle sections were scanned using a QiCAM digital camera fitted to an E50i Nikon microscope with a motorized stage using the ×10 objective. Image Pro Plus 5.1 software (Media Cybernetics, Marlow, UK) was used to reconstruct the entire muscle section at a high resolution.
Protein extraction and western blot analysis. Collected muscle sections were lysed with 150 µl protein extraction buffer containing 1% NP40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and protease inhibitors (RIPA buffer). After a 5-minute centrifugation at 13,000 r.p.m., the supernatant was collected and the protein concentration measured using a colorimetric assay (Biorad DC protein assay kit; Bioad, Hemel Hempstead, UK). Samples containing 100 µg of protein were solubilized in 10% sodium dodecyl sulfate sample buffer, boiled for 3 minutes, and fractionated on a 6% polyacrylamide gel as described previously.35,43 Proteins were transferred to polyvinylidene fluoride membranes, incubated with a 5% nonfat milk solution in phosphate-buffered saline/0.05% Tween-20 and probed with a monoclonal antibody against dystrophin (NCL-DYS1; Novocastra, Newcastle, UK) at a dilution of 1:200 in phosphate-buffered saline/0.05% Tween-20. All membranes were simultaneously probed with a monoclonal antibody against vinculin (1:100,000, hVIN-1; Sigma, Poole, UK). This was chosen as a loading control as vinculin occupies a similar subsarcolemmal position to dystrophin. Secondary antibody goat anti-mouse IgG conjugated to horseradish peroxidase (BioRad) was used at a dilution of 1:5,000. Antibodies were detected by enhanced chemiluminescence (ECL; Amersham Biosciences, Chalfont St Giles, UK). The blots were exposed to X-ray film (GRI, Braintree, UK) and developed using an automatic X-ray film processor (Processor X-ograph Imaging Systems, Malmesbury, UK). Densitometric quantification of band intensity was measured using Image J software (National Institute of Mental Health, Bethesda, MD).
SUPPLEMENTARY MATERIAL Figure S1. The effect of delivering increasing doses of PMO on TA muscle weight in aged mdx mice.
Acknowledgments
This study was funded by The Department of Health, the Muscular Dystrophy Campaign, Parent Project Muscular Dystrophy and the Big Lottery Fund.
Supplementary Material
The effect of delivering increasing doses of PMO on TA muscle weight in aged mdx mice.
REFERENCES
- Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM., and, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C., and, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH., Jr, and, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP., and, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., and, Campbell KP. Dystrophin-associated proteins are greatly reduced in skeletal muscle from mdx mice. J Cell Biol. 1991;115:1685–1694. doi: 10.1083/jcb.115.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM., and, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Wong S., and, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid MB., and, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H., and, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA., and, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- Gebski BL, Mann CJ, Fletcher S., and, Wilton SD. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet. 2003;12:1801–1811. doi: 10.1093/hmg/ddg196. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD., and, Wilton SD. Dystrophin expression in the mdx mouse after localised and systemic administration of a morpholino antisense oligonucleotide. J Gene Med. 2006;8:207–216. doi: 10.1002/jgm.838. [DOI] [PubMed] [Google Scholar]
- Heemskerk HA, de Winter CL, de Kimpe SJ, van Kuik-Romeijn P, Heuvelmans N, Platenburg GJ, et al. In vivo comparison of 2′-O-methyl phosphorothioate and morpholino antisense oligonucleotides for Duchenne muscular dystrophy exon skipping. J Gene Med. 2009;11:257–266. doi: 10.1002/jgm.1288. [DOI] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Malerba A, Thorogood FC, Dickson G., and, Graham IR. Dosing regimen has a significant impact on the efficiency of morpholino oligomer-induced exon skipping in mdx mice. Hum Gene Ther. 2009;20:955–965. doi: 10.1089/hum.2008.157. [DOI] [PubMed] [Google Scholar]
