Abstract
Duchenne muscular dystrophy (DMD) is associated with mutations in the dystrophin gene that disrupt the open reading frame whereas the milder Becker's form is associated with mutations which leave an in-frame mRNA transcript that can be translated into a protein that includes the N- and C- terminal functional domains. It has been shown that by excluding specific exons at, or adjacent to, frame-shifting mutations, open reading frame can be restored to an out-of-frame mRNA, leading to the production of a partially functional Becker-like dystrophin protein. Such targeted exclusion can be achieved by administration of oligonucleotides that are complementary to sequences that are crucial to normal splicing of the exon into the transcript. This principle has been validated in mouse and canine models of DMD with a number of variants of oligonucleotide analogue chemistries and by transduction with adeno-associated virus (AAV)-small nuclear RNA (snRNA) reagents encoding the antisense sequence. Two different oligonucleotide agents are now being investigated in human trials for splicing out of exon 51 with some early indications of success at the biochemical level.
Introduction
From the moment of its identification, the Duchenne muscular dystrophy (DMD) gene, was clearly going to test the ingenuity of would-be gene therapists. The need to achieve body-wide distribution of the largest known gene is compounded by its structural role as the keystone of a transmembrane cell–surface protein complex; removing the possibility, even with a fully functional protein, of the amplifying effect of an enzyme and implying the need for near-normal molar concentrations to approach normal function. Strange then, that one of the more promising strategies for treating DMD, the skipping of mutated sites, is actually facilitated by the large size and modular structure of dystrophin: its major functional binding sites being separated by a long stretch of rod-like “spacer” that carries no essential function and is the site of the more common DMD mutations.
Use of antisense oligonucleotides to modulate splicing of the dystrophin gene so as to restore a translatable mRNA transcript was mooted some years ago on the basis of in vitro data1,2 but firm evidence for practical utility of this approach awaited studies in the mdx mouse model of DMD.3,4,5,6 These, in turn, set in train a concerted effort to advance the technology toward human trials, as summarized in the following accounts of work presented and discussed at a meeting held in the Banbury Center at Cold Spring Harbor from the 14th to the 17th of October 2008.
Chemistry and Modifications: Crucial for Realizing Therapeutic Potential
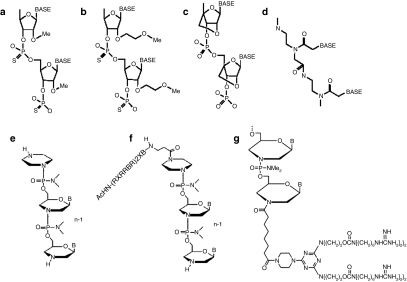
Progressive advances in exon skipping for DMD have been related to the application of new antisense oligomer chemistries and their modification for improved delivery (Figure 1). The most widely used chemistry is the 2′O-methylphosphorothioate-modified (2′OMePS) antisense oligoribonucleotide (AON). This modification provides resistance to nuclease degradation while retaining negative charge to facilitate effective delivery in cell culture systems by most delivery reagents.7 The potential of this chemistry for treating DMD was initially demonstrated in dystrophic mdx mice5,6 and more recently by intramuscular injection in DMD patients.8 However, for systemic delivery, 2′OMePS showed limited efficiency in the mdx mouse. Three intravenous (i.v.) injections of 2 mg 2′OMePS/mouse (~60–80 mg/kg) at weekly intervals did induce detectable dystrophin expression in all skeletal muscles, but only in sparse focal patches of muscle fibers within each muscle and never at >5% of normal levels. Disappointingly too, little or no dystrophin expression was seen in cardiac muscle. No toxicity to liver or kidney was observed. Thus, assuming that the preclinical model recapitulates precisely the efficiency and pharmacokinetics of administration to DMD boys, 2′OMePS appear safe but it is uncertain whether their systemic use would induce sufficient dystrophin expression to have a therapeutic impact in DMD boys.4
Figure 1.
Chemistries of antisense oligomers. (a) 2′-O-Methylphosphorothioate (2′OMePS AON); (b) 2′-O-methoxyethyl phosphorothioate; (c) locked nucleic acid (LNA); (d) peptide nucleic acid (PNA); (e) phosphorodiamidate morpholino oligomers (PMO); (f) AcHN-(RXRRBR)2XB peptide-tagged PMO (R, arginine, X, 6-aminohexanoic acid and B, ®- alanine) (PPMO); G, octa-guanidine PMO.
