Abstract
Despite having potent oncolytic activity, in vitro, direct intratumoral injection of oncolytic vesicular stomatitis virus (VSV) into established AE17ova mesothelioma tumors in C57Bl/6 mice had no therapeutic effect. During studies to combine systemic cyclophosphamide (CPA) with VSV to suppress the innate immune reaction against VSV, we observed that CPA alone had highly significant antitumor effects in this model. However, against our expectations, the combination of CPA and VSV consistently reduced therapeutic efficacy compared to CPA alone, despite the fact that the combination increased intratumoral VSV titers. We show here that CPA-mediated therapy against AE17ova tumors was immune-mediated and dependent upon both CD4 T cells and natural killer (NK) cells. However, intratumoral VSV induced a transforming growth factor-β (TGF-β)-dependent suppressive activity, mediated by CD11b+GR-1+ cells that significantly inhibited both antigen-specific T-cell activation, and CPA-activated, NK-dependent killing of AE17ova tumor cells. Overall, our results show that treatment with oncolytic viruses can induce a variety of immune-mediated consequences in vivo with both positive, or negative, effects on antitumor therapy. These underexplored immune consequences of treatment with oncolytic viruses may have significant, and possibly unexpected, impacts on how virotherapy interacts in combination with other agents which modulate antitumor immune effectors.
Introduction
Malignant mesothelioma is an aggressive neoplasm of the lining of the pleura or peritoneum for which conventional treatments are only poorly effective.1 Since mesothelioma is often localized and amenable to direct injection,2 the use of replication competent viruses, which replicate selectively in tumor cells,3,4 is attractive as a novel therapy. In theory at least, even low levels of a replication competent oncolytic virus accessing a tumor will allow rapid spread of the virus, lysis of the tumor cells and reductions in tumor burdens.3,4 Through either natural, or engineered, selectivity for tumor cells, viral replication should be extinguished in normal cells.3 Of the many different oncolytic viruses which have been developed,3,5 vesicular stomatitis virus (VSV) has been shown to be a potent oncolytic in a wide variety of cancer models, including mesothelioma.6,7,8,9,10 Replication of VSV in normal cells is rapidly extinguished due to induction of antiviral type-I interferon (IFN) responses (IFN-α/β). However, many tumor cells have defects in their IFN response8,11,12 allowing free-ranging infection and lysis.13,14
A significant experimental advantage of VSV is that there are fully immune-competent rodent models in which the interactions between the virus, the tumor, and the immune system can be tested.6,7,10,15,16,17,18 In general, the host immune system is viewed as an inhibitor of virotherapy because the innate immune response restricts viral spread.15,19,20 However, we have shown in the B16 melanoma model that the efficacy of VSV-mediated virotherapy is mediated by host immune effectors responding to an immunogenic virus at the tumor site.6,16,17,21 Therefore, the immune system may also play a positive role—both to prevent viral spread and toxicity22 and as an effector of antitumor therapy.6,16,17,20,21,23,24,25
Therefore, efforts directed toward suppressing the innate response to oncolytic viruses need to be carefully designed so that increased viral replication can be achieved without loss of antitumor immune effects induced by the virus. We, and others, have shown that immunosuppression with cyclophosphamide (CPA) enhances delivery/efficacy of oncolytic viruses through reductions in neutralizing antibodies, suppression of innate immune effectors,19,22,26,27,28,29 depletion of regulatory T cells (Treg)30,31 and activation of immune cells.27,28,32 Therefore, the pleiotropic immunomodulatory effects of CPA on the immune system make it an attractive candidate for combination with oncolytic viruses. However, in vivo administration of oncolytic viruses is also itself highly immunomodulatory, both locally and systemically.16,17,33 Therefore, the immune perturbations induced by agents such as CPA may well be significantly affected by the immune modulations induced by oncolytic viruses in ways that have been poorly studied to date.
In the present study, we tested the therapeutic efficacy of VSV for the treatment of the murine AE17ova mesothelioma in immune-competent C57Bl/6 mice. Despite potent oncolytic efficacy in vitro, VSV had no significant therapeutic effects in vivo. In order to improve the efficacy of the virus locally, we combined intratumoral VSV with systemic CPA.19,26,27,28 In this model, CPA exhibited significant immune-mediated efficacy against AE17ova tumors as a single agent, dependent upon CD4+ T cells and natural killer (NK) cells. To our surprise, however, combination with VSV consistently decreased the antitumor efficacy of CPA alone. We show here that treatment with VSV induces systemic immune suppressive effects, which inhibit the CPA-activated NK-mediated killing of AE17ova tumors. Our data show that treatment with oncolytic viruses induces a variety of immune-mediated consequences in vivo, which may have a significant impact on therapeutic outcome when used both alone, or in combination with other agents, which modulate antitumor immune effectors.
