Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder typified by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Recent evidence indicates that neuroinflammation may play a critical role in the pathogenesis of PD, particularly tumor necrosis factor (TNF). We have previously shown that soluble TNF (solTNF) is required to mediate robust degeneration induced by 6-hydroxydopamine (6-OHDA) or lipopolysaccharide. What remains unknown is whether TNF inhibition can attenuate the delayed and progressive phase of neurodegeneration. To test this, rats were injected in the SNpc with lentivirus encoding dominant-negative TNF (lenti-DN-TNF) 2 weeks after receiving a 6-OHDA lesion. Remarkably, when examined 5 weeks after the initial 6-OHDA lesion, no further loss of nigral DA neurons was observed. Lenti-DN-TNF also attenuated microglial activation. Together, these data suggest that TNF is likely a critical mediator of nigral DA neuron death during the delayed and progressive phase of neurodegeneration, and that microglia may be the principal cell type involved. These promising findings provide compelling reasons to perform DN-TNF gene transfer studies in nonhuman primates with the long-term goal of using it in the clinic to prevent the delayed and progressive degeneration of DA neurons that gives rise to motor symptoms in PD.
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder typified by the loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Although the mechanisms underlying PD pathogenesis are not fully understood, studies from multiple groups indicate that inflammation plays an integral role in the degeneration of nigral DA neurons.1,2
Tumor necrosis factor (TNF) is a cytokine produced by activated immune cells in all areas of the body, including the central nervous system.3 In patients with PD, TNF mRNA and protein levels are elevated in postmortem brain and cerebrospinal fluid.4,5 In addition, single-nucleotide genetic polymorphisms in the TNF promoter that increase transcriptional activity and TNF production are associated with earlier onset of disease in patients with idiopathic PD.6 Furthermore, these studies are supported by animal studies showing that fewer DA cells die in response to mitochondrial complex I inhibitor, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine, administration in TNF-deficient mice.7,8,9
We have previously demonstrated that soluble TNF (solTNF) is the ligand species mediating the cytotoxic effects on DA neurons in two separate rat models of nigral cell death using two different approaches to deliver TNF inhibitors. In the first experiment, the inhibitor protein dominant-negative TNF (DN-TNF) was chronically infused into the SNpc via osmotic minipump.10 In the second, a lentivirus encoding DN-TNF (lenti-DN-TNF) was used to generate DN-TNF within the SNpc.11 Regardless of the method employed, DN-TNF successfully attenuated (by nearly 50%) locomotor deficits and nigral degeneration in unilateral 6-hydroxydopamine (6-OHDA)-lesioned rats. However, because the neuroprotective effects obtained in these studies were achieved by coadministration of the inhibitors at the time of 6-OHDA lesion, it was difficult to ascertain whether solTNF-dependent toxicity occurred both in the acute stage of cell death induced by the neurotoxin and during the progressive stage of degeneration, a period known to be characterized by persistent neuroinflammation.12 Indeed, the importance and significance of identifying the mechanism(s) governing the progressive loss DA neurons cannot be understated and are critical for successful development of new disease-modifying therapies. Therefore, the objective of this study was to determine whether the progressive phase of nigral cell loss can be attenuated by inhibiting solTNF after the initial acute loss of DA cells due to neurotoxin. Second, we examined whether DN-TNF administration attenuated microglia activation in the progressive phase of the lesion. In order to answer these critical questions, we administered lenti-DN-TNF 2 weeks after an intrastriatal 6-OHDA lesion and performed stereological estimates of nigral DA neuron number, microglia activation, and locomotor deficits.
Our results demonstrate that delayed DN-TNF gene therapy in the SNpc can halt the progressive loss of 6-OHDA-induced nigral degeneration. These findings have exciting implications for clinical development of anti-TNF gene therapy as a neuroprotective strategy in PD.
