Abstract
Despite intense efforts to develop treatments against pancreatic cancer, agents that cure this highly resistant and metastasizing disease are not available. Considerable attention has focused on broccoli compound sulforaphane (SF), which is suggested as combination therapy for targeting of pancreatic cancer stem cells (CSCs). However, there are concerns that antioxidative properties of SF may interfere with cytotoxic drugs—as suggested, e.g., for vitamins. Therefore we investigated a combination therapy using established pancreatic CSCs. Although cisplatin (CIS), gemcitabine (GEM), doxorubicin, 5-flurouracil, or SF effectively induced apoptosis and prevented viability, combination of a drug with SF increased toxicity. Similarly, SF potentiated the drug effect in established prostate CSCs revealing that SF enhances drug cytotoxicity also in other tumor entities. Most importantly, combined treatment intensified inhibition of clonogenicity and spheroid formation and aldehyde dehydrogenase 1 (ALDH1) activity along with Notch-1 and c-Rel expression indicating that CSC characteristics are targeted. In vivo, combination treatment was most effective and totally abolished growth of CSC xenografts and tumor-initiating potential. No pronounced side effects were observed in normal cells or mice. Our data suggest that SF increases the effectiveness of various cytotoxic drugs against CSCs without inducing additional toxicity in mice.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death for both men and women in the United States.1 In half of patients with pancreatic adenocarcinoma, metastasis is detectable already at the time of diagnosis. Only in 20% of patients surgical resection is possible and the prognosis is poor. Over the last years increasing evidence points to the possibility that cancer may be based on a stem cell disease.2 Cancer stem cells (CSCs) might be responsible for tumor initiation, growth, metastasis, and relapse of pancreatic cancer after treatment. Like their normal counterparts, putative CSCs show remarkable resistance to radiation and chemotherapy.3 Markers for CSCs have been identified in different tumor entities including pancreatic cancer4,5 and their presence correlates to the extreme aggressiveness of this malignancy. Our recent data and results of other researchers demonstrated that long-term treatment with gemcitabine (GEM) as a standard cytotoxic therapy leads to an enrichment of pancreatic CSCs associated with enhanced therapy resistance.6,7 Thus considerable attention has focused on defining new therapeutic agents for targeted elimination of pancreatic CSCs. Recently, we identified the plant compound isothiocyanate sulforaphane (SF), present in high concentration in broccoli and other cruciferous vegetables, as effective experimental therapeutic to sensitize pancreatic CSCs to TRAIL (tumor necrosis factor–related apoptosis inducing ligand)-induced apoptosis.6 SF sensitized established pancreatic CSC lines by inhibiting binding of transactivation competent NF-κB complexes. Importantly, SF was nontoxic to normal cells such as human fibroblasts and mesenchymal stromal cells and had no side effects on nude mice. Thus, SF combination with chemotherapy may be a more effective therapeutic strategy for pancreatic cancer. However, SF protects, e.g., from DNA damage and induces the expression of phase 2 metabolism enzymes. This leads to a cytoprotection against the toxicity of electrophiles and reactive oxygen species.8,9 Because radiotherapy and many chemotherapeutic agents act by producing free radicals, combination with SF may be counterproductive. There are concerns that SF prevents oxidative damage and thereby lowers the effectiveness of cytotoxic therapy.
In the present work, we investigated for the first time the effect of combined SF treatment with chemotherapy using an established pancreatic CSC line. Combination of SF with different cytotoxic drugs had an additive effect and strongly increased cell death and eliminated CSC characteristics including tumor-initiating potential, clonogenicity, spheroidal growth, and aldehyde dehydrogenase 1 (ALDH1) activity. Most importantly, we confirmed the enhanced anti-CSC effect of SF combination therapy in established prostate CSCs, suggesting that SF combination with cytotoxic therapy may be effective in several tumor entities.
Results
SF increases drug effects toward pancreatic CSCs
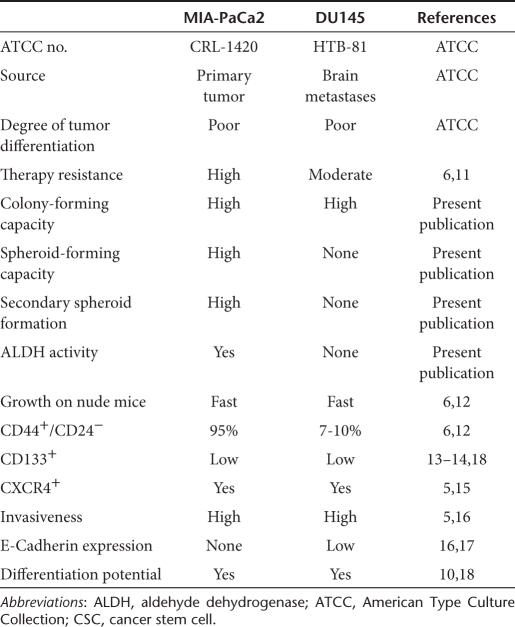
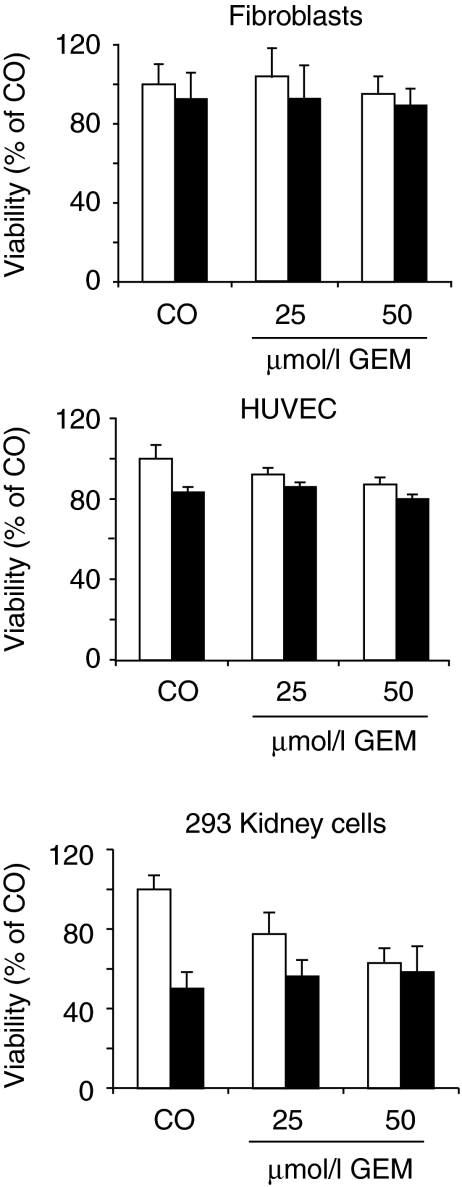