Results
VSV is cytotoxic in vitro but has no significant therapy in vivo
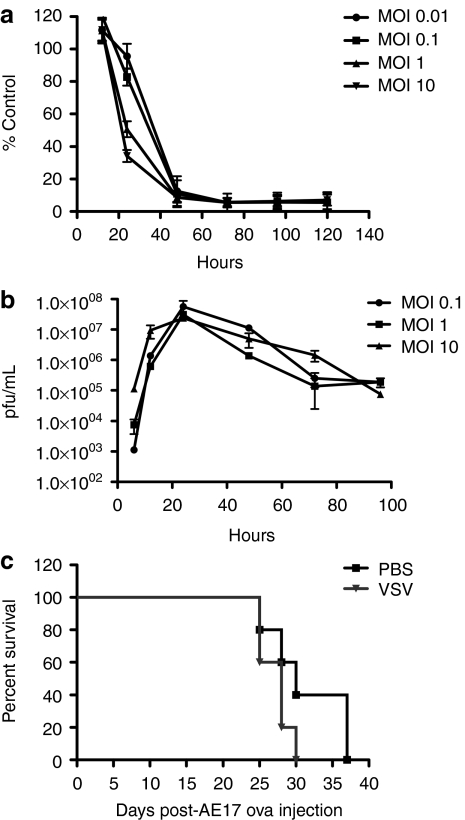
VSV-green fluorescent protein (GFP) induced rapid and extensive cell killing following infection of AE17ova cells at MOI ranging from 0.01 to 10 (Figure 1a), associated with ongoing replication of the virus (Figure 1b). While a decrease in cell killing was observed at 24 hours for the two lowest MOIs, by 48 hours postinfection, >90% of the AE17ova cells were killed at each MOI (Figure 1a). In contrast, direct injection of even multiple doses of VSV-GFP into established subcutaneous AE17ova tumors did not improve overall survival of virus-treated mice compared to control-treated mice administered heat-inactivated virus (data not shown) or phosphate-buffered saline (PBS) (Figure 1c). Moreover, virus titers recovered from injected tumors consistently decreased with time following injection, never exceeded the levels of input virus, and became undetectable within 3 days of the injection (data not shown). Taken together, these data suggested that intratumoral injection of VSV in this tumor model did not result in progressive viral replication, spread, and oncolysis.
Figure 1.
Oncolytic properties of VSV in vitro and in vivo. (a) MTT assays were performed on AE17ova cells infected with various MOI of VSV-GFP (data as percentage of control cell survival ± s.d). (b) Virus titers were determined from AE17ova cells infected with VSV-GFP at indicated MOIs. (c) Six-day AE17ova tumors in C57Bl/6 mice were injected with PBS or VSV-GFP every 2 days for four injections. Survival (tumors reaching a size of 1.0 cm in any diameter) is shown with time. GFP, green fluorescent protein; MOI, multiplicity of infection; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Combination of CPA with VSV detracts from therapy with CPA alone
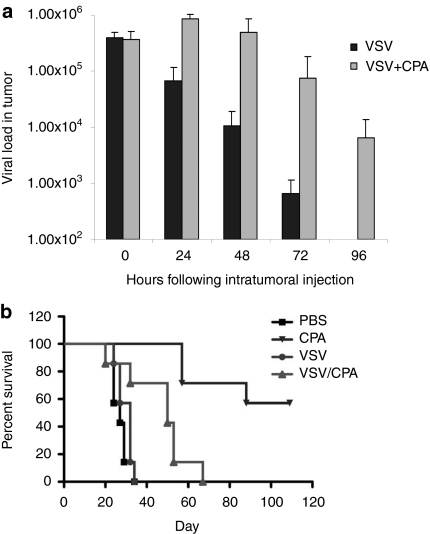
We hypothesized that combination with CPA would enhance the antitumor efficacy of VSV through its ability to suppress the innate immune response which inhibits replication of oncolytic viruses in vivo.19,26,27,28 Consistent with this hypothesis, over a period of 92 hours following direct intratumoral injection of VSV, we never observed a viral burst (increasing levels of virus detected following injection, followed by a fall off with time) that characterizes models in which vigorous viral replication is associated with tumor cures by oncolytic viruses.3,4 Pretreatment with CPA significantly enhanced the levels of virus detected in AE17ova tumors at 24, 48, 72, and 96 hours following viral injection, compared to no CPA, suggesting that CPA was, indeed, suppressing the innate immune response which inhibits replication of oncolytic viruses in vivo.19,26,27,28 (Figure 2a)
Figure 2.
CPA has antitumor activity in the AE17ova model. (a) Six-day AE17ova tumor-bearing C57Bl/6 mice were treated with CPA (3 mg/mouse, i.p.) or PBS followed by intratumoral VSV-GFP 24 hours later (n = 3/group). 15 minutes after injection (0 hours), 24, 48, 72, or 96 hours later, virus from tumor lysates was tittered on BHK-21 cells (total pfu of VSV/tumor). (b) Six-day AE17ova tumor-bearing C57Bl/6 mice were treated with CPA (3 mg/mouse, i.p.) or PBS followed by intratumoral PBS or VSV-GFP 24 hours later (n = 7/group). This cycle of CPA/VSV was repeated two times, every 6 days. Survival is shown with time. Data is representative of three different experiments. CPA, cyclophosphamide; i.p., intraperitoneal; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
In vivo, treatment with CPA alone significantly enhanced survival both over mice treated with PBS (P < 0.0001) and over mice treated with VSV alone (P = 0.0002) (Figure 2b). However, although the combination of CPA, followed by intratumoral VSV, significantly prolonged the survival of tumor-bearing mice compared to VSV treatment alone (P = 0.0152), we consistently observed that combination therapy decreased the efficacy of CPA treatment alone (P = 0.0011) (Figure 2b).