Results
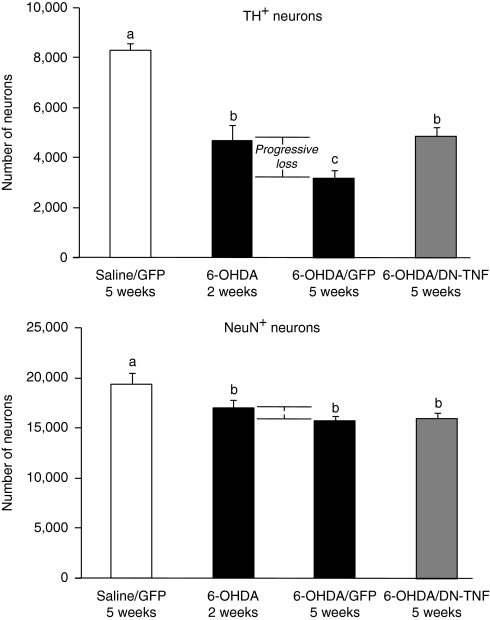
The primary objective of this study was to determine whether the loss of DA neurons during the progressive phase of a 6-OHDA-induced lesion could be attenuated with anti-solTNF gene transfer. To do this, we used a model in which injection of 6-OHDA into the striatal terminals causes axonal retraction and death of cell bodies in the SNpc over a period of several weeks beginning with an acute cell death phase during the first 12 days followed by a chronic phase between the 2nd and 3rd week after the initial 6-OHDA lesion during which nigral DA neuron cell death progresses more slowly from 40 to 65% relative to the contralateral (unlesioned) side.12,13,14 To selectively inhibit solTNF signaling specifically after the acute but before the progressive phase, rats were injected with lenti-DN-TNF 2 weeks after receiving a unilateral 6-OHDA (or saline) injection within the striatum (Figure 1). To confirm that a progressive loss of DA neurons occurred after the acute cell death phase, DA cell loss was examined in a subset of rats at 2 weeks after 6-OHDA injection. Thus, the number of tyrosine hydroxylase-positive (TH+)/neuronal nuclei-positive (NeuN+) cells that were lost between 2 and 5 weeks constitute progressive DA cell death. A one-way analysis of variance (ANOVA) was used to compare potential differences between mock-lesioned/mock-treated [saline/lenti-green fluorescent protein (lenti-GFP)] and 6-OHDA-lesioned groups receiving lenti-DN-TNF or lenti-GFP as a negative control. As predicted, statistically significant differences between groups were observed in TH+ (F3,23 = 27.01, P < 0.001) and NeuN+ (F3,23 = 4.99, P < 0.01) cells (Figure 2). Fisher's protected least significant difference post hoc analyses revealed a significant, 43% reduction in TH+ cells 2 weeks after receiving 6-OHDA compared to saline-injected controls (P < 0.001). By 5 weeks, the percentage of TH+ neurons was reduced 61% compared to controls (P < 0.001). Thus, 32% of TH+ cells were lost during the progressive phase (P < 0.05). However, when lenti-DN-TNF was injected 2 weeks after 6-OHDA, there was no further loss of TH+ cells compared to the loss measured at 2 weeks after 6-OHDA (4,683 TH+ cells at 2 weeks; 4,855 TH+ cells at 5 weeks DN-TNF). Similarly, the 6-OHDA lesion reduced the number of NeuN+ cells compared to saline-injected rats (all P < 0.05). There were no differences in the number of NeuN+ cells between the various 6-OHDA-treated rats (P > 0.05). These effects, however, become significant if the data are expressed and analyzed as a percentage of contralateral SNpc (see Supplementary Figure S1 for more detail). Compared to the raw values presented in Figure 2, expressing the data in this manner allows us to control for the natural variability in DA neurons that exist between rats and is therefore a better representation of the magnitude of the changes that are occurring following these treatments. Taken together, these data strongly implicate solTNF as the primary factor underlying the loss of DA neurons during the progressive phase of cell death.
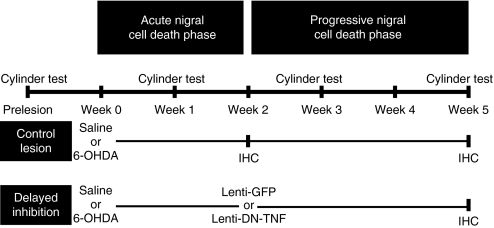
Figure 1.
Experimental design and outcome measures. Rat striata were injected unilaterally with saline or 6-OHDA at week 0. Before surgery and again at weeks 1, 3, and 5, motor asymmetry was assessed using the cylinder test. A subset of rats (delayed inhibition) received a second injection of either lenti-GFP or lenti-DN-TNF 2 weeks after the striatal 6-OHDA lesion. Unbiased stereology was used to estimate DA neuron number within the substantia nigra pars compacta at 2 or 5 weeks after the 6-OHDA lesion. IHC, immunohistochemistry; DN-TNF, dominant-negative tumor necrosis factor; GFP, green fluorescent protein; 6-OHDA, 6-hydroxydopamine.
Figure 2.
Effects of delayed DN-TNF gene transfer on nigral DA neuron survival. Total numbers of TH and NeuN double-positive cells at 2 and 5 weeks after 6-OHDA lesion were estimated by unbiased stereology and analyzed using a one-way analysis of variance. Data are reported as the mean ± SEM. Fisher's post hoc test was used to assess significance. Bars with different letters are statistically significantly different from each other (P < 0.05). Group N's: saline/GFP–5 weeks (n = 6); 6-OHDA– 2 weeks (n = 5); 6-OHDA/GFP–5 weeks (n = 7); 6-OHDA/DN-TNF–5 weeks (n = 9). DA, dopaminergic; DN-TNF, dominant-negative tumor necrosis factor; GFP, green fluorescent protein; NeuN, neuronal nuclei; 6-OHDA, 6-hydroxydopamine; TH, tyrosine hydroxylase.
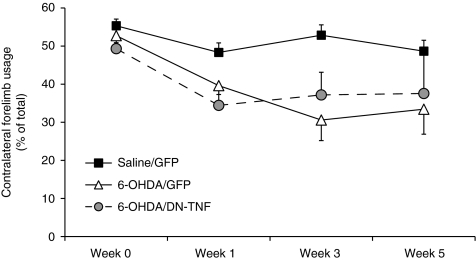
To determine whether the rescue of nigral DA neurons was accompanied by an improvement in locomotor deficit, we measured forelimb asymmetry using the cylinder test developed by Schallert et al.15 Forelimb asymmetry was tested before the 6-OHDA lesion and 1, 3, and 5 weeks postlesion (Figure 3). A between-subject (treatment; saline/GFP; 6-OHDA/GFP; 6-OHDA/DN-TNF) repeated measures ANOVA (week 0, 1, 3, and 5) was used to determine whether administration of DN-TNF ameliorated 6-OHDA-induced forelimb asymmetry in the rats. No significant interaction was observed although a main effect of treatment (F2,6 = 4.61, P < 0.05) and week of treatment (F3,6 = 6.62, P < 0.05) were noted. Specifically, DN-TNF did not significantly attenuate the forelimb asymmetry induced by 6-OHDA, at least not within 5 weeks.