More recently, phosphorodiamidate morpholino oligomers (PMO) have been explored for exon skipping in the dystrophin gene. In PMO, the phosphodiester bond is replaced by phosphorodiamidate linkage and the ribose replaced by a morpholino moiety (Figure 1). PMOs are charge-neutral and refractory to biological degradation. This chemistry has long been used for translational blockade in zebrafish; penetrating the cells of the developing fishes relatively easily.9 It has also been applied to cultured mammalian cells10 where its delivery appears to be impeded by its nonionic nature. In response to this problem, “scrape-loading” (creating pores in the membrane) and “leashing” (complexing PMO with negatively charged complementary DNA sequences) were then developed to enhance delivery by use of commercially available delivery reagents, such as polyethyleneimine and lipofectin.11 However, on direct injection into muscles the leash adjunct proved toxic and was therefore not tested by i.v. administration. Despite the poor entry of unmodified PMO into cells in tissue culture, it was later found to enter muscle fibers better than 2′OMePS in vivo in the dystrophic mdx mouse. A single intramuscular injection of 10 µg PMO induced significantly higher levels of dystrophin expression than the same PMO complexed with leash and lipofectin.3 Furthermore, regular weekly i.v. injections of PMO targeting mouse dystrophin exon 23 induced up to 50% of normal levels of dystrophin in body-wide skeletal muscles in the mdx mice, with improved muscle pathology, decreased serum levels of muscle creatine kinase and partial restoration of normalized muscle strength. Even after systemic administration for 1 year, no toxicity has been detected in muscles or other organs. A more recent investigation at higher dosages12 confirmed that PMO produced higher levels of exon 23 skipping than 2′OMePS and thus appears to be a promising antisense oligomer chemistry for the treatment of DMD patients.3
Although both 2′OMePS and PMO induce exon skipping systemically, it was disappointing to find that dystrophin expression was highly variable within and between muscles, even after repeated i.v. injections.3,4,12 Why this is so, is not clearly understood, but may be due to the reliance on passive diffusion for entry into muscle fibers. For PMO, the lack of charge may present less of an impediment to cell surface contact thus allowing more efficient entry than 2′OMePS into muscle fibers, particularly those with leaky membranes as seen in dystrophic muscles. Such dependence on muscle damage for effective delivery of AONs, would have the advantage of limiting the amount of AON entering untargeted and undamaged nonmuscle cells, thus diminishing possible side effects. However, for long-term effective treatment of DMD, it would carry the disadvantage that muscle fibers rescued by PMO-induced exon skipping would have to re-enter a myopathic state to permit further PMO entry. Such a requirement for recurring cycles of rescue and degeneration in treated muscles could severely limit the value of antisense therapy for DMD patients.
The requirement of muscle damage for effective delivery and AON induced dystrophin expression is further demonstrated by the relative lack of dystophin expression in cardiac muscle of mdx mice after systemic injection of either 2′OMePS AON or PMO.3,4,12 Cardiac muscles in the mice are less affected than skeletal muscle by the dystrophic process and neither conspicuous pathological change nor functional impairment are seen until late stages. Consistently, only trace amounts of dystrophin are detected in cardiac muscle even after repeated injections into mdx mice of either 20′ MePS or PMO AON3,4,12 even with doses of PMO that induce high levels of dystrophin in skeletal muscles. However, direct injection of AON or adeno-associated virus (AAV)-mediated AON delivery induced effective dystrophin expression in cardiac muscles, suggesting that efficiency of delivery rather than of exon-skipping is the critical factor in this organ.13
One way of enhancing intracellular delivery is to employ cell-penetrating peptides or polymers to provide active transport of AON into muscle fibers. Earlier studies showed that conjugation to an arginine-rich peptide significantly improved PMO-mediated antiviral activity14 as well as delivery of PMO for dystrophin exon skipping in cell cultures and on intramuscular injection into muscles.15 More recently, Jearawiriyapaisarn et al.16 used a transgenic mouse that expresses enhanced green fluorescent protein as a positive readout for the efficiency of exon exclusion to evaluate the potency, functional biodistribution, and toxicity of PMOs conjugated to various arginine-rich cell-penetrating peptides containing 6-aminohexanoic acid (X) and/or β-alanine. The greatest restoration of enhanced green fluorescent protein expression in both skeletal and cardiac muscles was observed with PMO tagged with a peptide of (RXRRBR)2XB (PPMO). When applied to the dystrophic mdx mice model of DMD, a single i.v. injection of 30 mg/kg of PPMO restored dystrophin in all skeletal muscles to almost normal levels17 that were maintained by regular biweekly administration over 12 weeks and accompanied by improvement in muscle strength and pathology, with significant lowering of serum creatine kinase levels. Most importantly, i.v. injections of PPMO elicited near-normal levels of dystrophin in cardiac muscle (Figure 2) and prevented dobutamine-induced cardiac failure. Efficient exon skipping was also achieved in smooth muscles in other organs such as the esophagus. Treatment with the PPMO did not cause detectable toxicity. Recently, this PPMO has been shown to considerably ameliorate the severe pathology in the dystrophin–utrophin double null mouse.18 Together, these findings illustrate the theoretical feasibility of using PPMO to rescue dystrophin expression in both skeletal and cardiac muscles of DMD patients.