Immune consequences of CPA treatment in vivo
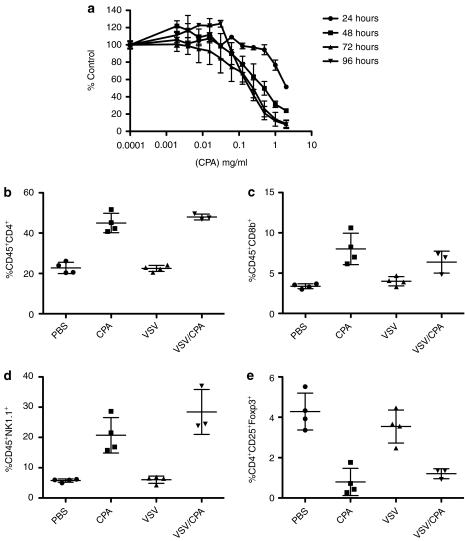
One explanation for this unexpected result could be that CPA may kill the substrate for virus replication (the tumor cells) more rapidly than the injected virus can replicate, thereby prematurely extinguishing viral replication, spread, and oncolysis. Consistent with such a model, in vitro treatment with CPA eradicated AE17ova cells (Figure 3a), with an IC50 of ~0.2 mg/ml at 72 and 96 hours post-treatment. However, because CPA treatment in vivo enhanced virus replication in AE17ova tumors (Figure 2a), it seemed unlikely that the directly cytotoxic effects of CPA were inhibiting VSV-mediated oncolysis. Therefore, we investigated the immune sequelae of CPA treatment in this model, with or without VSV, to understand the mechanisms of inhibition of CPA-mediated antitumor therapy by VSV.
Figure 3.
CPA modulates immune subsets in vivo. (a) MTT survival assay for AE17ova cells treated with different concentrations of CPA (percentage of control cell survival ± s.d). (b–f) Spleens from tumor-bearing mice treated with PBS, CPA, VSV, or CPA/VSV according to the schedule of Figure 2b, were analyzed by flow cytometry for (b) CD4+ T cells, (c) CD8+ T cells, (d) NK1.1 cells, or (e) CD4+CD25+FoxP3+ Treg. CPA, cyclophosphamide; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
In this respect, treatment of C57Bl/6 mice with CPA induced significant increases in systemic levels of CD4+ and CD8+ T cells, as well as NK cells, compared to PBS-treated mice (Figure 3b–d). Consistent with previous reports,31 CPA also led to significant depletion of Tregs (P = 0.0026 wrt PBS) (Figure 3e). In contrast, intratumoral injection of VSV alone did not significantly alter systemic levels of CD4+, CD8+, NK, or Treg cells compared to PBS-treated controls (Figure 3b–e). When CPA was combined with intratumoral injection of VSV, the significant CPA-induced increases in CD4+, CD8+, and NK cell levels, as well as the reduction in Treg numbers were preserved (Figure 3b–e).
Antitumor efficacy of CPA is immune mediated
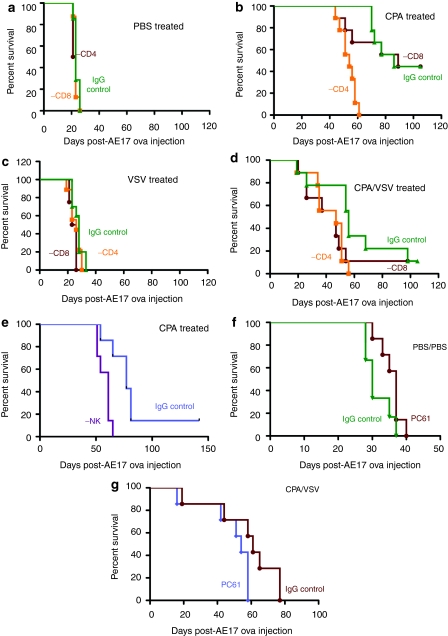
Depletion of neither CD4+ nor CD8+ T cells alone affected growth of AE17ova tumors compared to treatment with immunoglobulin G (IgG) control antibody (Figure 4a). When combined with either CPA alone (Figure 4b), VSV alone (Figure 4c) or CPA+VSV (Figure 4d), depletion of CD8+ T cells did not significantly affect survival compared to nondepleted controls (Figure 4a). In contrast, depletion of CD4+ T cells significantly decreased survival of mice treated with CPA, either with (Figure 4d) or without (Figure 4b) VSV, compared to similarly treated control IgG-treated mice. In addition, depletion of NK cells significantly reduced CPA-mediated therapy of AE17ova tumors both in the absence (Figure 4e), or presence (data not shown), of VSV treatment compared to IgG control-treated animals (P = 0.0044 CPA; P = 0.023 CPA/VSV) (Figure 4e).
Figure 4.
Antitumor efficacy of CPA depends upon CD4+ and NK cells. (a–d) AE17ova tumor-bearing C57Bl/6 mice (n = 7) were depleted of CD8 cells (Lyt 2.43), or CD4 cells (GK1.5) or with IgG control. At day 6–7, mice were treated with (a,c) PBS or (b,d) CPA followed by i.t. (a,b) PBS or (c,d) VSV-GFP 24 hours later. This cycle of CPA/VSV treatment was repeated two times, every 6 days. (e) AE17ova tumor-bearing C57Bl/6 mice (n = 7) were depleted of NK cells (anti-asialo-GM-1) or with IgG control. At day 6–7, mice were treated with CPA followed by i.t. PBS 24 hours later. This cycle of CPA/PBS treatment was repeated two times, every 6 days. (f,g) AE17ova tumor-bearing C57Bl/6 mice were depleted of Treg cells (PC61 antibody) or with IgG control. At day 6–7, mice were treated with PBS followed by i.t. PBS 24 hours later (f) or with CPA followed by i.t. VSV 24 hours later (g). This cycle of CPA/PBS treatment was repeated two times, every 6 days. CPA, cyclophosphamide; IgG, immunoglobulin G; i.t., intratumoral; NK, natural killer; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
PC61-mediated Treg depletion alone consistently slowed the development of AE17ova tumors, but this did not reach significance compared to control IgG-treated mice (P = 0.069 for PC61 compared to IgG, Figure 4f). Similarly, we did not observe any significant changes in survival of mice depleted with PC61 antibody and treated with CPA, VSV, or CPA/VSV compared to the corresponding, mock-depleted groups—P = 0.064 for PC61 compared to nondepleted, CPA/VSV-treated mice (Figure 4g).