Figure 3.
Impact of delayed DN-TNF gene transfer on motor asymmetry in 6-OHDA lesioned rats. At weeks 0, 1, 3, and 5, 6-OHDA-lesioned animals were tested for deficits in forelimb asymmetry using the cylinder test. A repeated measures analysis of variance was used to examine potential differences between groups. Data are plotted as contralateral forelimb use as a percentage of total and expressed as mean ± SEM. Group N's: saline/GFP–5 weeks (n = 6); 6-OHDA–2 weeks (n = 5); 6-OHDA/GFP–5 weeks (n = 7); 6-OHDA/DN-TNF–5 weeks (n = 9). DN-TNF, dominant-negative tumor necrosis factor; GFP, green fluorescent protein; 6-OHDA, 6-hydroxydopamine.
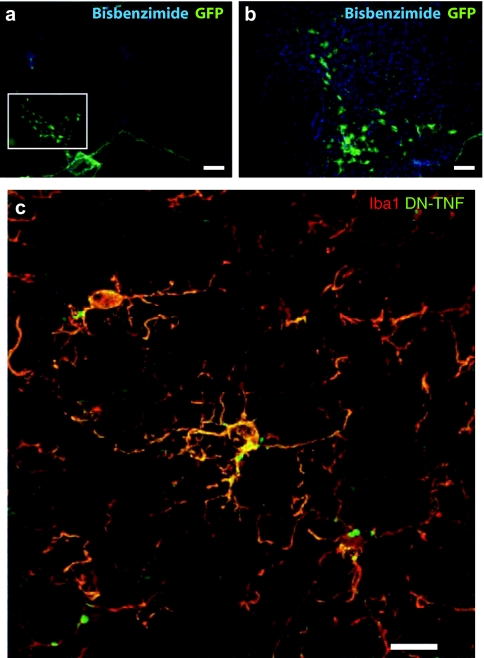
Next, we investigated the extent of lentivirus transduction in glial cells in SNpc following a single intranigral injection. Because the lentiviral vector expressing DN-TNF has an internal ribosome entry site followed by the sequence for GFP, we were able to determine the area of SNpc where the DN-TNF protein was being expressed following in vivo transduction with lenti-DN-TNF-IRES-GFP. Immunofluorescence analysis of brain sections from 6-OHDA/lenti-DN-TNF-injected rats with an antibody specific for GFP revealed the presence of GFP+ cells with glial morphology in the SNpc (Figure 4a,b). To confirm that these cells were indeed microglia that were co-expressing DN-TNF protein, we performed double-labeling analysis with an antibody against the microglial marker Iba1 and an antibody against human TNF, which recognizes the human DN-TNF; a human TNF variant that contains two point mutations (A145R/I97T). Detectable expression of DN-TNF protein (green) in Iba1+ cells (red) was confirmed using confocal microscopy (Figure 4c). These findings suggest that in vivo transduction with lenti-DN-TNF results in microglial-derived DN-TNF expression to allow neutralization of native solTNF bioactivity.
Figure 4.
Immunofluorescent localization of GFP+ cells, Iba1+ microglia, and DN-TNF protein in rats receiving DN-TNF gene transfer. (a,b) Anti-GFP immunofluorescence staining (green) in cells of glial morphology transduced in substantia nigra pars compacta (SNpc; white box). (c) Confocal microscope Z-stack of cells immunopositive for the microglial marker Iba1 (Alexa-546, red) and for hDN-TNF (Alexa-488, green) in SNpc. a, bar = 400 µm; b, bar = 200 µm; c, bar = 20 µm. DN-TNF, dominant-negative tumor necrosis factor; GFP, green fluorescent protein.