To test whether SF increases chemotherapy-mediated inhibition of tumor cell viability, the established pancreatic CSC cell line MIA-PaCa2 was evaluated. As summarized in Table 1, this cell line rapidly grows upon injection in nude mice, has a very invasive growth pattern, along with self-renewal potential, ALDH1 activity, differentiation potential, high apoptosis resistance, a characteristic surface expression of CSC markers (CD44+/CD24−, EpCAM+, CD133+, CXCR4+) and no E-Cadherin expression.5,6,10 Furthermore, we recently demonstrated that the CD44+/CD24− population is most likely responsible for tumorigenic potential6 by comparing highly resistant MIA-PaCa2 cells (95% CD44+/CD24−; 37% ALDH1 activity; colony- and spheroid-forming potential) with sensitive pancreatic BxPc-3 cells (17% CD44+/CD24−; 0.3% ALDH1 activity; no colony- and spheroid-forming potential). We found that 103 MIA-PaCa2 formed much faster tumors on mice compared to BxPc-3 cells. Also, continuous treatment of BxPc-3 cells with GEM led to enrichment of chemoresistant cells along with enrichment of the CD44+/CD24+ population to 42%. Because MIA-PaCa2 cells contain already 95% of a CD44+/CD24+ population associated with all characteristics of tumorigenicity, we did not further enrich CD44+/CD24+ cells but refer in the following these cells as CSChigh cells. CSChigh cells were treated with SF or cisplatin (CIS), GEM, doxorubicin and 5-flurouracil alone or in combination and 72 hours later viability was analyzed by morphological inspection and MTT assay. Although combination of SF with CIS, doxorubicin, or GEM targeted 60% of the tumor cells, co-treatment with SF and 5-flurouracil was most effective and targeted even 80% of the cell population (Figure 1a). As GEM is the standard chemotherapy for treatment of pancreatic cancer, the therapeutic potential of GEM and SF co-treatment was further evaluated in detail. For evaluation of the clonogenic potential, CSChigh cells were treated with SF or GEM alone or in combination. GEM was administered at a concentration of 5 nmol/l based on dose–response experiments demonstrating that this concentration is a sublethal dose (data not shown). Seventy-two hours after treatment, surviving cells were re-seeded. Although GEM reduced clonogenicity to 90%, SF prevented colony formation in 50% compared to untreated controls (Figure 1b). Combination of GEM with SF showed an additive effect and reduced colony formation to 35%. To examine apoptosis resistance, pancreatic CSChigh cells were treated with SF or GEM alone or with both agents together. Seventy-two hours later early apoptosis was investigated by staining with annexin V followed by flow cytometry. Single treatment with GEM induced apoptosis in 30% of cells (Figure 1c). Combined treatment with SF and GEM increased apoptosis up to 40%. Similarly, combined treatment with SF and CIS was also significantly more effective than each agent alone. In conclusion, SF enhances the therapeutic effect of cytotoxic drugs toward the CSC characteristics clonogenicity and apoptosis resistance in pancreatic CSChigh cells.
Table 1. CSC characteristics of MIA-PaCa2 and DU145 cells.
Figure 1.
Sulforaphane (SF) increases drug-mediated effects on cell viability, clonogenicity and induction of apoptosis in pancreatic cancer stem cells (CSCs). (a) CSChigh pancreatic cancer cells were left untreated (CO), or were treated with SF (5 µmol/l), cisplatin (CIS), gemcitabine (GEM), doxorubicin (DOX) or 5-flurouracil (5-FU) in concentrations indicated alone or in combination with SF as indicated. Seventy-two hours later viability was determined by MTT assay (upper panel). Data are presented as mean ± SD (*P < 0.05 compared with treatment in the absence of SF). Representative pictures of cells were taken at ×100 magnification (lower panel). (b) To examine clonogenic cell division, CSChigh pancreatic cancer cells were seeded in 6-well tissue culture plates and treated with SF (5 µmol/l) and GEM (5 nmol/l) alone or in combination (SF+GEM). Seventy-two hours later cells were trypsinized and re-plated at low density in 6-well plates. Ten days later colonies containing >50 cells were counted under a dissecting Zeiss Stemi DV4 microscope. Data are presented as mean ± SD. Photographs of the fixed and stained colonies are presented on the left panel. (c) MIA-PaCa2 cells were seeded in 6-well tissue culture plates and treated similar to the previous MTT assay. Induction of apoptosis was evaluated by annexin V staining of the cells and flow cytometry. Induction of apoptosis is presented as percentage of annexin-positive cells. Data are presented as mean ± SD (*P < 0.05).