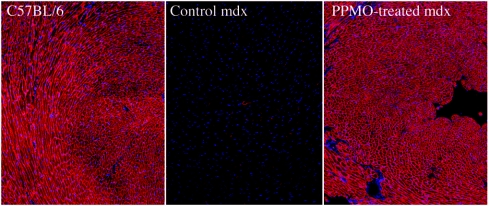
Figure 2.
Restoration of dystrophin in cardiac muscles of mdx mice after six intravenous injections (at biweekly intervals) of 30 mg/kg of the PPMOE23 targeting mouse dystrophin exon 23. Muscles were examined 2 weeks after the last injection. Left panel, muscles from heart of normal C57BL/6. Middle panel, muscle from heart of control mdx mouse. Right panel, PPMO-treated mdx. Dystrophin was detected by immunochemistry with the polyclonal rabbit antidystrophin antibody, P7, and visualized with Alexa 594 tagged goat-anti-rabbit Igs. Blue nuclear staining with DAPI.
However, use of peptides to enhance delivery raises the possibility of an immune response that may prevent repeated administration or cause rejection of targeted tissues or both, especially because DMD patients would require regular life-long administration. Although no immune response was observed in the above study17 or in previous reports with similar peptides in animal models,14,19 immunogenicity varies considerably between species, arguing for longer-term studies in a range of animal models. But final verification can come only from clinical trials. It is, therefore, important to develop nonpeptide alternatives to enhance delivery of oligomers. The known sequence and structure of the peptide used in the PPMO provides a basis for modeling such nonpeptide polymers as delivery vehicles with similar or improved function. With this in mind, Wu et al. exploited a nonlinear, nonpeptidic dendrimer as a transporter for delivery of PMO. This consists of eight guanidinium head groups bonded to a trifunctional triazine as a core scaffold, which is then conjugated to PMO targeting exon 23 (ref. 6) (termed Vivo-PMO).20 The study demonstrated that the Vivo-PMO targeting mouse dystrophin exon 23 (Vivo-PMOE23) is highly effective for exon skipping and dystrophin induction in mdx mice. A single i.v. injection of 6 mg/kg Vivo-PMOE23 generated dystrophin expression in skeletal muscles at levels equivalent to the injection of 300 mg/kg unmodified PMOE23. Repeated injections of 6 mg/kg Vivo-PMOE23 achieved ~50% and 10% wild-type levels of dystrophin expression in body-wide skeletal muscles and in cardiac muscle respectively, without eliciting a detectable immune response. Vivo-PMOs showed no signs of toxicity at the effective dosage regime that reduced the serum levels of creatine kinase significantly.20 These results thus offer prospects for the development of new nonpeptide delivery moieties with improved function and low toxicity.
Multiexon Skipping in Dystrophic Dogs
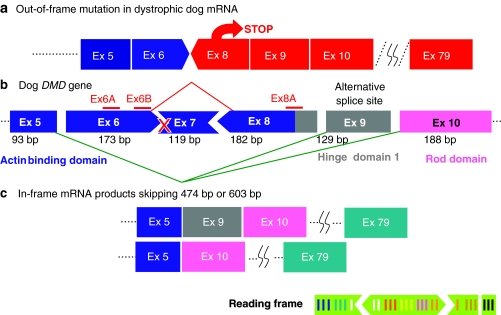
Although antisense-mediated exon skipping clinical trials currently conducted in United Kingdom and Netherlands targeting exon 51 show promising results,8,21 such single exon skipping covers only a proportion of DMD patients. Even if antisense oligos against most exons in the DMD gene become available, approximately half of DMD patients will require multiexon skipping by targeting of more than one exon, depending, not on the size but on the type of mutation (e.g., deletion, duplication, point mutation, etc.) and the “phase” of the mutated exon and its neighboring exons. For example, to treat a patient with deletion of exon 7, one needs to target at least two exons (e.g., both exon 6 and exon 8) to put the mutation back in frame (Figure 1). In fact, canine X-linked muscular dystrophy harbors such mutation22 (i.e., a splice site mutation in intron 6 that excludes exon 7 from the mRNA transcript (Figure 3)) and is therefore, a good model for testing the efficacy and efficiency of double-exon skipping.23 The dystrophic dog has several further advantages over the mdx mouse. First, it provides the prospect of more detailed analyses of clinical condition, such as clinical grading, magnetic resonance imaging, three-dimensional-echocardiography, and electrocardiogram.23 Second, the canine X-linked muscular dystrophy model, is closer in clinical phenotype than the mdx mouse model to human DMD. Indeed, it shows, if anything, a more severe progression than DMD; this, in combination with its similarity in body weight, makes it especially useful for physiological and toxicological studies.23 Finally, there may be some advantage in the fact that many target sites for exon skipping show identity of DNA (mRNA) sequences between dog and human. Drug regulation authorities such as US Food and Drug Administration are inclined to regard antisense oligonucleotides (AOs) of different sequences as different drugs; thus, to target the range of mutations encountered in DMD patients, many AO sequences will need to be designed, tested, and approved. Between man and mouse targeting homologous sites has little predictive value, perhaps due to minor sequence differences.24 Dogs and humans however, share considerable sequence identity; for most exons in the DMD gene one can design a single 20–25mer antisense sequence that is applicable to both and comparison of targeting efficiencies between these species should be explored further.