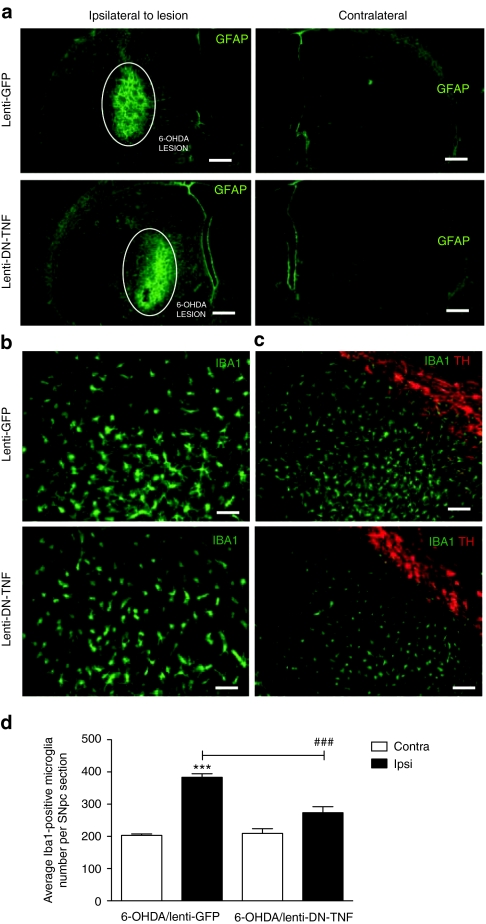
In previous in vitro studies, transduction of primary microglia with lenti-DN-TNF efficiently blocked solTNF-induced microglia activation.11,16 Therefore, in order to determine the extent to which transduction with lenti-DN-TNF blocked 6-OHDA-induced astrogliosis and microgliosis in vivo, we immunolabeled the brain sections from the striatum and SNpc with antibodies specific for the microglial marker Iba1 and the astrocyte marker glial fibrillary acidic protein. As expected, transduction of cells in SNpc with lenti-DN-TNF or lenti-GFP did not result in gross differences in astrogliosis in the striatum, the site of the 6-OHDA lesion (Figure 5a). In contrast, intranigral lenti-DN-TNF significantly attenuated the microglial response induced by 6-OHDA in the SNpc compared to that of the lenti-GFP-injected/6-OHDA-lesioned animals (F1,1 = 19.46, P < 0.05; Figure 5b,c). Specifically, the average number of microglia in SNpc sections of lenti-GFP/6-OHDA-lesioned animals was twice that of SNpc sections from the contralateral SNpc (P < 0.05), whereas the average microglia number in SNpc sections of lenti-DN-TNF/6-OHDA-lesioned animals was not significantly different from that in SNpc sections from the contralateral side (P > 0.05; Figure 5d). In addition, it is possible that lenti-DN-TNF therapy may have reduced the activation state of microglia in SNpc, as evidenced by the less extensive branching morphology in Iba1+ cells in 6-OHDA/lenti-DN-TNF animals versus 6-OHDA/lenti-GFP (Figure 5b,c).
Figure 5.
Effects of nigral DN-TNF gene transfer on microgliosis and astrogliosis. (a) Activated astrocytes were identified by GFAP immunostaining (green) in the striatum. The area lesioned by the 6-OHDA neurotoxic is highlighted with a white circle. (b,c) Microglia were identified by Iba1 immunostaining (green) in the substantia nigra pars compacta (SNpc) area identified by tyrosine hydroxylase immunostaining (red). a, bar = 400 µm; b, bar = 100 µm; in c, bar = 200 µm. (d) Quantification of microglia in SNpc of 6-OHDA/lenti-GFP and 6-OHDA/lenti-DN-TNF-injected rats. Values represent the mean ± SEM of Iba1+ microglia within SNpc calculated from three separate brain sections (12 random fields/section) using threshold analysis in three animals per treatment group. Two-way analysis of variance for comparison of 6-OHDA/lenti-GFP and 6-OHDA/lenti-DN-TNF with Fisher's protected least significant difference post hoc test (###P < 0.001). DN-TNF, dominant-negative tumor necrosis factor; GFP, green fluorescent protein; 6-OHDA, 6-hydroxydopamine.
Discussion
We demonstrate here that DN-TNF gene therapy in the SNpc results in transduction of glial cells and has robust neuroprotective effects during the progressive phase of DA cell death. Specifically, nigral DA neuron number in the 6-OHDA/lenti-GFP group displayed a progressive decline from 57 to 39% of contralateral side between week 2 and 5, whereas the 6-OHDA/lenti-DN-TNF group remained stable at 59% of contralateral, leading us to conclude that administration of lenti-DN-TNF at week 2 slowed the progression of 6-OHDA-induced death and stabilized nigral DA neuron number (Figure 2). Interestingly, a single nigral injection delivered 2 weeks after the 6-OHDA lesion afforded as much protection as a single nigral injection of lenti-DN-TNF coadministered at the time of the 6-OHDA intrastriatal lesion.11 Thus, these data strongly support a role for solTNF signaling during the delayed phase of nigral cell death during which there is progressive worsening of locomotor deficits.
To this last point, however, DN-TNF did not rescue the locomotor deficit produced by 6-OHDA lesion. This is not altogether surprising as behavioral deficits are only reliably detected when the vast majority of neurons have already died. In humans, this number roughly corresponds to about two-thirds DA cell loss.17 Thus, the 32% difference in DA cell numbers between 6-OHDA/GFP- and 6-OHDA/DN-TNF-treated rats is likely not great enough to reflect behavioral changes, at least not within 5 weeks.