SF increases taxol-induced toxicity toward established prostate CSCs
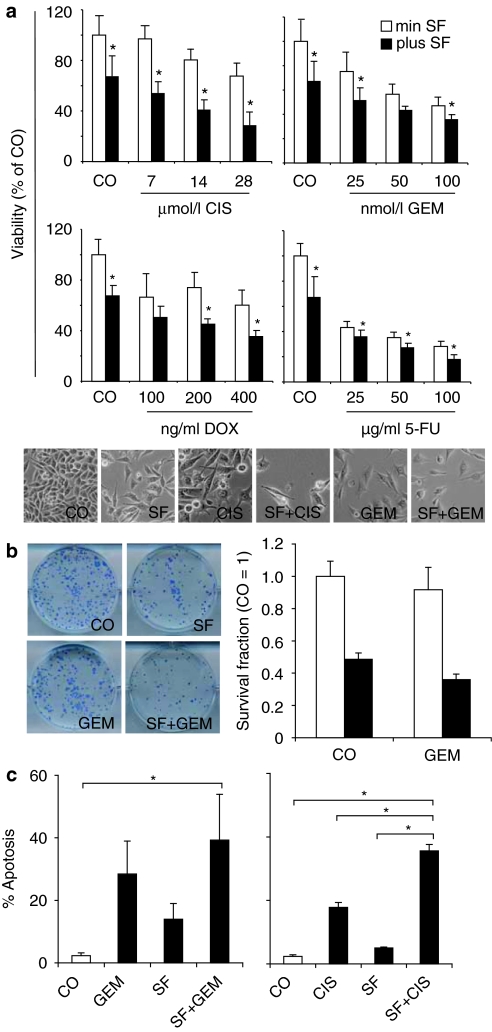
To evaluate whether SF may also potentiate the cytotoxicity of chemotherapy to CSC characteristics in other tumor entities, we used the prostate cancer cell line DU145, which comprises cells with CSC properties such as self-renewal, differentiation potential, high proliferative, tumorigenic and invasive potential, therapy resistance, low E-Cadherin expression, and a typical CSC marker expression (CD44+/α2β1/CD133+, CD44+/CD24−, CXCR4+) on the cell surface (Table 1).12,14,15,16,17,18 These cells were treated with taxol (TAX)—a standard chemotherapeutic drug for prostate cancer, alone or combined with SF. Likewise, CIS was tested alone or in combination with SF. Seventy-two hours after treatment viability was measured by MTT assay and evaluated by morphology. The presence of SF clearly potentiated CIS-mediated inhibition of viability (Figure 2a). Similar results were observed for low doses of TAX of 2.5 and 5 nmol/l, which are relevant in patients. However, a combination of SF with a high dose of TAX (10 nmol/l) did not further reduce the viability of CSChigh cells, but SF rather inhibited the TAX effect. We do not know the reason for this unexpected observation, which occurred repeatedly in our assays. However, since a dose of 10 nmol/l TAX is physiologically not relevant, this observation may be neglected, especially since combined treatment with TAX and SF in long-term treatment abrogated clonogenicity completely (Figure 2b). Similarly, combination of SF with TAX or SF with CIS significantly increased apoptosis compared to treatment with each agent alone (Figure 2c). In conclusion, SF and chemotherapy act in concert to inhibit viability and clonogenicity and to reduce apoptosis resistance in CSC-enriched DU145 prostate cancer cells.
Figure 2.
Sulforaphane (SF) increases chemotherapeutic drug effects in prostate cancer cells. (a) Prostate cancer cells DU145 were left untreated (CO) or were treated with SF (5 µmol/l) alone or in combination with taxol (TAX) or cisplatin (CIS) at doses indicated. Viability was determined 72 hours later as described above (*P < 0.05). Images of cells treated for 72 hours are shown in the lower panel. (b) DU145 cells were seeded in 6-well tissue culture plates and treated with SF (5 µmol/l) and TAX (5 nmol/l) alone or in combination. Seventy-two hours later, cells were trypsinized and re-plated at low density in 6-well plates. Ten days later colonies were stained with Coomassie blue and images of colonies were taken (left panel). Colonies containing >50 cells were counted under a dissecting Zeiss Stemi DV4 microscope and the amounts of the survival fractions are presented (right panel). (c) Prostate cancer cells were treated with SF, TAX, CUS or SF combined with a cytotoxic drug for 72 hours. Apoptosis induction was evaluated by annexin V staining and flow cytometry and is shown as percentage of annexin-positive cells. Data are presented as mean ± SD (*P < 0.05).
Cytotoxic effects of SF and drug combination are lower in normal cells
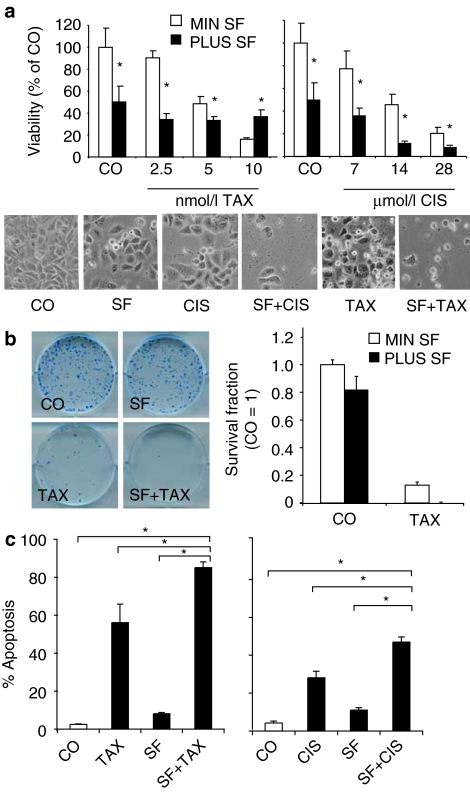
To examine whether a combination of SF with chemotherapeutic agents is toxic for normal cells, human primary skin fibroblasts, human umbilical vein endothelial cells and human embryonic kidney cells (293) were treated with SF, GEM, or both together. Seventy-two hours later, viability was evaluated by MTT assay (Figure 3). Although neither GEM nor SF alone nor the combination had significant toxic effects on nonmalignant fibroblasts and human umbilical vein endothelial cells, we observed a strong SF-induced toxicity in immortalized 293 cells, which increases GEM-mediated toxicity. These results suggest that normal mesenchymal or endothelial cells are not affected by SF, while viability of immortalized or malignant cells is reduced.