Figure 3.
Diagram to illustrate the exon-skipping strategy to restore open reading frame at the mutation site in the CXMD dystrophic dog. A point mutation in the acceptor splice site in intron 6 preceeding exon 7 (X) leads to exclusion of exon 7 from the transcript and loss of open reading frame when exon 6 is spliced to exon 8. To restore open reading frame, requires the loss of at least two further exons: 6 and 8. In the event, exon 9 is also excluded from the transcript but, because it contains a whole number of codon triplets, this does not disrupt the translation of the resultant mRNA. CXMD, canine X-linked muscular dystrophy.
Overall, the dog experiments provide a promising message for DMD patients. McClorey and colleagues transfected cultured myotubes from dystrophic dogs in vitro with a cocktail of antisense oligos targeting exons 6 and 8, noting restoration of reading frame in mRNA.25 Recently, we sought to test efficacy and toxicity of i.v. PMO induced exon skipping in vivo in the DMD dog model.26 We identified a cocktail that, by either intramuscular injection or systemic i.v. delivery, resulted in extensive dystrophin expression to therapeutic levels. Weekly or biweekly systemic i.v. injections, over the course of 5–22 weeks, with a three-morpholino cocktail (120–200 mg/kg in total of three oligos/injection) targeting exon 6 and exon 8, induced therapeutic levels of dystrophin expression throughout the body, to an average of 26% of normal levels. Expression of dystrophin was associated with significant functional and clinical stabilization, being accompanied by reduced inflammation as observed histologically and by magnetic resonance imaging, improved or stabilized clinical symptoms and timed running tests. Histology and blood tests indicated no evidence of toxicity. Dystrophin expression was also detected in cardiac muscles by immunohistochemistry but, as in the mdx mouse,3,4 less than in skeletal muscles and concentrated in small patches. Recently, we have found that an i.v. injection of peptide-conjugated morpholinos (PPMOs) at 12 mg/kg elicited increased dystrophin expression in the canine heart, as detected by western blotting (Yokota et al., data not shown).
An unexpected observation in the dog study was that, in tissue culture, either of the two antisense oligonucleotide components of the cocktail directed against exon 6 were able, alone, to efficiently induce the desired exon 5–10 splicing in the absence of the sequence against exon 8. By contrast, they did not do this in vivo. In addition, excision of exon 8 by the exon 6-specific sequences alone occurred only in the context of the mutant exon 7 splice site (i.e., it did not occur in wild-type dog cells). Similarly, AO administration to human cells produced some disparities in skipping between patients carrying small mutations in the DMD gene and wild-type cells.27 The differences between patterns of skipping in vivo versus in vitro and between wild-type versus mutant genotypes indicate that the pattern of exon skipping is greatly influenced by variables other than the local presence of target sequence. Thus, it is prudent to consider testing of selected target sequences in multiple systems before committing to a specific sequence for subsequent clinic trials.
Significance of Multiexon Skipping
Theoretically, multiple exon skipping could restore open reading frame in >80% both of deletion and nonsense mutations in the DMD gene.28,29,30,31 Moreover, since some in-frame deletions are associated with milder phenotypes than others, selective skipping of more exons than are required for simple restoration of reading frame offers the prospect of selecting options that optimize the functionality of the resultant dystrophin protein. Thus, it has been proposed that a cocktail of AOs targeting exons 45–55, a deletion associated with a high percentage of asymptomatic or mild BMD clinical phenotypes32 would potentially be applicable to 63% of patients with dystrophin deletions. Currently, techniques for skipping 11 exons simultaneously are not available but might be achieved in future by improved efficacy of AO chemistry or more efficient delivery methods.
AAV U7 Generation of Antisense Oligonucleotides
Perhaps the most efficient way to achieve long lasting exon skipping, without recurrent infusions of antisense oligonucleotides, would be to generate the antisense agent within the target cells. Current studies have used gene vectors expressing modified U7 or U1 small-nuclear RNAs as antisense shuttles (AS-snRNAs).33,34,35 Because these expression cassettes are very small (AS-U7 is about 400 nucleotides) there is sufficient room within gene vectors to combine several copies of different AS-snRNAs designed to target multiple exons within a gene or even different genes simultaneously.