Comparison of the neuroprotective effects achieved by delayed solTNF inhibition compared to co-inhibition of solTNF signaling at the time of a 6-OHDA lesion11 suggests that solTNF is more important for development of the delayed and progressive phase of nigral degeneration than for acute nigral cell death induced by the lesion. However, because these studies did not analyze the effects of selective solTNF inhibition exclusively during the acute phase, it is not known whether that alone might have prevented development of the progressive lesion. But it is important to note that previous studies with this lentiviral vector backbone demonstrated that central nervous system expression of the transgene lasts up to 12 months18 and expression in the SNpc is maintained for at least 6 months (M.G. Tansey unpublished results). Therefore, the slight differences in neuroprotection achieved by co-inhibition in previous studies compared to that achieved by delayed TNF inhibition is unlikely to be the result of differences in DN-TNF expression levels at the study end point (5 weeks postlesion). In summary, given that inhibition of TNF signaling before the start of the acute and delayed/progressive phase of cell death11 and inhibition of TNF signaling after the end of the acute phase in the present studies yielded similar results, we conclude that TNF is needed for development of the delayed and progressive phase of nigral cell death. Population-based case–control studies indicate that homozygous carriers of single-nucleotide polymorphisms in the TNF promoter that increase transcriptional activity and result in higher than normal TNF production have an increased risk for early onset (before the age of 50 years) PD.6,19,20 Therefore, we speculate that timely delivery of DN-TNF in the SNpc may be neuroprotective in individuals at high risk for development of PD by successfully halting the progressive degeneration of DA neurons so the threshold of 70% DA loss needed to develop clinical symptoms is never reached.
Although we did not directly measure the levels of DN-TNF produced intranigrally by human TNF enzyme-linked immunosorbent assay, it is likely that we achieved similar glial-derived DN-TNF production as that reported in our co-inhibition studies in which the same lentiviral construct was used.11 We base this assertion on the fact that comparable extent of neurohistological rescue was achieved in the present studies compared to our previous studies and those studies demonstrated that a single intranigral injection of this same lenti-DN-TNF virus injected resulted in variable expression of DN-TNF protein levels ranging from 0.6 to 2 ng/per midbrain.11
Based on our analysis of microglial burden, the mechanism by which lenti-DN-TNF achieved rescue is likely to be a combination of neutralization of solTNF bioactivity (predicted based on the mode of action of the DN-TNF inhibitor) as well as attenuation of microglia activation in the SNpc (Figure 5). The latter mechanism would be expected to result in reduced levels of other microglial-derived mediators (including reactive oxygen and nitrogen species) likely to have had synergistic toxic effects with solTNF on nigral DA neurons. It should be noted that although solTNF binds TNFRI receptor with higher affinity than TNFRII receptor, these studies could not distinguish whether the solTNF bioactivity blocked by lenti-DN-TNF administration was mediated exclusively via TNFRI without any contribution from TNFRII (see ref. 21 for review).
In recent years, approaches targeting pro- and anti-inflammatory cytokines and cyclooxygenase-2 have emerged and undergone rigorous testing in preclinical 6-OHDA rat models.14,22 Although most manipulations attenuated nigral DA neuron loss when administered simultaneously with the lesion, few have shown efficacy specifically in a progressive phase of neuron loss.14 Although the 6-OHDA model used in our studies is considered to be modeling “preclinical” stages of PD,23 the intrastriatal 6-OHDA lesion achieved significant loss of nigral DA neurons (60%) and a behavioral correlate before DN-TNF transduction. It should be noted that before DN-TNF transduction in the SNpc at week 2, all animals in the study displayed amphetamine-induced rotational behavior corresponding to a 40–50% striatal DA depletion and ~30–50% loss of nigral DA neuron cell bodies.24
At this time, pharmacological and surgical therapies target and alleviate the nonmotor and motor symptoms of PD, however, there are no therapies that can slow or halt the progression of PD.25 Currently, anti-TNF biologics are US Food and Drug Administration approved for the treatment of patients with peripheral autoimmune disorders characterized by chronic systemic inflammation; however, these anti-TNF biologics inhibit both solTNF and transmembrane TNF signaling and have been shown to increase the patient's risk for opportunistic infections.26,27,28 By contrast, the DN-TNF inhibitor used in our studies has been shown to preserve transmembrane TNF function thereby protecting against endotoxin-mediated hepatotoxicity and preserving immunity against Mycobacterium bovis and Mycobacterium tuberculosis infections in mice.29,30 Therefore, if solTNF is the species promoting inflammation in the SNpc as suggested by this and previous work from our group,10,11 selective targeting of solTNF with DN-TNF inhibitors in the central nervous system may prove to be the safer approach to avoid suppression of neuroimmune responses mediated by transmembrane TNF. More importantly, given the fact that both detrimental and protective effects of TNF have been described in brain regions affected in multiple sclerosis,31,32 ischemia,33 and Alzheimer's disease-like pathology,34,35 anti-TNF therapy in PD patients will have to be targeted extremely carefully to the desired region (i.e., SNpc) and the patients monitored extremely closely for possible adverse effects.