Figure 3.
Sulforaphane (SF) does not significantly increase cytotoxic drug effects to normal cells. Primary human fibroblasts, primary human umbilical vein endothelial cells (HUVECs) or human immortalized embryonic kidney (293) cells were treated with SF (5 µmol/l), gemcitabine (GEM) (5 nmol/l) or both together (SF+GEM). Viability was measured 72 hours later by MTT assay as described above.
SF increases chemotherapeutic effects to self-renewal and ALDH activity
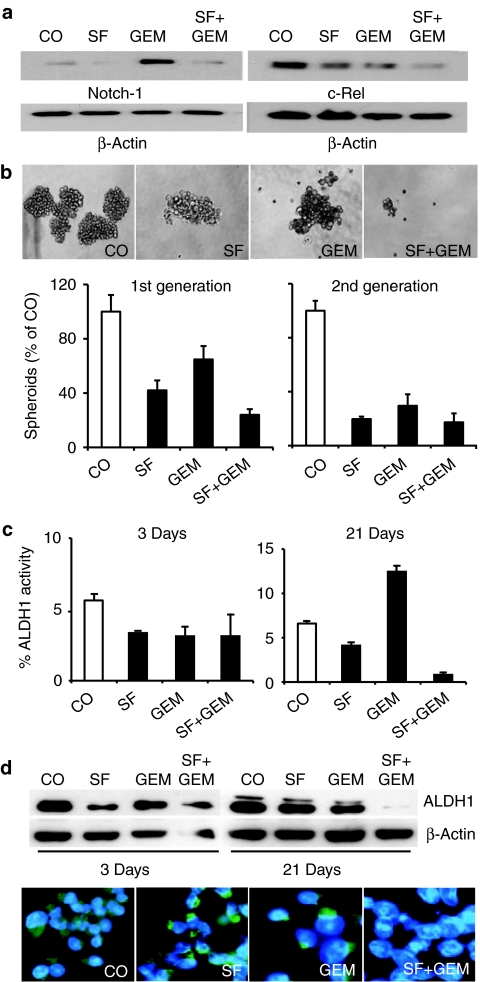
To analyze whether SF interferes with pathways involved in self-renewal, we evaluated its effects on Notch-1 expression, since the Notch signaling pathway plays an important role in stem cell self-renewal.19 Treatment of CSChigh cells for 48 hours with SF downregulated basal Notch-1 expression, while GEM led to strong induction of Notch-1 (Figure 4a). Importantly, combination treatment totally prevented the GEM-induced Notch-1 upregulation. Downregulation of Notch-1 was associated with downregulation of c-Rel expression, which was strongest for the combination treatment. Since c-Rel is a very transactivation-potent subunit of the NF-κB transcription factor, these results suggest that Notch-1 may be involved in SF-induced inhibition of NF-κB signaling, as we recently demonstrated6 and is according to recent findings demonstrating NOTCH-1-mediated regulation of the NF-κB pathway.20 To investigate direct effects to self-renewal, we treated spheroidal growing cells with SF or GEM alone or in combination. While single treatment with SF and to a less extent with GEM reduced spheroid formation, combined treatment inhibited spheroid formation most effectively (Figure 4b, pictures and 1st generation). Spheroid formation could be further reduced upon dissociation of the treated first-generation spheroids, re-plating equal amounts of live cells, followed by a second round of treatment of fully formed spheroids for 3 days (Figure 4b, 2nd generation). In these short-term assays the effectivity of GEM in reducing spheroid formation was similar to SF. Likewise, GEM or SF alone reduced ALDH1 activity, (Figure 4c, left panel), a marker associated with CSCs.21 In contrast, long-term treatment with GEM for 21 days led to a selection of ALDH1-active cells (Figure 4c, right panel), while SF alone reduced ALDH1-positive cells and prevented selection of this CSC population by GEM in short- and long-term assays. Similar results were obtained upon examination of ALDH1 expression by western blot analysis and immunofluorescence staining of cells followed by fluorescence microscopy (Figure 4d). In conclusion, SF increases reduction of self-renewal and ALDH1 activity by GEM.
Figure 4.
Sulforaphane (SF) enhances cytotoxic drug effects to pancreatic cancer stem cell (CSC) properties. (a) Cells were treated for 48 hours with SF (5 µmol/l) and gemcitabine (GEM) (25 nmol/l) or both agents together (SF+GEM). Expression of Notch-1 and c-Rel was evaluated by western blot analysis. (b) Pancreatic CSChigh cells were seeded at clonal density in low adhesion plates for spheroid formation. Twenty-four hours later cells were treated with SF (5 µmol/l), GEM (25 nmol/l), or both agents together (SF+GEM). Spheroids were photographed at day 7 under ×100 magnification or quantified (1st generation). Thereafter, 1st generation spheroids were dissociated to single cells and equal numbers of live cells pretreatment group were re-plated. Upon spheroid formation cells were treated as described above and 3 days later spheroid formation was quantified (2nd generation). (c) Pancreatic CSChigh were treated as described above. Three or 21 days later aldehyde dehydrogenase 1 (ALDH1) activity was analyzed by flow cytometry and the percentage of ALDH1-positive cells is presented. Data are presented as mean ± SD. (d) Likewise, proteins were harvested and expression of ALDH1 protein was analyzed by western blot. Expression of β-actin served as loading control. Lower panel: Twenty-one days after treatment cells were subjected to immunofluorescence analysis for ALDH1. Randomly chosen fields were examined under ×400 magnification using a Nikon Eclipse TS100 microscope and photographs were taken.