Although a number of viral vectors could be used for the delivery of such AS-snRNA chimeras in tissue culture as well as in vivo, AAV have come to the fore, offering the advantage of stable long-term expression. Current AAV8-, AAV1- and AAV6-capsids effect efficient and widespread transduction of muscles in mice after tail vein administration,36 with promising new serotypes pending.37,38 Systemic delivery of AAV vectors harboring AS-U1 in the mdx mouse resulted in effective body-wide dissemination of the therapeutic construct and significant improvement of muscle function suggestive of overall maintenance of muscle mass and strength.39 Similar results have been obtained with the AS-U7 system:35 sustained dystrophin rescue to near wild-type levels and restoration of normal levels of muscle resistance to mechanical stress. In addition, no immune response has been reported, against the rescued dystrophin, due perhaps to fact that the rescued truncated dystrophin is represented in the repertoire of pre-existing revertant fibers, which naturally occur in dystrophic mice. However, while the long-term stability of corrected fibers was clearly demonstrated in the mdx mouse,13,40 the AAV(AS-snRNA) approach still faces problems arising from immune sensitization against AAV, that would prevent the application of repeated treatment unless an effective regime of immunomodulation can be developed.36
For most myopathic disorders, to be of practical clinical therapeutic value, a genetic therapy would, ideally, provide treatment of the whole skeletal and cardiac musculature. As has been demonstrated by initial experimental trials in murine models, this cannot be achieved by intramuscular injections; only a systemic injection can approach this objective. Such a systemic delivery procedure is not without risk and entails long and expensive development, in particular to overcome the immune problems.41,42 First, production of the large quantity of vector required to treat even a single patient is a daunting task that is the objective of a number of methods for large scale AAV production currently being developed.43 Second, practicability of the AAV(AS-snRNA) technology requires development of a safe and effective protocol for systemic administration. This needs to be tested in a large animal, such as the canine X-linked muscular dystrophy dog, to permit evaluation of the dose range and the protocols of administration of the vectors required to achieve therapeutic effectiveness while remaining safe. In order to anticipate, on a rational basis, the adaptation of such a protocol to trials in man, it is important to conduct such studies in conditions that mimic clinical practice as closely as possible.
Ongoing Therapeutic Trials Using Antisense Oligonucleotides
Two European consortia are involved in clinical trials using two different antisense oligonucleotide chemistries. One group is based in Holland, closely associated with the Leiden University Medical School (Prof Gert van Ommen and Dr Jan Verschuuren) and works in close collaboration with the company Prosensa, which also sponsored these studies. The second group is based in United Kingdom, where a consortium of four Universities (MDEX consortium) is led by F.M., and works in close collaboration with AVI Biopharma, which is sponsoring the present study.
Both groups are targeting exon 51, although using two different primary sequences, and different backbones. The Dutch study utilizes a 21-mer 2′OMePS,7 whereas the MDEX Consortium is employing a 30 PMO.44 Both groups elected to study patients with deletions who would benefit from exon 51 skipping (50, 52, 52–63, 45–50, 48–50, and 49–50), both because cumulatively these account for 13% of all DMD deletions,7,28 and more especially because the resulting protein has been clearly demonstrated to be extremely functional, as suggested by several multigenerational families deleted for the same domains with no symptoms whatsoever.45,46,47
The Dutch consortium have completed and published in 2007 the result of a proof of concept study in which four DMD boys have received a single injection of the 2′OMePS into the tibialis anterior. This was well tolerated and accompanied by specific skipping of exon 51 as well as detection of sarcolemmal dystrophin in 64–97% of myofibers of the biopsied muscle; the amount of dystrophin ranged from 3 to 12% of that found in the normal control muscle and with intensities in individual fibers ranging from 17 to 35%.8
The MDEX consortium performed a similar study using the PMO AO, but with a different design: a dose escalation study in seven DMD boys, who received either 0.09 or 0.9 mg in one of the two extensor digitorum brevis muscles, whereas the contralateral muscle received saline. The results, recently published,21 demonstrated clearly detectable dystrophin expression in 44–79% of myofibers, with intensity of dystrophin staining averaging 17% greater than the levels in the contralateral muscle and, in the most positive fibers, up to 42% of that in healthy muscle fibers.21
Both studies have been followed by repeated systemic administration studies. The Dutch consortium recently completed a study in which four group of DMD boys received escalating doses of the 2′OMePS antisense to skip exon 51, subcutaneously, at doses of 0.5, 2.0, 4.0, and 6.0 mg/kg, weekly for 5 weeks. All 12 children (3/group) had a muscle biopsy at the beginning and the end of the study. While the results of this study have not yet been published, Dr Goemans reported at the World Muscle Society meeting in 2009 (Geneva)48 that the study drug was well tolerated and that a dose–response in exon skipping and dystrophin production was observed. All boys who received the 2′OMePS AO have been enrolled in an extension study that is currently underway.