Clinical trials are currently underway to investigate the therapeutic relevance of viral delivery of proteins designed to target many pathways in PD (reviewed in refs. 25,36,37). Our studies support the feasibility and efficacy of viral delivery mechanisms to slow down the progression of nigral DA neuron loss in PD and perhaps the associated locomotor symptoms. Viral technology is generally safe to use in human and animal models and can be manipulated to infect and integrate DNA into the host genome of specific brain regions, allowing for continuous expression of desired proteins and perhaps in the near future may become a viable alternative therapeutic approach for PD patients. For example, glutamic acid decarboxylase is the key enzyme in the synthesis of gabaminobutyric acid when delivered to the subthalamic nucleus in PD patients and nonhuman primate models with adeno-associated virus can increase production of gabaminobutyric acid, therefore, increasing the amount of inhibitory neuron firing and reducing motor symptoms associated with PD (reviewed in ref. 25). Although glutamic acid decarboxylase gene therapy may be effective in reducing motor symptoms, unlike DN-TNF it would not be expected to slow down the disease progression. Currently, lentiviral vectors have been approved as vehicles for clinical gene transfer of aromatic amino acid decarboxylase, TH, and GTP cyclohydrolase 1, all of which are genes required for DA synthesis.38
In summary, in a progressive rat model of PD, anti-inflammatory DN-TNF gene therapy reduces microgliosis in the SNpc and halts the progressive loss of nigral DA neurons. Although the exact mechanisms for inflammation-induced nigrostriatal degeneration of neurons have not been firmly delineated, we provide neurohistological evidence that virally delivered anti-TNF therapy selectively targeted to the nigral region of the midbrain attenuates microglia activation and halts the progressive loss of DA neurons. These promising findings provide compelling reasons to advance this line of investigation forward into studies to establish the safety and efficacy of DN-TNF gene therapy in nonhuman primate models of PD with the long-term goal of delivering it during the preclinical stages of PD once early diagnosis of this devastating disease becomes a reality.
Materials and Methods
Cloning and preparation of lentiviral stocks. The human full-length DN-TNF (TNF variant A145R/I97T) DNA sequence or GFP sequence were subcloned into a constitutive self-inactivating lentiviral vector and validated in vitro and in vivo according to previously published reports.11,16 The GFP-expressing lentivirus has been previously described and validated.18,39 Both DN-TNF and GFP lentiviral stocks were produced and purified according to previously published protocols.18 The final titer was 102 µg/ml p24 and 1 × 108 IU/ml for the lenti-GFP control and 84 µg/ml and 2 × 108 IU/ml for lenti-DN-TNF. All viruses were diluted in Hank's balanced salt solution (Invitrogen, Carlsbad, CA).
Animal studies. Young adult female Sprague-Dawley SASCO rats (200–250 g) were purchased from Charles River Laboratories (Wilmington, MA). All rats were housed in a pathogen-free, climate-controlled facility at The University of Texas Southwestern Medical Center. All studies and animal protocols were approved and guided by the Institutional Animal Care and Use Committee at UT Southwestern.
Surgical procedures. Immediately before surgical procedures, 6-OHDA (Sigma, St Louis, MO) was dissolved at 5 mg/ml with sterile saline. Lentivirus stocks were diluted in the ratio of 1:2 in sterile Hank's balanced salt solution) and kept on ice, protected from light throughout the entire procedure. Striatal 6-OHDA lesions were performed as described previously.12 Briefly, young adult female Sprague-Dawley rats (200–250 g) were anesthetized by a single intraperitoneal injection of a ketamine/xylazine cocktail (100 mg/kg ketamine, 20 mg/kg xylazine, and 10 mg/kg acepromazine) and placed into a stereotaxic frame. Burr holes were drilled to allow a single unilateral injection of 20 µg 6-OHDA (Sigma) or sterile saline at the following stereotaxic coordinates: anteroposterior, 1.0 mm from bregma; mediolateral, −3.0 mm; dorsoventral, −4.5 mm below surface of the dura. A 4 µl of 6-OHDA or saline was infused into the right striatum at the rate of 0.5 µl/minute. For the co-inhibition groups, a second burr hole was drilled to allow a single unilateral injection of 2 µl of lenti-DN-TNF or lenti-GFP using a 28-gauge needle at a rate of 0.5 µl/minute into the right SNpc at the following coordinates: anteroposterior, −5.3 mm from bregma; mediolateral, −2.3 mm; dorsoventral, −7.3 mm below surface of dura. For the delayed inhibition groups, this procedure was performed 2 weeks after the 6-OHDA lesion. Following all intracranial injections, a 5-minute waiting period and slow needle retraction were employed to allow proper diffusion of 6-OHDA and/or virus. Postoperatively and for the following 3 days, animals received subcutaneous injections of the buprenorphine HCL (0.05 mg/kg) and were monitored closely for signs of pain or discomfort.
Amphetamine-induced rotational behavior. Two weeks after 6-OHDA lesion, animals were tested for amphetamine-induced rotational behavior according to a previously published protocol10,11 as an indirect measure of striatal dopamine depletion. Animals received a single intraperitoneal injection of 𝒹-amphetamine (2.5 mg/kg in normal saline; Sigma) and rotational asymmetry was scored for 20 minutes continuously during the hour following the intraperitoneal injection. Complete ipsilateral rotations were scored as rotations/minute. Rats injected with 6-OHDA that did not meet the inclusion criteria for a successful lesion12 were omitted from the studies.
Forelimb asymmetry test. Before surgery and at weeks 1, 3, and 5 postlesion, animals were tested for forelimb asymmetry according to previously published protocols40 with the following modifications. Animals were allowed to explore in an unfamiliar glass cylinder (20 cm in diameter) and videotaped for 3–5 minutes undisturbed and rearing postures (leaning at least one forepaw against the cylinder wall) are observed. Hemiparkinsonian rats with lesion in the right dorsal striatum present a significant impairment in the contralateral (left) forepaw use, indicative of forepaw use asymmetry. The number of left and right forepaw contacts counted, and the data presented as percent left (contralateral) forepaw contacts.