Therefore, CPA-mediated therapy of AE17ova tumors is immune mediated and depends upon both CD4+ T cells and NK cells.
Intratumoral VSV activates suppressor activity
Although depletion of Treg did not affect CPA/VSV-mediated therapy (Figure 4), we observed significant toxicity in mice depleted of Treg and treated with VSV alone. These animals quickly became extremely lethargic with forced breathing, which eventually required euthanasia. Therefore, we were not able to determine the contribution of Treg to survival of mice treated with VSV alone. However, these observations indicated that Treg provide an important protection to mice infected with VSV. Moreover, the fact that we did not observe such toxicity in VSV-treated, nondepleted mice suggested that VSV infection may activate suppressor-like activities in vivo to protect from toxicity.
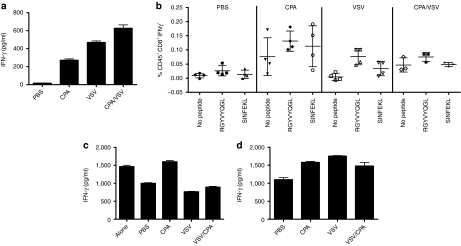
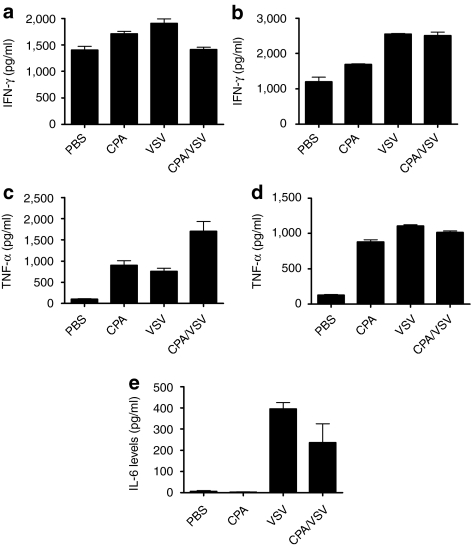
To investigate these effects further, we studied the effects of intratumoral VSV on T-cell activation. Splenocytes from mice treated with VSV alone secreted significantly higher levels of IFN-γ than splenocytes from mice treated with PBS (P < 0.001) (Figure 5a), confirming our previous observations that VSV induces generalized immune cell activation.16 CPA also induced increased splenocyte activity (P < 0.01 compared to PBS) (Figure 5a), an effect maintained in mice treated with both CPA and VSV (Figure 5a). Further dissection of this immune activation indicated that mice treated with VSV prime T-cell responses specific for viral proteins (P = 0.0038 for responses to VSV-N compared to controls) (Figure 5b). We consistently observed a trend toward priming of tumor antigen-specific responses (anti-ova) following intratumoral VSV, although these were not always significant (P = 0.08 for responses to OVA compared to controls) (Figure 5b).
Figure 5.
VSV induces immune suppressors. (a) Splenocytes from mice treated with two rounds of PBS, CPA, VSV, or CPA/VSV were harvested 24 hours following the final injection of VSV or PBS and 106 cells were plated, in triplicate from each mouse. Forty-eight hours later, supernatants were harvested and assayed for IFN-γ by ELISA. (b) Splenocytes prepared as in a pulsed with no peptide, SIINFEKL (ova), or RGYVYQGL (VSV-N protein) were assayed for IFN-γ-producing cells by ELISPOT. (c) Naive OT-I CD8+ T cells activated with H-2Kb-restricted ova peptide SIINFEKL either alone, or with splenocytes from mice treated with PBS, CPA, VSV, or CPA/VSV 24 hours following the final injection, were assayed for IFN-γ by ELISA. The suppressive activity in splenocyte cultures is reflected by inhibition of IFN- γ responses of naive OT-I T cells activated by SIINFEKL. (d) The experiment of c was repeated in the presence of recombinant human TGF-β sRII/Fc chimera added to each OT-I/splenocyte culture in order to inhibit the activity of TGF-β which may be being secreted by the added splenocytes. CPA, cyclophosphamide; IFN-γ, interferon-γ ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot; PBS, phosphate-buffered saline; TGF-β, transforming growth factor-β VSV, vesicular stomatitis virus.
Consistent with the hypothesis that generalized immune activation by VSV (Figure 5a,b) may include activation of suppressor activities, splenocytes from mice treated with VSV moderately suppressed the activation of OT-I T cells compared to splenocytes from PBS-treated mice (P = 0.02) (Figure 5c). In contrast, splenocytes from CPA treated mice enhanced antigen-specific T-cell activation (P < 0.01 compared to PBS-treated mice) (Figure 5c), probably associated with the ability of CPA to deplete Treg (Figure 5c). Significantly, however, in splenocytes from mice treated with CPA and VSV, VSV-mediated suppressive effects were dominant (P = 0.001 compared to CPA-treated mice) (Figure 5c).
Since splenocytes from VSV-treated mice secrete relatively high basal levels of IFN-γ (Figure 5a,b), the apparently moderate suppressive activity of these splenocytes on antigen-specific T-cell activation in Figure 5c is probably underestimated in this assay. When transforming growth factor (TGF)-β was blocked in these cultures, the inhibition exerted by splenocytes from VSV- or CPA/VSV-treated mice on antigen-specific T-cell activation (Figure 5c) was completely reversed (P < 0.001 for both VSV and CPA/VSV-treated mice compared to PBS-treated mice) (Figure 5d). Indeed, in the presence of TGF-β blockade, splenocytes from VSV-treated mice significantly enhanced antigen-specific T-cell activation of OT-I over that seen with splenocytes from untreated mice (Figure 5d).