Encouraging results have also been announced by the analysis of the first four cohorts of the boys recruited into the MDEX systemic study using the PMO. In this study, seven groups of DMD boys received escalating doses of PMO (0.5, 1.0, 2.0, 4.0, 10, and 20 mg/kg) for a period of 12 weeks. All patients had a pretreatment and post-treatment muscle biopsy. At the time of writing only the first four cohorts have completed the study, and the preliminary analysis indicates that in the three patients in the 2.0 and 4.0 mg/kg cohorts there was accurate skipping of exon 51. In one of the patients at the 2.0 mg/kg dose, the appearance of skipped mRNA was accompanied by a several fold increase in expression of dystrophin protein in the post-treatment samples using both western blotting and immunofluorescent analysis (fivefold on western blot and approximately sevenfold on immunocytochemistry). While the results of the patients recruited into the last two cohorts will not be available until the 2nd quarter of 2010, both these results, and those from the Dutch consortium are very encouraging. Two pivotal multicentric phase III studies are currently being planned, one by Prosensa/GSK, using the 2′OMePS AO, and one by AVI Biopharma, using the PMO AO, and are both likely to start in 2010. The design will be a randomized placebo controlled study which is likely to last for ~1 year. Additional studies are also being planned by Prosensa (a multicentre phase I/II study targeting exon 44 with a 2′OMePS, whereas target optimization for exon 43, 45, 46, and 52 are being pursued, possibly followed by further clinical studies in 2011–2012). In addition AVI Biopharma has initiated a preclinical program which is anticipated to lead to an IND/IMPD filing in early 2010 for it's lead peptide-conjugated PMO (PPMO) to skip exon 50 and thus into a clinical study which is currently being planned.
Prospects
As attested by the above accounts, the potential for use of exon skipping as a therapeutic strategy for DMD has developed from a plausible notion in the mid-1990's1,2 to the point where early clinical trials show that it holds realistic prospects of providing genuine therapeutic benefit. There remain, however, substantial barriers: some scientific, some regulatory, with occasional interaction between the two.
The major scientific issues concern the choice of sequence for any given exon and the enhancement of delivery and effectiveness of that sequence to the majority, ideally all, of the muscle fibers in the body.
Although, effective sequences that promote skipping of a number of exons have been identified, we have no reliable method for determining whether any given sequence is optimal. A thorough screen for optimal sequences alone and in combination requires the ease of use of a tissue culture system and although a broad correspondence has shown between the in vitro and in vivo activities of different chemistries and adjuncts49,50 it is evident from the canine studies26 that myogenic cultures cannot be relied upon to inform us accurately as to the in vivo activity of various sequences. A recent study of equivalent sequences that target human and mouse exons confirms the view that the efficacy of targeting is highly context dependent24 and that we should be wary of generalizing the applicability of specific results from one test system.
As to delivery, most work in the mdx mouse favors PMO over 2′OMePS backbone chemistry, but neither shows great promise for entering cardiac muscle in useful amounts and even in skeletal muscle, effectiveness is patchy. We are therefore in need of developments such as the addition of cell-penetrating moieties which, in turn, will entail extensive animal studies to establish dosage regimes that provide efficacy with minimal toxicity.
For the AAV(AS-snRNA) approach, the ideal would be a single body-wide delivery to 100% of cardiac and skeletal muscle cells, with the reasonable expectation that this would need to be repeated rarely, perhaps never if we are lucky. At present, such efficient delivery does not seem to be possible with a single infusion and the potential immune complications; generation of neutralizing antibodies and of cell-mediated response to residual viral antigens mandates a thorough appraisal of multiple delivery protocols.
For regulatory bodies, antisense induced exon skipping represents an extreme example of agents that are highly targeted to the individual patient, and poses a potentially educative challenge to the appropriateness of standard procedures. The combination of a need for at least one different oligonucleotide sequence for each target exon and the large number of different exons, together with the small numbers of patients who might benefit from skipping of some specific exons, raises considerable obstacles to the conduct of standard safety and efficacy regimes. The problem is further compounded by the fact that sequence-specific side effects are likely to be species-specific and therefore not reliably assessable on animal models. A requirement for a full toxicological workup of each individual sequence would be a major disincentive for manufacturers to extend their interests beyond a small number of the more widely applicable target exons or even to seek to optimize sequences for the commoner exon targets. Moreover, many target exons would be relevant to too few patients to permit conduct of any form of conventionally designed trial. Thus, imposition of the normal regulatory processes would constitute a major impediment to the application of exon-skipping therapy across the range of patients who might benefit from it. A positive exploration of these issues would act as a trailblazer to the benefit of the progress of personalized medicine in general.