Tissue processing. At 5 weeks postlesion, animals were deeply anesthetized with Euthasol (Butler, Dublin, OH; 100 mg in 500 µl sterile saline) and transcardially perfused with 250 ml of heparinized (1 ml/l) phosphate-buffered saline pH 7.4 followed by 500 ml of 4% paraformaldehyde in phosphate-buffered saline pH 7.4. Brains were postfixed for 24 hours in 4% paraformaldehyde and then dissected out and placed into a 20% sucrose solution in phosphate-buffered saline for 26–28 hours. Brains were cryosectioned coronally on a Leica1650 cryostat (cut thickness: 30 µm); sections were collected serially throughout the striatum and SNpc, placed into tissue collection solution (50% 0.1 mol/l phosphate buffer, 25% glycerol, 30% ethylene glycol), and stored at −20 for further analysis.
Bright-field immunohistochemistry. Free-floating immunohistochemistry on SNpc sections was performed using previously published 3,3′-diaminobenzidine (DAB) protocols.18 Briefly, sections were labeled overnight at 4 °C with primary antibodies: TH at 1:5,000 (Millipore, Billerica, MA) followed by NeuN at 1:2,000 dilution (Millipore). Sections were then labeled with the appropriate biotinylated secondary antibody diluted at 1:200 (Vector Laboratories, Burlingame, CA) for 2 hours at room temperature and incubated in neutravidin–horseradish peroxidase–conjugated antibody (Thermo Scientific, Waltham, MA) at 1:5,000 dilution for 1 hour at room temperature. The enzymatic reaction for TH was allowed to proceed for 4–6 minutes in a 0.05% 3,3′-diaminobenzidine, 0.01% hydrogen peroxide, and 0.04% nickel chloride solution. The reaction for NeuN was allowed to proceed for 4–6 minutes in a 0.05% 3,3′-diaminobenzidine and 0.01% hydrogen peroxide solution. Immunolabeled sections were mounted on glass slides (SuperFrost Plus; Fisher Scientific, Pittsburgh, PA) and dehydrated/coverslipped as previously described.41
Stereological estimate of neuron number. Unbiased stereological estimates of DA (TH+ cell) and total neuron (NeuN+ cell) numbers were performed using StereoInvestigator analysis software (MicroBrightField, Williston, VT) and the optical fractionator method42 according to previously published reports.11 Boundaries in the SNpc were defined according to previously defined anatomical analysis in the rat43 and cells were counted from ~24 sections (to ensure coefficient of errors <0.1) by investigators blinded to treatment history under a ×40 oil-immersion objective on a Nikon 80i microscope (Nikon Melville, NY). Stereological parameters were designated as previously described11 with the following modifications: average mounted thickness, 20 µm; optical dissector, 16 µm; and upper and lower guard zones, 2 µm.
Fluorescence immunocytochemistry. Free-floating immunohistochemical analyses of SNpc and striatal sections were performed as previously described.18 Sections were labeled with glial fibrillary acidic protein antibody diluted 1:1,000 (Dakocytomation; Dako North America, Carpinteria, CA), Iba1 antibody diluted 1:600 (AbCam, Cambridge, MA), GFP antibody diluted 1:1,000 (Rockland, Gilbertsville, PA), or huTNF antibody diluted 1:1,000 (R&D Systems, Minneapolis, MN) overnight at 4 °C. Appropriate Alexa-conjugated secondary antibodies diluted 1:1,000 (Invitrogen) were used at room temperature for 2.5 hours. Sections were mounted onto glass slides and coverslipped using BioMeda Gel-Mount. Images were captured with a Photometrics CoolSnap CCD ES monochromatic digital camera and analyzed with MetaMorph software (Universal Imaging Systems, West Chester, PA).
Confocal microscopy. Images of Iba1+ microglia and hDN-TNF-expressing cells were acquired using an Olympus FV1000 Laser Scanning Confocal system attached to an Olympus IX81 microscope (Center Valley, PA). Images (12 bits/pixel) and optical slices for a Z-stack series were obtained using a ×100 (1.45 numerical aperture) objective to capture microglia cell bodies and processes (Alexa-546+) and hDN-TNF+ cells (Alexa-488+) at the best resolution (0.38-µm interval step slices at a sampling speed of 20 µs/pixel) and processed for analysis using Olympus Fluoview FV10-ASW (version 01.07.02.02; Center valley, PA) software and Adobe Photoshop CS3 (version 10.0.1; San Jose, CA).
Quantification of microglia. Images of midbrain sections double-labeled with antibodies specific for Iba1 or TH were captured under ×4 objective lens on a Nikon 90i fluorescence microscope (Nikon, Melville, NY). Quantification of Iba1+ cells was performed using thresholding analysis on Nikon Elements 5 software from images taken with a ×20 PlanFluor objective with Alexa-546 filter set. Values represent the mean ± SEM of Iba1+ microglia within SNpc calculated from three separate brain sections (12 random fields/section) using threshold analysis in three animals per treatment group.