VSV-induced suppression of CPA-activated NK effectors
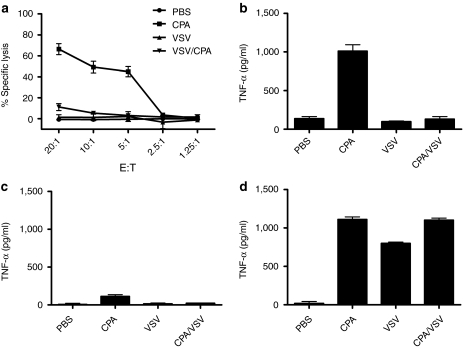
Consistent with the importance of NK cells to CPA-mediated therapy (Figures 3d and 4), splenocytes from mice treated with CPA had high levels of cytolytic activity against AE17ova tumor targets (Figure 6a). In contrast, splenocytes from mice treated only with VSV had no increased cytolytic activity against AE17ova (Figure 6a). Significantly, however, splenocytes from mice treated with CPA/VSV had significantly reduced levels of cytolytic activity against AE17ova compared to mice treated with CPA alone (Figure 6a). The CTL assay of Figure 6a correlated very well with levels of tumor necrosis factor-α (TNF-α) released from splenocytes from mice treated with PBS, CPA, VSV, or CPA+VSV (Figure 6b). Thus, although splenocytes from CPA-treated mice released high levels of TNF-α upon coculture with AE17ova (Figure 6b), this activity was completely inhibited in splenocytes from mice treated with both CPA and VSV (P < 0.0001 for CPA/VSV-treated mice compared to CPA-treated mice) (Figure 6b). When the assay of Figure 6b was repeated using splenocytes depleted of NK cells, the ability of splenocytes from CPA-treated mice to release TNF-α in response to AE17ova was lost (Figure 6c) confirming our in vivo results (Figure 4) that NK cells mediate tumor cell killing in the AE17ova model. In addition, and consistent with the immune activation data of Figure 5, blockade of TGF-β in splenocyte/tumor cell cocultures from VSV-treated mice (±CPA) restored levels of NK-mediated TNF-α release to levels comparable to those of splenocytes from CPA-treated mice alone (no significant difference between CPA-treated and CPA/VSV-treated mice) (Figure 6d).
Figure 6.
CPA activates NK-mediated cytotoxicity against AE17ova. (a) 51Cr-labeled AE17ova tumor cell targets were cocultured with splenocytes and tumor-draining lymph nodes from mice treated as shown. % lysis of target cells is shown. (b) Supernatants from AE17ova tumor cells cocultured with splenocytes from mice treated with PBS, CPA, VSV, or CPA/VSV, as in Figure 5, were assayed for TNF-α (effector:target ratio of 10:1). (c,d) The experiment of b was repeated using splenocytes predepleted of NK cells (c) or in the presence of recombinant human TGF-β sRII/Fc chimera to inhibit TGF-β (d). CPA, cyclophosphamide; PBS, phosphate-buffered saline; TGF-β, transforming growth factor-β TNF-α, tumor necrosis factor-α VSV, vesicular stomatitis virus.
These data indicate that intratumoral VSV induces TGF-β-dependent suppressive activity in vivo, which inhibits both antigen-specific T-cell activation (Figure 5) and CPA-activated NK tumor cell killing (Figure 6). Importantly, this VSV-induced suppressive activity was dominant in mice treated with both CPA and VSV (Figure 5c,d) and mirrored the in vivo suppressive effects observed when CPA was combined with VSV for treatment of AE17ova tumors.
Intratumoral VSV induces CD11b+GR-1+ suppressors
Splenocytes from both VSV- and CPA/VSV-treated mice inhibited IFN-γ production from antigen-activated OT-1 T cells (Figure 5c, columns 2 versus 4 and 5). However, depletion of GR-1+ cells from those splenocyte populations relieved that inhibition (Figure 7a, columns 1 versus 3 and 4) to an extent that closely mimicked the effect produced by inhibiting TGF-β (Figure 5d, columns 1 versus 3 and 4). Similar relief of inhibition of NK cell activation against AE17ova targets was also observed by depletion of GR-1+ cells from splenocytes of mice treated with either VSV or VSV/CPA (Figure 6b compared to Figure 7c). Again, depletion of GR-1+ cells closely mirrored the effects of inhibition of TGF-β on NK cell activation against AE17ova (Figure 6d compared to Figure 7c).
Figure 7.
VSV-induced suppression is mediated by CD11b+GR-1+ cells. (a,b) Naive OT-I CD8+ T cells were activated with SIINFEKL peptide and cocultured with splenocytes from mice treated with PBS, CPA, VSV, or CPA/VSV predepleted of either (a) GR-1+ or (b) CD11b+ cells. Seventy-two hours later, supernatants were assayed for IFN-γ. Both CPA and VSV treatments are statistically different from PBS treatment (P = 0.03 and P < 0.01, respectively in (a); all three treatments are significantly different (P < 0.01) from PBS in (c). (c,d) Supernatants from AE17ova cells cocultured with splenocytes (effector:target ratio 10:1) from mice treated with PBS, CPA, VSV, or CPA/VSV and predepleted of either (c) GR-1+ or (d) CD11b+ cells were assayed for TNF-α. In both (c) and (d) all three treatments are significantly different from PBS, P < 0.001 in all cases. (e) Tumors from mice treated as shown were assayed for IL-6. CPA, cyclophosphamide; IFN-γ, interferon-γ IL, interleukin; PBS, phosphate-buffered saline; TNF-α, tumor necrosis factor-α VSV, vesicular stomatitis virus.