SF sensitizes CSC to cytotoxic therapy in vivo
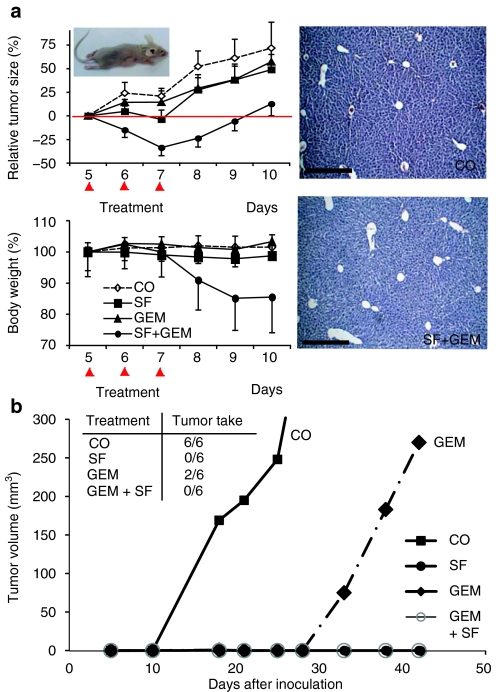
In order to address whether SF might influence sensitivity of CSC xenografts toward chemotherapy, we transplanted CSChigh pancreatic cancer cells subcutaneously into nude mice. Mice were left untreated or were treated with SF, GEM or both agents together and tumor growth was measured during a period of 10 days (Figure 5a, left upper panel). Administration of SF or GEM alone only marginally inhibited tumor growth, compared to untreated controls. Co-treatment with SF and GEM, however, had an additive effect on the inhibition of tumor growth compared to each single treatment. Combined treatment with SF and GEM reduced body weight about 15% (Figure 5a, left lower panel), while single treatments had no effect, indicating that the double treatment is stressful for the mice, but tolerated. Histology of livers from untreated mice or mice treated with SF and GEM combination revealed no necrotic areas, suggesting that combined treatment is not liver toxic (Figure 5a, right panel).
Figure 5.
Sulforaphane (SF) enhances gemcitabine (GEM)-mediated cytotoxic effects by prevention of tumor-initiating potential in mice. (a) Pancreatic CSChigh cells [4 ×106 cells in 200 µl phosphate-buffered saline (PBS)] were injected subcutaneously into nude mice. After the tumors had reached a mean diameter of 8–10 mm, SF, GEM or both agents together (SF+GEM) were administered at days 5, 6, and 7 after tumor cell implantation (indicated by red arrows). Control animals (CO) received PBS injections only. The tumor volume was measured daily (upper left panel). Body weight of each individual mouse was set to 100% before treatment. Mice were weighted daily and relative changes in body weight are shown (lower left panel). Data are presented as mean of 6 animals ± SEM (n = 6) (*P < 0.05 compared with control and single treatment). A representative H&E staining of liver tissue after three consecutive treatments with SF and GEM is shown (scale bar = 200 µm) (left panel). (b) Pancreatic CSChigh cells were pretreated with SF (5 µmol/l), GEM (25 nmol/l) or both together in vitro. Control cells (CO) were left untreated. Seventy-two hours later an equal amount (5.7 × 103) of live cells were transplanted subcutaneously with 50% Matrigel into the right anterior flank of 5–6 weeks old NMRI-nu (nu/nu) female mice, 6 mice per treatment group. External tumor size was measured using a caliper at time points indicated. The number of tumors, which start to re-grow in each group is indicated (tumor take). Data are presented as mean of six animals in the control group and as mean of two growing tumors in the GEM-treated group.
In order to investigate whether combination treatment affects the tumor initiating potential, CSChigh pancreatic cancer cells were pretreated in vitro with SF, GEM or both agents together. Seventy-two hours later, an equal number of live cells was transplanted into nude mice and tumor development was measured during 50 days (Figure 5b). After 10 days fast growing tumors were visible in mice transplanted with control cells. Twenty-eight days later mice transplanted with GEM-treated tumor cells developed tumors. In contrast, no tumor growth was observed in mice transplanted with SF-treated cells or cells treated with a combination of SF and GEM. These results suggest that GEM delays tumor growth by diminishing the number of differentiated tumor cells while sparing the highly resistant CSCs. In contrast, SF targets also the highly therapy resistant CSC population and thus completes the therapeutic effect of GEM.
Discussion
In the present work, we show that the phytochemical SF increases chemotherapeutic drug-mediated cytotoxicity in pancreatic CSCs in vitro and in vivo. Moreover, combined treatment with SF and GEM targeted CSC characteristics, e.g., self-renewal and ALDH1 and inhibited by this way relapse of tumor growth after treatment. Related observations were made in an established prostate CSC line suggesting that a combination of SF with chemotherapy is a promising strategy for enhancing the cytotoxic drug effects against CSCs in pancreatic cancer and other tumor entities.