Statistical analyses. All data are expressed as mean ± SEM. TH+ and NeuN+ neurons were analyzed by one-way ANOVA (Figure 2 and Supplementary Figure S1). Behavioral data from the cylinder test was analyzed by repeated measures ANOVA (Figure 3). Significant effects were further examined by Fisher's protected least significant difference post hoc tests. A 2 (ipsilateral versus contralateral) × 2 (lenti-GFP versus lenti-DN-TNF) ANOVA was used to examine potential differences in Iba1+ microglia (Figure 5d). Significant effects were further examined using Fisher's protected least significant difference post hoc tests (Figure 5d). All statistical analyses were performed with the use of Statistica software, version 9 (Statsoft, Tulsa, OK). All parametric tests passed both the normality and equal variance test. The value for α was set at P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Survival of dopaminergic (DA) neurons in ipsilateral SNpc expressed as a percent of DA neurons in the contralateral SNpc.
Acknowledgments
We thank Xi Chen for technical advice with microglia quantification, Eric Eaton and Mike Sawchuck for technical help with confocal microscopy, and members of the Tansey lab for helpful discussions. Funding for this work was generously provided by The Michael J. Fox for Parkinson's Research and 5R01NS094933 (MGT) from NINDS/NIH. M.G.T. is a former employee of Xencor Inc., but does not hold significant financial stake in the company and does not consult for the company. Xencor Inc. did not provide any funding for these studies.
Supplementary Material
Survival of dopaminergic (DA) neurons in ipsilateral SNpc expressed as a percent of DA neurons in the contralateral SNpc.
REFERENCES
- Frank-Cannon TC, Alto LT, McAlpine FE., and, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases. Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL., and, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Samanta A, Feldmann M.2000. TNFα. In: Oppenheim JJ, Feldman M (eds) Cytokine Reference. Academic: New York. 414–434.
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y., and, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K., and, Nagatsu T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, et al. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B., and, Feldon J. Genetic ablation of tumor necrosis factor-α (TNF-α) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI., and, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI., and, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-α. FASEB J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A., and, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H., and, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, During MJ. Gene Therapy of the Central Nervous System: From Bench to Bedside. Academic Press: Amsterdam; Boston; 2006. [Google Scholar]
- Sánchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL., and, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T., and, Jones TA. “Exuberant” neuronal growth after brain damage in adult rats: the essential role of behavioral experience. J Neural Transplant Plast. 1993;4:193–198. doi: 10.1155/NP.1993.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner WJ. Motor fluctuations in Parkinson's disease. Rev Neurol Dis. 2006;3:101–108. [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH., and, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecka M, Klodowska-Duda G, Kurzawski M, Slawek J, Gorzkowska A, Opala G, et al. Interleukin-10 (IL10) and tumor necrosis factor α (TNF) gene polymorphisms in Parkinson's disease patients. Parkinsonism Relat Disord. 2008;14:636–640. doi: 10.1016/j.parkreldis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM., and, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- McCoy MK., and, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P., and, Isacson O. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson's disease. J Neuroinflammation. 2008;5:8. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C., and, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sauer H., and, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Stern MB., and, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72 21 Suppl 4:S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- Robinson WH, Genovese MC., and, Moreland LW. Demyelinating and neurologic events reported in association with tumor necrosis factor α antagonism: by what mechanisms could tumor necrosis factor α antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis. Arthritis Rheum. 2001;44:1977–1983. doi: 10.1002/1529-0131(200109)44:9<1977::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Sambamurti K., and, Greig NH. TNF-α inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- van Oosten BW, Barkhof F, Truyen L, Boringa JB, Bertelsmann FW, von Blomberg BM, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996;47:1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]
- Olleros ML, Guler R, Vesin D, Parapanov R, Marchal G, Martinez-Soria E, et al. Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-guerin and Mycobacterium tuberculosis infections. Am J Pathol. 2005;166:1109–1120. doi: 10.1016/S0002-9440(10)62331-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, et al. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK., and, Ting JP. TNF α promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Kassiotis G, Bauer J, Akassoglou K, Lassmann H, Kollias G., and, Probert L. A tumor necrosis factor-induced model of human primary demyelinating diseases develops in immunodeficient mice. Eur J Immunol. 1999;29:912–917. doi: 10.1002/(SICI)1521-4141(199903)29:03<912::AID-IMMU912>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Mohand-Said S, Hanoteau N, Fuchs C, Pfizenmaier K., and, Eisel U. Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. J Neurosci. 2002;22:RC216. doi: 10.1523/JNEUROSCI.22-07-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger SW, Hörster D, Furukawa K, Goodman Y, Krieglstein J., and, Mattson MP. Tumor necrosis factors α and β protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a κB-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, et al. Chronic neuron-specific tumor necrosis factor-α expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG. Parkinson disease: another player in gene therapy for Parkinson disease. Nat Rev Neurol. 2010;6:7–8. doi: 10.1038/nrneurol.2009.214. [DOI] [PubMed] [Google Scholar]
- Lim ST, Airavaara M., and, Harvey BK. Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res. 2010;61:14–26. doi: 10.1016/j.phrs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, et al. Dopamine gene therapy for Parkinson's disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009;1:2ra4. doi: 10.1126/scitranslmed.3000130. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Ikawa M, Dayn Y., and, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci USA. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-García AM, Zhao X, Liu SJ, Jones TA., and, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, et al. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L., and, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- German DC., and, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival of dopaminergic (DA) neurons in ipsilateral SNpc expressed as a percent of DA neurons in the contralateral SNpc.