A similar, but even more marked, relief of inhibition of both antigen-specific T-cell activation (Figure 7b), and NK cell activation against AE17ova (Figure 7d), was observed upon depletion of CD11b+ cells from splenocytes from mice treated with VSV or CPA/VSV compared to those from mice treated only with PBS (Figure 7b,d).
These data indicate that intratumoral VSV-induced CD11b+GR-1+ suppressor cells, suggesting that these cells may be myeloid-derived suppressor cells (MDSC).34,35 Consistent with this hypothesis, intratumoral VSV induced increased levels of CD11b+GR-1+ cells in the tumor-draining lymph nodes, both with (P = 0.03 compared to PBS-treated mice), or without (P = 0.01), CPA treatment. A trend toward increased intratumoral infiltration with CD11b+GR-1+ cells was detected in tumors, although there was considerable variation between tumors and no statistically significant difference could be seen over several experiments. In vivo depletion experiments to investigate the role of VSV-induced GR1+ve suppressor cells on CPA-mediated therapy of AE17ova tumors were highly variable and inconsistent in their results. Finally, cytokine multiplex assays showed that interleukin-6 (IL-6), a known inducer of MDSC activity,36 was induced at high levels in tumors of mice treated with VSV, in comparison to control PBS- or CPA-treated mice (P = 0.002 for both) (Figure 7e).
Taken together, these data show that intratumoral VSV induces a MDSC-like suppressor activity mediated by CD11b+GR-1+cells, which can be recovered from the lymphoid organs of virus-treated mice, which may be mediated, at least in part, by a strong induction of IL-6 by virus injection in vivo.
Discussion
We show here that, despite potent oncolytic activity in vitro (Figure 1a,b), VSV generated no significant therapy in vivo in the AE17ova mesothelioma model (Figure 1c). Upon direct intratumoral injection, we could not detect appreciable ongoing viral replication, suggesting that innate immune reactivity to VSV restricted viral replication, spread, and oncolysis.15,16,17,19,20,26 Therefore, we tested combination therapy with CPA based on its multiple immunomodulatory activities, including suppression of innate immunity.19,22,26,29 Unexpectedly, CPA alone generated considerable therapy, consistently curing about 50% of mice (Figure 2b). However, even though virus replication was enhanced within tumors by the combination (Figure 2a), treatment with both CPA and VSV significantly reduced therapy compared to CPA alone, suggesting that factors other than direct viral oncolysis are important in this model.
Therefore, we sought to understand why intratumoral VSV inhibited the immune-mediated therapy of CPA. Splenocytes from CPA-treated mice lysed AE17ova targets very efficiently (Figure 7a) and secreted TNF-α in response to the tumor cells (Figure 7b), an activity which disappeared upon depletion of NK cells (Figure 7c). Taken together with the in vivo depletion results (Figures 4 and 5), these data indicated that CPA-activated immune effectors, of which NK cells are a major component, which could kill AE17ova tumors directly both in vitro and in vivo. VSV treatment in vivo also induced generalized T-cell activation, evidenced by increased IFN-γ secretion by splenocytes16 (Figure 6a,b). Since VSV inhibited CPA-activated, NK-mediated tumor cell killing (Figures 2b and 7a), we hypothesized that VSV-induced immune activation may also include activation of suppressive cells. Consistent with this, although Treg were not increased in the spleens of VSV-treated mice (Figure 3e), VSV treatment induced a TGF-β-dependent activity in splenocytes which suppressed both antigen-specific T-cell activation (Figure 6c,d) and CPA-activated NK tumor cell killing (Figure 7b–d). Studies in SCID mice of the VSV/CPA combination therapy were difficult to interpret because of significant toxicities associated with virus dissemination from injected tumors.
VSV-induced suppression was dominant in splenocytes from mice treated with both CPA and VSV (Figures 6c,d and 7b–d), similar to the in vivo data showing suppression of CPA-mediated tumor therapy when CPA was combined with VSV (Figure 2b). We do not believe that this suppressive activity was mediated through conventional Treg. This is because CPA treatment at the levels used here inhibit Treg activity in vivo27,28 and yet the suppressive activity was still present in mice treated with both CPA and VSV. Second, treatment with PC61 depleting antibody did not alleviate the VSV-induced suppression of CPA therapy (Figure 4f,g). Finally, VSV-induced suppression was efficiently relieved by depletion of both GR-1+ and, more effectively, CD11b+ cells from splenocytes of mice treated with either VSV alone or with CPA/VSV (Figure 7). These data suggest that VSV treatment in vivo induces a population of MDSC, which expand in vivo in pathological conditions—such as infection with viruses37—with a well-documented ability to suppress immune responses.34,35 In mice, MDSCs are CD11b+GR-1+ cells although there are functionally distinct subsets within this population.34 Consistent with the VSV-mediated induction of suppressive MDSC-like effectors, we also observed that intratumoral VSV induced high levels of IL-6, which has been shown to mediate the expansion and activation of MDSC in vivo.36 Finally, we show here a close correlation between depletion of CD11b+GR-1+ cells, blockade of TGF-β and relief of VSV-induced suppressive activity (Figures 6 and 7).