Therapeutic efficacy of SF
In our previous study, we demonstrate that SF targets CSC-like pancreatic cancer cells and increases therapeutic effects of TRAIL, quercetin and sorafenib.6,17,10 Recent studies confirm that SF eliminates pancreatic and prostate cancer cells and those of other tumor entities such as breast and colon cancer cells.9,22,23,24,25,26,27,28,29,30 In the present study, SF increased chemotherapeutic drug-mediated cytotoxicity in pancreatic and prostate cancer cell lines. Correspondingly, SF enhanced radiosensitivity of HELA cervix carcinoma cells.31 Thus, our initial idea that the antioxidative properties of SF may lead to inhibition of therapeutic cytotoxicity does not prove true. The reason may be the more indirect antioxidative properties of SF, which are mediated through activation of phase 2 enzymes.8 Most importantly, SF exhibited no pronounced toxicity to nonmalignant cells like mesenchymal stromal cells and fibroblasts nor did it reduce body weight or mediate liver toxicity in mice as demonstrated in the present and our recent studies.6,17,10 To our knowledge, we are the first to demonstrate that SF potentiates the cytotoxic effect of GEM toward pancreatic CSCs. In detail, SF increased targeting of self-renewal activity in vitro. Our data suggest that this is most likely due to downregulation of Notch-1 and c-Rel expression. In long-term in vitro experiments, co-treatment with SF prevented GEM-induced selection of cells positive for the CSC-marker ALDH1. These features of SF may have been responsible for the observed blocking of re-growth of GEM-treated tumor cells upon transplantation into immunodeficient mice. However, in contrast to treatment with single agents, combination of GEM and SF led to a 15% reduction of body weight of mice. This indicates that combination treatment is stressful for the mice but tolerated, since no liver necrosis occurred. Based on the favorable toxicological profile of SF along with its other benefits in tumor cell elimination, Fimognari et al. suggested the design of innovative clinical studies to investigate whether SF co-administration can indeed enhance efficacy of cytotoxic drug-based regiments in the clinic.33
Molecular mechanism of SF-mediated targeting of CSCs
Mechanistically, we recently demonstrated that SF sensitizes pancreatic CSCs by downregulation of NF-κB binding activity.6,10 As exemplified in our present study, prevention of Notch-1 expression by SF correlates to inhibition of NF-κB and self-renewal activity in pancreatic CSCs. These results correspond to recent data demonstrating that Notch-1 is an upstream regulator of NF-κB and involved in stem cell resistance and self-renewal.19,34 Our data are confirmed by Wang et al.,32 who found a similar mechanism upon combination of dietary isoflavones with curcumin in inhibition of growth of pancreatic cancer cells. These authors showed that the inhibition of cell growth and induction of apoptosis was significantly higher the combination group than that could be achieved by either agent alone. These changes were associated with decreased Notch-1 expression and DNA binding activity of NF-κB and its target genes Cyclin D1, Bcl-2, and Bcl-xL.
In our previous work, we showed that GEM increases the percentage of CSC-marker positive cells in pancreatic cancer cells.6 Consistently, Mueller et al.7 and Jimeno et al.35 suggested that GEM preferentially targets more differentiated and rapidly proliferating tumor cells. In the present work we observed that long-term in vitro treatment with GEM results in an enrichment of ALDH1-positive pancreatic CSChigh cells in contrast to cells treated with SF alone or with a combination of SF and GEM. Likewise transplantation of GEM-treated pancreatic CSChigh cells to immunodeficient mice led to tumor re-growth. This is in contrast to transplantation of SF- or GEM and SF-treated CSChigh cells, where no relapse occurred. These findings correspond to recent suggestions that the GEM-resistant cell population refers to the tumorigenic and chemotherapy-resistant cell population.21,36,37 These findings suggest that SF abrogates the tumor-initiating potential of CSCs, which may be the reason for the observed increased therapeutic effect of GEM upon combination with SF. Another interesting compound for targeting tumor-initiating potential of CSCs is the Hedgehog inhibitor cyclopamine. Consistent with our results, Jimeno et al.35 showed, that cyclopamine co-treatment potentiated the antitumor effect of GEM in pancreatic cancer. A further promising strategy for targeting of CSCs is the induction of differentiation.38 A recent publication shows that SF mediates differentiation of human promyelocytic cells.39 These data suggest that SF may also be an interesting candidate for differentiation therapy of CSCs, although this point was not studied in the present work.
SF-mediated targeting of self-renewal of CSCs
The tumorigenic potential is associated with the self-renewal capacity of CSC,37 which initiates tumor growth and spread. The percentage of CSCs is usually measured by the ability to form tumorospheres, which grow in an anchorage-independent manner. Since sphere formation is directly proportional to the amount of self-renewing cells, this method is adequate to evaluate the effect of treatment on CSCs.37 In the present work, we could show that pancreatic CSChigh cell lines form spheroids in serial passages when cultured under conditions that favor proliferation of undifferentiated cells. Combined treatment with SF and GEM, however, depleted spheroid-forming capacity. Dysregulation of pathways involved in the process of self-renewal such as Notch-1 signaling is suggested to lead to uncontrolled self-renewal and to resistance toward chemotherapy.37 Therefore, SF-mediated downregulation of basal Notch-1 expression and prevention of GEM-induced Notch-1 expression might have contributed to resensitization of CSCs to chemotherapeutic drugs in our study. Interestingly, Hunakova et al. showed that in breast carcinoma cells SF significantly downregulates Twist1 and POU5F1, which are transcription factors that mediate epithelial mesenchymal transition and self-renewal of undifferentiated embryonic stem cells.40 Targeting of self-renewal is however not restricted to SF but seems to be characteristic for several other food ingredients. Kakarala et al. showed in sphere assays that the natural products curcumin and piperine target self-renewal in breast CSCs.41
Conclusions
In the present work, we demonstrate that SF does not diminish cytotoxic effects of drugs, but, in contrast, strongly increases their anticancer efficacy against pancreatic and prostate CSCs. Combination of SF with a cytotoxic drug efficiently induced apoptosis along with inhibition of self-renewing potential, ALDH1 activity, clonogenicity, xenograft growth and relapse of GEM-treated tumor cells in nude mice. Combination of SF with cytotoxic therapy may be of therapeutic benefit in clinical settings. Since our data and those of others suggest that SF potentiates the effect mediated by chemo- and radiotherapy in vitro and in animals, lower doses of these regimens might be successfully applied to patients under SF co-treatment.