- Vitiello L, Bassi N, Campagnolo P, Zaccariotto E, Occhi G, Malerba A, et al. In vivo delivery of naked antisense oligos in aged mdx mice: analysis of dystrophin restoration in skeletal and cardiac muscle. Neuromuscul Disord. 2008;18:597–605. doi: 10.1016/j.nmd.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P, et al. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet. 2008;17:3909–3918. doi: 10.1093/hmg/ddn293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Schray RC, Sirsi SR., and, Lutz GJ. Nanopolymers improve delivery of exon skipping oligonucleotides and concomitant dystrophin expression in skeletal muscle of mdx mice. BMC Biotechnol. 2008;8:35. doi: 10.1186/1472-6750-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellorusso C, Crawford RW, Chamberlain JS., and, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- DelloRusso C, Scott JM, Hartigan-O'Connor D, Salvatori G, Barjot C, Robinson AS, et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci USA. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Head SI., and, Morley JW. Branched fibers in dystrophic mdx muscle are associated with a loss of force following lengthening contractions. Am J Physiol, Cell Physiol. 2007;293:C985–C992. doi: 10.1152/ajpcell.00128.2007. [DOI] [PubMed] [Google Scholar]
- Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS., and, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LV, Davison K, Falkous G, Harwood C, O'Donnell E, Slater CR, et al. Dystrophin in skeletal muscle. I. Western blot analysis using a monoclonal antibody. J Neurol Sci. 1989;94:125–136. doi: 10.1016/0022-510x(89)90223-2. [DOI] [PubMed] [Google Scholar]
- Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S, et al. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Betts C, Seow Y, Boutilier J, Iverson PL, et al. A fusion peptide directs enhanced systemic dystrophin exon skipping and functional restoration in dystrophin-deficient mdx mice. Hum Mol Genet. 2009;18:4405–4414. doi: 10.1093/hmg/ddp395. [DOI] [PubMed] [Google Scholar]
- Wu B, Li Y, Morcos PA, Doran TJ, Lu P., and, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton HM., and, Moulton JD.2010Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy Biochim Biophys Actaepub ahead of print). [DOI] [PubMed]
- Head SI, Williams DA., and, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci. 1992;248:163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Lovering RM, Michaelson L., and, Ward CW. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am J Physiol, Cell Physiol. 2009;297:C571–C580. doi: 10.1152/ajpcell.00087.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danko I, Chapman V., and, Wolff JA. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992;32:128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Wells DJ, Wells KE, Asante EA, Turner G, Sunada Y, Campbell KP, et al. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum Mol Genet. 1995;4:1245–1250. doi: 10.1093/hmg/4.8.1245. [DOI] [PubMed] [Google Scholar]
- Phelps SF, Hauser MA, Cole NM, Rafael JA, Hinkle RT, Faulkner JA, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- Neri M, Torelli S, Brown S, Ugo I, Sabatelli P, Merlini L, et al. Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul Disord. 2007;17:913–918. doi: 10.1016/j.nmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Li D, Yue Y., and, Duan D. Preservation of muscle force in Mdx3cv mice correlates with low-level expression of a near full-length dystrophin protein. Am J Pathol. 2008;172:1332–1341. doi: 10.2353/ajpath.2008.071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechavala-Gomeza V, Kinali M, Feng L, Brown SC, Sewry C, Morgan JE, et al. Immunohistological intensity measurements as a tool to assess sarcolemma-associated protein expression. Neuropathol Appl Neurobiol. 2010;36:265–274. doi: 10.1111/j.1365-2990.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- Sharp PS, Dick JR., and, Greensmith L. The effect of peripheral nerve injury on disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neuroscience. 2005;130:897–910. doi: 10.1016/j.neuroscience.2004.09.069. [DOI] [PubMed] [Google Scholar]
- Foster H, Sharp PS, Athanasopoulos T, Trollet C, Graham IR, Foster K, et al. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther. 2008;16:1825–1832. doi: 10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
- Gollins H, McMahon J, Wells KE., and, Wells DJ. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther. 2003;10:504–512. doi: 10.1038/sj.gt.3301927. [DOI] [PubMed] [Google Scholar]
- Wells DJ, Wells KE, Walsh FS, Davies KE, Goldspink G, Love DR, et al. Human dystrophin expression corrects the myopathic phenotype in transgenic mdx mice. Hum Mol Genet. 1992;1:35–40. doi: 10.1093/hmg/1.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of delivering increasing doses of PMO on TA muscle weight in aged mdx mice.