It is clear, therefore, that CPA has multiple pleiotropic effects in vivo which contribute to the antitumor efficacy that we observed in this model. Both we, and others, have previously reported that CPA induces the depletion of Treg,27,28,38 that it mediates antitumor effects through activation of NK cells,27,32 and that both of these mechanisms are effective against murine mesothelioma.32,38 However, a novel aspect of our current studies is the critical role for CD4+ T cells in CPA-mediated therapy of AE17ova tumors (Figure 4). Our ongoing studies suggest that CPA functionally inhibits a population of Treg cells, which releases suppression of both CPA-activated NK cells and of a subset of CD4+ T cells with direct cytotoxic activity against tumor cells.39 Therefore, depletion of CD4+ T cells (Figure 4) most likely both depleted endogenous Treg (thereby enhancing the activity of CPA-activated NK cells with antitumor activity) and removed cytotoxic CD4+T cells, which are themselves direct effectors of therapy. Further characterization of these functionally important subsets of CD4+ T cells, and the effects of CPA and VSV on their activation, are ongoing.
Taken together, our data support a model in which CPA activates various immune cells with direct antitumor activity, of which NK cells are a major component. However, when CPA is combined with intratumoral virus, VSV induces a CPA-insensitive, TGF-β-dependent, MDSC-like activity, possibly through IL-6 induction. We believe that this suppressive activity is separate from Treg, but may activate additional Treg in vivo through TGF-β. We propose that this MDSC activity then inhibits CPA-activated, antitumor NK cell effectors, through TGF-β production, leading to decreased therapy compared to CPA alone. Our data from Figure 2 also suggest that the CPA-mediated suppression of innate immune responses to VSV induces increased intratumoral viral titers, which allow for enhanced viral replication and, potentially, better antitumor effects compared to no CPA. However, the suppressive activity induced by VSV is still dominant in reducing overall antitumor therapy of CPA+VSV compared to CPA alone.
In light of our results, along with other published data,6,7,8,9,10 it is clear that the efficacy of intratumoral VSV can differ significantly between tumor models. We have observed that the suppressive activity induced by VSV that we describe here is not dependent upon the tumor model used in the C57Bl/6 mice. In contrast, however, the therapeutic outcome of intratumoral VSV does depend closely upon the phenotype of the tumor being treated, both in its sensitivity to viral replication and/or to immune-mediated antiviral effectors (such as NK cells), which are also induced by VSV administration in vivo. Interestingly, CPA has significant efficacy against B16ova tumors, which are sensitive to NK-mediated killing; in contrast, it has no therapeutic effect against the B16 melanoma model, which is largely insensitive to NK-mediated killing due to lack of innate immune sensor molecules, such as the receptor for IL-28.6,16,17,20,21 In this respect, we have shown recently that the lack of expression of IL-28r by AE17ova cells also represents one mechanism by which these tumors are unable to respond to innate antiviral immune signaling to confer sensitivity to VSV-induced immune clearance (C. Willmon, manuscript in preparation).
Our results are significant in several respects. We show that administration of an oncolytic virus in vivo, even intratumorally, leads to multiple immune modulatory effects, which can have both positive and negative consequences for tumor therapy.40 In particular, virus activates local innate immune responses, which can negatively impact viral replication, spread, and oncolysis, but which can also have significant antitumor effects.6,16,17,20,21 However, as we demonstrate here, antiviral immune responses can also include immune suppressive components. Virus-induced activation of suppressor cells is important during natural viral infections to control, and restrict, the extent of the antiviral immune response.34,35 However, such suppressive effectors can also inhibit concomitantly acting antitumor, immune-mediated therapies,40 induced either by the virus itself or, as is the case in the present study, by an additional agent used in combination with the virus. Thus, although immune modulators, including CPA, have been successfully combined with oncolytic virotherapy,19,22,26,27,28,29 it is clear that oncolytic viruses themselves induce a variety of (often underexplored) immune-mediated consequences in vivo, which can have both positive, or negative, effects on antitumor therapy. As combinations between oncolytic virus and other treatment modalities become more common,41 the immune sequelae of administration of oncolytic viruses should be considered more closely—especially when the additional therapeutic agents activate antitumor immune effector mechanisms.
Materials and Methods
Virus and cell lines. AE17ova cells are mouse mesothelioma cells transduced with chicken ovalbumin42 (provided by Dr Delia Nelson, University of Western Australia). VSV-GFP (Indiana serotype) was a gift from Dr Glen Barber (University of Miami, FL).7
MTT assays. Viability of cells infected with different MOIs of VSV-GFP, or treated with dilutions of CPA (Baxter, Deerfield, IL), was assessed at indicated time points as per the manufacturer's instructions (Promega, Madison, WI).
ELISPOT analysis. 1 × 105 cells from spleens or tumor-draining lymph nodes were stimulated for 48 hours with appropriate peptides (5 µg/ml) (SIINFEKL, chicken ovalbumin and RGYVYQGL, VSV N protein, synthesized at the Mayo Foundation Core Facility, Rochester, MN). IFN-γ+ spots were quantified by a computer-assisted image analyzer.
51Chromium cytotoxicity assay. AE17ova tumor cell targets labeled with 20 µl 51Cr for 1.5 hours were cocultured with splenocytes/tumor-draining lymph nodes at different effector:target ratios. After 4 hours, cells were spun down and 35 µl of supernatant was transferred to scintillation plates. % lysis was calculated using the formula: % lysis = 100 × (cpm experiment – cpm spontaneous release)/(cpm maximum release – cpm spontaneous release).