Materials and Methods
Established cell lines. As models for pancreatic CSChigh cancer cells the established cell line MIA-PaCa2 and the prostate cancer cell line DU145 were used (compare Table 1). Both were obtained from the American Type Culture Collection (Manassas, VA). Primary skin fibroblasts were kindly provided by Dr. H.-J. Stark (DKFZ, Heidelberg, Germany). MIA-PaCa2, fibroblasts and human immortalized embryonic kidney (293), cells were cultured in Dulbecco's modified Eagle medium and DU145 cells in RPMI medium (PAA Laboratories, Pasching, Austria). Media were supplemented with 10% fetal calf serum (Sigma-Aldrich, St Louis, MO) and 10 mmol/l HEPES (PAA Laboratories, Pasching, Austria). Human umbilical vein endothelial cells (PromoCell, Heidelberg, Germany) were cultured in ready-to-use endothelial cell growth medium (PromoCell).
Cytotoxic agents. GEM (kind gift from Eli Lilly, Indianapolis, IN) was diluted in phosphate-buffered saline to a 50 µmol/l stock. Stock solutions of SF were prepared in ethanol. Doxorubicin (Sigma, Deisenhofen, Germany) was diluted in 90% ethanol. Stock solutions of 5-flurouracil, CIS and TAX (Sigma, Deisenhofen, Germany) were prepared in dimethyl sulfoxide. Final concentrations of the solvents in medium were 0.1% or less.
Treatment of cells with cytotoxic agents. Twenty-four hours before treatment, established cells were seeded in 6-well tissue culture plates (2 ml cell suspension per well) and 96-well plates (100 µl cell suspension per well) at a density of 15 × 104 and 3 × 103 cells per well, respectively. Cells were treated with SF in the absence or in the presence of chemotherapeutic agents for 72 hours. For treatment cytotoxic drugs were added directly into the culture medium.
MTT assay. Cells were resuspended at a density of 3 × 104 to 5 × 104 cells per ml in 96-well plates, 100 µl per well. Twenty-four hours later, cells were treated with SF and/or chemotherapeutic agents for 72 hours. After treatment, the MTT assay was performed as described.6
Colony-forming assay. Tumor cells were seeded at a density of 1.5 × 105 cells per well in 6-well tissue culture plates. Twenty-four hours later, the cultures were treated with SF and/or GEM for 72 hours. Subsequently, the cultures were trypsinized, plated at a density of 400 cells per well in 6-well tissue culture plates and incubated for 10 days without changing medium. For determination of colony formation, cultures were fixed (3.7% paraformaldehyde and 70% ethanol), and stained with 0.05% Coomassie blue. The number of colonies with >50 cells was counted under a dissecting microscope. The percentage of cell survival was calculated (plating efficiency of nontreated cultures = 1).
Annexin V staining and apoptosis detection. Cells were treated with fluorescein-isothiocyanate-conjugated annexin V (Invitrogen, Camarillo, CA) after respective treatment. Externalization of phosphatidylserine was identified by flow cytometry (FACScan, BD Biosciences, Heidelberg, Germany).6 Briefly, 1.5 × 105 cells were seeded per well in a 6-well plate. Twenty-four hours later, cells were treated with SF and/or GEM for 72 hours. All cells were collected and were washed with annexin buffer (10 mmol/l HEPES, 150 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 1.8 mmol/l CaCl2) followed by staining with fluorescein-isothiocyanate-conjugated annexin for 20 minutes on ice and in the dark (2 µl annexin in 100 µl annexin buffer per sample). For flow cytometric analysis cells were resuspended in fresh annexin buffer.
Spheroid assay. For formation of spheroids, cells were cultured in NeuroCult NS-A basal serum-free medium (human) (StemCell Technologies, Vancouver, Canada), supplemented with 2 µg/ml Heparin (StemCell Technologies), 20 ng/ml hEGF (R&D Systems, Wiesbaden-Nordenstadt, Germany), 10 ng/ml hFGF-b (PeproTech, Hamburg, Germany) and NeuroCult NS-A Proliferation Supplements (StemCell Technologies). Cells were seeded at low densities (5 × 102 to 2 × 103 cells/ml) in 12-well low adhesion plates, 1 ml per well. For quantification of the percentage of spheroid forming cells, cells were seeded at one cell per well in 96-well plates. Wells with more than one cell were excluded from evaluation.
Detection of ALDH1 activity. ALDEFLUOR substrate (5 µl, Aldagen, Durham, NC) was added to 1 × 106 tumor cells in 1 ml assay buffer, and incubated for 30 minutes at 37 °C. Incubation of cells with ALDEFLUOR substrate in presence of the ALDH inhibitor diethylamino-benzaldehyde served as a negative control. Cells were analyzed by flow cytometry according to the instructions of the manufacturer.
Immunofluorescence. Cells were treated with SF, GEM or a combination of both agents for 21 days and plated on coverslips for 24 hours. Cells were fixed in aceton, blocked with 10% normal goat serum and incubated with primary mouse anti-human ALDH1 Ab (BD Biosciences, San Jose, CA) followed by washing in phosphate-buffered saline/Tween 0.2% and incubation with secondary goat anti-mouse Alexa 488 IgG (molecular probes, Karlsruhe, Germany).
Western blot analysis. Whole cell extracts were prepared by a standard protocol as described6 and proteins were detected by western blot analysis using mouse polyclonal Ab anti ALDH1 (BD Biosciences, San Jose, CA), mouse polyclonal Ab anti c-Rel (Santa Cruz Biotechnology), and mouse monoclonal Ab anti Notch-1 (Abcam, Cambridge, UK).