REFERENCES
- Dunckley MG, Manoharan M, Villiet P, Eperon IC., and, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. doi: 10.1093/hmg/7.7.1083. [DOI] [PubMed] [Google Scholar]
- Takeshima Y, Nishio H, Sakamoto H, Nakamura H., and, Matsuo M. Modulation of in vitro splicing of the upstream intron by modifying an intra-exon sequence which is deleted from the dystrophin gene in dystrophin Kobe. J Clin Invest. 1995;95:515–520. doi: 10.1172/JCI117693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, et al. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci USA. 2005;102:198–203. doi: 10.1073/pnas.0406700102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. doi: 10.1038/nm897. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci USA. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ., and, van Deutekom JC. Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12 Suppl 1:S71–S77. doi: 10.1016/s0960-8966(02)00086-x. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Nasevicius A., and, Ekker SC. Effective targeted gene “knockdown” in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Bruno IG, Jin W., and, Cote GJ. Correction of aberrant FGFR1 alternative RNA splicing through targeting of intronic regulatory elements. Hum Mol Genet. 2004;13:2409–2420. doi: 10.1093/hmg/ddh272. [DOI] [PubMed] [Google Scholar]
- Gebski BL, Mann CJ, Fletcher S., and, Wilton SD. Morpholino antisense oligonucleotide induced dystrophin exon 23 skipping in mdx mouse muscle. Hum Mol Genet. 2003;12:1801–1811. doi: 10.1093/hmg/ddg196. [DOI] [PubMed] [Google Scholar]
- Heemskerk HA, de Winter CL, de Kimpe SJ, van Kuik-Romeijn P, Heuvelmans N, Platenburg GJ, et al. In vivo comparison of 2'-O-methyl phosphorothioate and morpholino antisense oligonucleotides for Duchenne muscular dystrophy exon skipping. J Gene Med. 2009;11:257–266. doi: 10.1002/jgm.1288. [DOI] [PubMed] [Google Scholar]
- Denti MA, Incitti T, Sthandier O, Nicoletti C, De Angelis FG, Rizzuto E, et al. Long-term benefit of adeno-associated virus/antisense-mediated exon skipping in dystrophic mice. Hum Gene Ther. 2008;19:601–608. doi: 10.1089/hum.2008.012. [DOI] [PubMed] [Google Scholar]
- Abes S, Moulton HM, Clair P, Prevot P, Youngblood DS, Wu RP, et al. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J Control Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Yin H, Lu Q., and, Wood M. Effective exon skipping and restoration of dystrophin expression by peptide nucleic acid antisense oligonucleotides in mdx mice. Mol Ther. 2008;16:38–45. doi: 10.1038/sj.mt.6300329. [DOI] [PubMed] [Google Scholar]
- Jearawiriyapaisarn N, Moulton HM, Buckley B, Roberts J, Sazani P, Fucharoen S. Sustained dystrophin expression induced by peptide-conjugated morpholino oligomers in the muscles of mdx mice. Mol Ther. 2008;16:1624–1629. doi: 10.1038/mt.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, et al. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyenvalle A, Babbs A, Powell D, Kole R, Fletcher S, Wilton SD, et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher S, Honeyman K, Fall AM, Harding PL, Johnsen RD, Steinhaus JP, et al. Morpholino oligomer-mediated exon skipping averts the onset of dystrophic pathology in the mdx mouse. Mol Ther. 2007;15:1587–1592. doi: 10.1038/sj.mt.6300245. [DOI] [PubMed] [Google Scholar]
- Wu B, Li Y, Morcos PA, Doran TJ, Lu P., and, Lu QL. Octa-guanidine morpholino restores dystrophin expression in cardiac and skeletal muscles and ameliorates pathology in dystrophic mdx mice. Mol Ther. 2009;17:864–871. doi: 10.1038/mt.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- Shimatsu Y, Yoshimura M, Yuasa K, Urasawa N, Tomohiro M, Nakura M, et al. Major clinical and histopathological characteristics of canine X-linked muscular dystrophy in Japan, CXMDJ. Acta Myol. 2005;24:145–154. [PubMed] [Google Scholar]
- Mitrpant C, Adams AM, Meloni PL, Muntoni F, Fletcher S., and, Wilton SD. Rational design of antisense oligomers to induce dystrophin exon skipping. Mol Ther. 2009;17:1418–1426. doi: 10.1038/mt.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClorey G, Moulton HM, Iversen PL, Fletcher S., and, Wilton SD. Antisense oligonucleotide-induced exon skipping restores dystrophin expression in vitro in a canine model of DMD. Gene Ther. 2006;13:1373–1381. doi: 10.1038/sj.gt.3302800. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitali P, Rimessi P, Fabris M, Perrone D, Falzarano S, Bovolenta M, et al. Exon skipping-mediated dystrophin reading frame restoration for small mutations. Hum Mutat. 2009;30:1527–1534. doi: 10.1002/humu.21092. [DOI] [PubMed] [Google Scholar]