Suppression of IFN-γ secretion from activated T cells. T cells of OT-I transgenic mice express the V2 chain of the OT-I T-cell receptor, which recognizes the SIINFEKL peptide from chicken ovalbumin in the context of H-2Kb as expressed by B16ova cells.43 To assay for T-cell suppressive activity, 250,000 freshly harvested splenocytes were plated with 105 naive OT-I CD8+ T cells with 1 µg/ml SIINFEKL peptide43 and 50 IU/ml human IL-2 (Mayo Clinic Pharmacy, Rochester, MN) as described.44 Splenocytes were isolated from mice 24 hours following the final injection of virus (or PBS) from mice treated with two rounds of (CPA/VSV)—that is 8 days following the first injection of CPA or PBS. Supernatants were assayed for IFN-γ by enzyme-linked immunosorbent assay. Suppressive activity in splenocyte cultures is reflected by their ability to inhibit IFN-γ responses of naive OT-I T cells when activated with SIINFEKL. Dependence of suppression on TGF-β45 was assayed using recombinant human TGF-β sRII/Fc chimera (R&D Systems, Minneapolis, MN), a 159-amino acid extracellular domain of human TGF-β receptor type II fused to the Fc region of human IgG1. Splenocyte/lymph node cultures were depleted by coculture with anti-MAC3, anti-asialo-GM-1 (Cedarlane, Burlington, NC), anti-CD8 (Lyt2.4.3), anti-CD4 (GK1.5) (Core Facility, Mayo Clinic, Rochester, MN), anti-CD11b (Miltenyi, Auburn, CA) or anti-GR-1 antibodies (BioXcell, West Lebanon, NH). Depletion was confirmed by fluorescence-activated cell sorting.
In vivo studies. All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals. To establish subcutaneous tumors, 1 × 106 AE17ova cells in 100 µl PBS were injected into the flank of C57Bl/6 mice. Once subcutaneous tumors reached ~200 mm3, intraperitoneal injections of CPA (3 mg/mouse) were followed 24 hours later by intratumoral injections (saline or 5 × 108 plaque-forming unit VSV-GFP) every 6 days. This regimen of CPA/virus was repeated every 6 days until the tumor reached 1.0 cm in the longest diameter or for a maximum of five rounds (five CPA or PBS injections and five virus or PBS injections). Mice were killed if tumor burden exceeded 1 × 1 cm.
Immune cell depletions were performed by intraperitoneal injections (0.1 mg/mouse) of anti-CD8 (Lyt 2.43) or anti-CD4 (GK1.5) (Core Facility, Mayo Clinic), anti-NK cell (anti-asialo-GM-1; Cedarlane), and IgG control (ChromPure Rat IgG; Jackson ImmunoResearch, West Grove, PA) 4 days after tumor implantation and then weekly. For Treg depletion, 0.5 mg of PC61 antibody (Core Facility, Mayo Clinic) was given intraperitoneal 4 days after tumor implantation and 3 days before viral injection. Fluorescence-activated cell sorting analysis of spleens/lymph nodes confirmed subset-specific depletions.
Cytokine analysis. Cytokines were assayed from tumors using the Procarta Cytokine Assay Kit (Panomics, Fremont, CA) on a Luminex 100 instrument (Bio-Rad, Hercules, CA).
Statistical analyses. For comparison of two individual data points, two-sided Student's t-test was applied to determine statistical significance. ANOVA with post-hoc testing was used for groups of three or more. Survival curves were plotted according the Kaplan–Meier method, and statistical significance in the different treatment groups was compared using the log-rank test.
Acknowledgments
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard M. Schulze Family Foundation, the Mayo Foundation, and by NIH grants CA107082, CA130878, and CA132734.
REFERENCES
- Sterman DH., and, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–283. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT, et al. A phase I trial of repeated intrapleural adenoviral-mediated interferon-β gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther. 2010;18:852–860. doi: 10.1038/mt.2009.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R, Miest T, Shashkova EV., and, Barry MA. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA., and, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D., and, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF., and, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Ebert O., and, Woo SL. Treatment of multi-focal colorectal carcinoma metastatic to the liver of immune-competent and syngeneic rats by hepatic artery infusion of oncolytic vesicular stomatitis virus. Int J Cancer. 2005;114:659–664. doi: 10.1002/ijc.20772. [DOI] [PubMed] [Google Scholar]
- Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J, et al. Expression of IFN-β enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Balachandran S., and, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J, et al. 2010Interference of CD40L-mediated Tumor Immunotherapy by Oncolytic VSV HumanGene Ther 21439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Huang TG, Meseck M, Altomonte J, Ebert O, Shinozaki K, et al. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy. Hum Gene Ther. 2008;19:635–647. doi: 10.1089/hum.2007.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Tian JP, Fulci G, Chiocca EA., and, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J, et al. Type III interferon IL-28 mediates antitumor efficacy of oncolytic virus VSV in immune competent mouse models of cancer. Cancer Res. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote D, Cattaneo R., and, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- Thomas DL., and, Fraser NW. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8:543–551. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Galivo F, Wongthida P, Diaz RM, Thompson J, Jevremovic D, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors by systemically delivered oncolytic virus. Mol Ther. 2008;16:1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ., and, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, Tuve S, Ni S, Hellström KE, Hellström I., and, Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most RG, Currie AJ, Cleaver AL, Salmons J, Nowak AK, Mahendran S, et al. Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth. PLoS ONE. 2009;4:e6982. doi: 10.1371/journal.pone.0006982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E., and, Rose JK. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol. 2004;78:9317–9324. doi: 10.1128/JVI.78.17.9317-9324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI., and, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S., and, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J., and, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen JL., and, Olson JK. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler's virus-induced demyelinating disease. J Immunol. 2009;183:6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, et al. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–1228. doi: 10.1007/s00262-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA., and, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Health WR, Howard JL, Bevan MJ., and, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Kottke T, Pulido J, Thompson J, Sanchez-Perez L, Chong H, Calderwood SK, et al. Antitumor immunity can be uncoupled from autoimmunity following heat shock protein 70-mediated inflammatory killing of normal pancreas. Cancer Res. 2009;69:7767–7774. doi: 10.1158/0008-5472.CAN-09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA., and, Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]