Nude mice and tumor xenografts. MIA-PaCa2 cells (4 × 106 in 150 µl) were injected subcutaneously into the right anterior flank of 4–6 weeks old BALBc (nu/nu) male mice (day 0). After the tumors had reached a mean diameter of about 8–10 mm, mice carrying tumor xenografts were randomly divided into groups of six animals each and treatment was started. Mice were treated intraperitoneally with SF (3 mg/kg) and/or GEM (3 mg/kg) on days 5, 6, and 7 after tumor implantation. Tumor growth was monitored by measuring two diameters with calipers daily. In the second in vivo experiment MIA-PaCa2 cells were pretreated with SF (5 µmol/l) and/or GEM (25 nmol/l) in vitro. Control cells were left untreated. After 72 hours of pretreatment an equal number (5.7 × 103 cells) of live cells was subcutaneously transplanted with 50% Matrigel into the right anterior flank of 5–6 weeks old NMRI-nu (nu/nu) female mice. In both experiments tumor volumes (V) were calculated using the formula V = ½ (length × width2). Mice were euthanized at tumor sizes >1,500 mm3. Animal experiments have been carried out in the animal facilities of the German Cancer Research Center (DKFZ) after approval by the authorities (Regierungspräsidium Karlsruhe, Germany).
Statistical analysis. For MTT and FACS-measurements data are presented as mean ± SD. Data were analyzed using the Student's t-test for statistical significance. P < 0.05 was considered significant. For xenografts on nude mice a distribution free test for tumor growth curve analyses for therapy experiments with xenografted cancer cells was used as described by Koziol et al.42
Acknowledgments
This study was supported by grants from the Bundesministerium für Bildung und Forschung (01GU0611), Tumorzentrum Heidelberg/Mannheim (D10027(6)350), Stiftung Chirurgie Heidelberg, Dietmar-Hopp Stiftung and Deutsche Krebshilfe. All authors disclose any commercial affiliations or consultancies, stock or equity interests, or patent-licensing arrangements that could be considered to pose a financial conflict of interest related to the submitted manuscript.
REFERENCES
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF., and, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ribacka C, Pesonen S., and, Hemminki A. Cancer, stem cells, and oncolytic viruses. Ann Med. 2008;40:496–505. doi: 10.1080/07853890802021342. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun-Davis T, Schneider-Broussard R., and, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M., and, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–1248. [PubMed] [Google Scholar]
- Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- Zhang C, Mattern J, Haferkamp A, Pfitzenmaier J, Hohenfellner M, Rittgen W, et al. Corticosteroid-induced chemotherapy resistance in urological cancers. Cancer Biol Ther. 2006;5:59–64. doi: 10.4161/cbt.5.1.2272. [DOI] [PubMed] [Google Scholar]
- Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB., and, Farrar WL. CD44+ CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Cai HH, Wei JS, An Y, Ji ZL, Lu ZP, et al. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol Rep. 2010;23:1375–1382. doi: 10.3892/or_00000774. [DOI] [PubMed] [Google Scholar]
- Pfeiffer MJ., and, Schalken JA. Stem cell characteristics in prostate cancer cell lines. Eur Urol. 2010;57:246–254. doi: 10.1016/j.eururo.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Matsubara A, Teishima J, Mutaguchi K, Yasumoto H, Dahiya R, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–663. doi: 10.1016/j.bbrc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37:551–561. doi: 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- Wei C, Guomin W, Yujun L., and, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line. Cancer Biol Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM., and, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipo C, Golde TE, Osborne BA., and, Miele LA. Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest. 2008;88:11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S., and, Srivastava RK. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin Cancer Res. 2008;14:6855–6866. doi: 10.1158/1078-0432.CCR-08-0903. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF., and, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC., and, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233:208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myzak MC, Tong P, Dashwood WM, Dashwood RH., and, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- Singh AV, Xiao D, Lew KL, Dhir R., and, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, Xiao D, Brown CK, et al. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- Pledgie-Tracy A, Sobolewski MD., and, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–1021. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- Jackson SJ., and, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25:219–227. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Chen C, Hebbar V., and, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- Yu D, Sekine-Suzuki E, Xue L, Fujimori A, Kubota N., and, Okayasu R. Chemopreventive agent sulforaphane enhances radiosensitivity in human tumor cells. Int J Cancer. 2009;125:1205–1211. doi: 10.1002/ijc.24480. [DOI] [PubMed] [Google Scholar]
- Wang Z, Desmoulin S, Banerjee S, Kong D, Li Y, Deraniyagala RL, et al. Synergistic effects of multiple natural products in pancreatic cancer cells. Life Sci. 2008;83:293–300. doi: 10.1016/j.lfs.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimognari C., and, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y., and, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- Dave B., and, Chang J. Treatment resistance in stem cells and breast cancer. J Mammary Gland Biol Neoplasia. 2009;14:79–82. doi: 10.1007/s10911-009-9117-9. [DOI] [PubMed] [Google Scholar]
- Sell S. Cancer stem cells and differentiation therapy. Tumour Biol. 2006;27:59–70. doi: 10.1159/000092323. [DOI] [PubMed] [Google Scholar]
- Fimognari C, Lenzi M, Cantelli-Forti G., and, Hrelia P. Induction of differentiation in human promyelocytic cells by the isothiocyanate sulforaphane. In Vivo. 2008;22:317–320. [PubMed] [Google Scholar]
- Hunakova L, Sedlakova O, Cholujova D, Gronesova P, Duraj J., and, Sedlak J. Modulation of markers associated with aggressive phenotype in MDA-MB-231 breast carcinoma cells by sulforaphane. Neoplasma. 2009;56:548–556. doi: 10.4149/neo_2009_06_548. [DOI] [PubMed] [Google Scholar]
- Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, Ginestier C, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol JA, Maxwell DA, Fukushima M, Colmerauer ME., and, Pilch YH. A distribution-free test for tumor growth curve analyses with application to an animal tumor immunotherapy experiment. Biometrics. 1981;37:383–390. [PubMed] [Google Scholar]