- Aartsma-Rus A, Fokkema I, Verschuuren J, Ginjaar I, van Deutekom J, van Ommen GJ, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- Yokota T, Duddy W., and, Partridge T. Optimizing exon skipping therapies for DMD. Acta Myol. 2007;26:179–184. [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Pistilli E, Duddy W., and, Nagaraju K. Potential of oligonucleotide-mediated exon-skipping therapy for Duchenne muscular dystrophy. Expert Opin Biol Ther. 2007;7:831–842. doi: 10.1517/14712598.7.6.831. [DOI] [PubMed] [Google Scholar]
- Yokota T, Takeda S, Lu QL, Partridge TA, Nakamura A., and, Hoffman EP. A renaissance for antisense oligonucleotide drugs in neurology: exon skipping breaks new ground. Arch Neurol. 2009;66:32–38. doi: 10.1001/archneurol.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béroud C, Tuffery-Giraud S, Matsuo M, Hamroun D, Humbertclaude V, Monnier N, et al. Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat. 2007;28:196–202. doi: 10.1002/humu.20428. [DOI] [PubMed] [Google Scholar]
- De Angelis FG, Sthandier O, Berarducci B, Toso S, Galluzzi G, Ricci E, et al. Chimeric snRNA molecules carrying antisense sequences against the splice junctions of exon 51 of the dystrophin pre-mRNA induce exon skipping and restoration of a dystrophin synthesis in Delta 48-50 DMD cells. Proc Natl Acad Sci USA. 2002;99:9456–9461. doi: 10.1073/pnas.142302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman L, Suter D, Emerick V, Schümperli D., and, Kole R. Stable alteration of pre-mRNA splicing patterns by modified U7 small nuclear RNAs. Proc Natl Acad Sci USA. 1998;95:4929–4934. doi: 10.1073/pnas.95.9.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Lorain S, Gross DA, Goyenvalle A, Danos O, Davoust J., and, Garcia L. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther. 2008;16:541–547. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Wang L., and, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- Yu CY, Yuan Z, Cao Z, Wang B, Qiao C, Li J, et al. A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 2009;16:953–962. doi: 10.1038/gt.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denti MA, Rosa A, D'Antona G, Sthandier O, De Angelis FG, Nicoletti C, et al. Chimeric adeno-associated virus/antisense U1 small nuclear RNA effectively rescues dystrophin synthesis and muscle function by local treatment of mdx mice. Hum Gene Ther. 2006;17:565–574. doi: 10.1089/hum.2006.17.565. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Poupiot J,, Goyenvalle A,, Perez N,, Garcia L,, Danos, O, et al. Noninvasive monitoring of therapeutic gene transfer in animal models of muscular dystrophies. Gene Ther. 2006;13:20–8.. doi: 10.1038/sj.gt.3302594. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Virag T, Cecchini S., and, Kotin RM. Producing recombinant adeno-associated virus in foster cells: overcoming production limitations using a baculovirus-insect cell expression strategy. Hum Gene Ther. 2009;20:807–817. doi: 10.1089/hum.2009.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arechavala-Gomeza V, Graham IR, Popplewell LJ, Adams AM, Aartsma-Rus A, Kinali M. Comparative analysis of antisense oligonucleotide sequences for targeted skipping of exon 51 during dystrophin pre-mRNA splicing in human muscle. Hum Gene Ther. 2007;18:798–810. doi: 10.1089/hum.2006.061. [DOI] [PubMed] [Google Scholar]
- Lesca G, Testard H, Streichenberger N, Pelissier JF, Lestra C, Burel E, et al. [Family study allows more optimistic prognosis and genetic counselling in a child with a deletion of exons 50-51 of the dystrophin gene] Arch Pediatr. 2007;14:262–265. doi: 10.1016/j.arcped.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Melis MA, Cau M, Muntoni F, Mateddu A, Galanello R, Boccone L, et al. Elevation of serum creatine kinase as the only manifestation of an intragenic deletion of the dystrophin gene in three unrelated families. Eur J Paediatr Neurol. 1998;2:255–261. doi: 10.1016/s1090-3798(98)80039-1. [DOI] [PubMed] [Google Scholar]
- Saengpattrachai M, Ray PN, Hawkins CE, Berzen A., and, Banwell BL. Grandpa and I have dystrophinopathy?: approach to asymptomatic hyperCKemia. Pediatr Neurol. 2006;35:145–149. doi: 10.1016/j.pediatrneurol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Goemans NM, Buyse G, Tulinius M,, Verschuuren JJG,, de Kimpe SJ,, van Deutekom JCT. A phase I/II study on antisense compound PRO051 in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2009;19:659–660. [Google Scholar]
- Wang Q, Yin H, Camelliti P, Betts C, Moulton H, Lee H, et al. In vitro evaluation of novel antisense oligonucleotides is predictive of in vivo exon skipping activity for Duchenne muscular dystrophy. J Gene Med. 2010;12:354–364. doi: 10.1002/jgm.1446. [DOI] [PubMed] [Google Scholar]
- Yin H, Moulton HM, Betts C, Merritt T, Seow Y, Ashraf S.2010et al. Functional Rescue of Dystrophin-deficient mdx Mice by a Chimeric Peptide-PMO Mol Ther 181822–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]