Abstract
We show here, for the first time to our knowledge, that the antitumor therapy of oncolytic vesicular stomatitis virus (VSV) in the B16ova model depends upon signaling through myeloid differentiation primary response gene 88 (MyD88) in host cells. VSV-mediated therapy of B16ova tumors was abolished in MyD88−/− mice despite generation of antigen-specific T cell responses similar to those in immune-competent mice. Mice defective in only toll-like receptor 4 (TLR4), TLR7, or interleukin 1 (IL-1) signaling retained VSV-induced therapy, suggesting that multiple, redundant pathways of innate immune activation by the virus contribute to antitumor immune reactivity. Lack of MyD88 signaling was associated with decreased expression of proinflammatory cytokines and neutrophil infiltration in response to intratumoral virus, as well as decreased infiltration of draining lymph nodes (LN) with plasmacytoid dendritic cells (pDCs) (CD11b−GR1+B220+) and myeloid-derived suppressor cells (CD11b+GR1+F4/80+). MyD88 signaling in response to VSV was also closely associated with a type I interferon (IFN) response. This inhibited virus replication within the tumor but also protected the host from viral dissemination from the tumor. Therefore, the innate immune response to oncolytic viruses can be, simultaneously, protherapeutic, antioncolytic, and systemically protective. These paradoxically conflicting roles need to be carefully considered in future strategies designed to improve the efficacy of oncolytic virotherapy.
Introduction
The development of oncolytic viruses has been based on the concept that even low levels of a replication competent virus introduced into a tumor will result in rapid spread, with subsequent lysis of the tumor cells, with tumor selective viral replication being possible through either natural, or engineered, selectivity.1,2,3,4,5,6 Based on this rationale, the immune system is often viewed as an inhibitor of virotherapy because an aggressive innate immune response against a viral pathogen—even in a tumor—restricts viral replication and oncolysis.7,8,9 However, where successful, the mechanisms by which oncolytic viruses lead to tumor clearance in vivo are clearly multifactorial and the immune system can contribute to effective therapy. Thus, the extent of viral replication does not necessarily correlate with therapy10,11,12 and tumor clearance often involves viral-, or immune-mediated, vascular destruction,7,13,14 as well as immune effectors against tumor, the invading viral pathogen, or both.9,10,11,15,16,17,18,19 In addition, the immune system acts as a safety blanket to prevent viral spread.20
Vesicular stomatitis virus (VSV), a negative strand Rhabdovirus, replicates in the cytoplasm, is highly cytolytic and highly sensitive to the antiviral actions of type I interferon (IFN)α/β in normal cells.21,22,23 However, many tumor cells have defects in their IFN response21,22,24 allowing free-ranging infection and lysis.2,25 As a result, VSV is a potent oncolytic in a wide variety of models.2,15,22,26,27,28 Previously, we have shown that virotherapy with VSV in the B16ova model rapidly induces a robust, antiviral innate immune response.10 Consistent with this, intratumoral VSV injection was not associated with a viral burst and replication was extinguished very rapidly.10 Moreover, a VSV which could undergo only a single cycle of gene expression, but no ongoing replication, was as effective against B16ova tumors as was a fully replication competent VSV.10 In addition, VSV-mediated therapy depends on natural killer, CD8+ T cells, and the type III IFN, interleukin-28 (IL-28).15,19 Therefore, the innate immune response acts both as a major inhibitor of viral replication, spread, and oncolysis,10,15,16,19 and also as a key effector of immune-mediated therapy in response to the introduction of an immunogenic viral pathogen at the tumor site.10,15,19
Invading pathogens, such as VSV, are detected by the innate immune system through activation of toll-like receptors (TLR).29 In this respect, the myeloid differentiation primary response gene 88 (MyD88) molecule is a central adaptor protein in the signal transduction pathways mediated by IL-1 and most TLRs, with the exception of TLR3.30 In isolated cell populations, TLR7 and MyD88 mediated recognition of VSV and production of type I IFN from plasmacytoid dendritic cells (pDCs). In addition, the TLR-MyD88 pathway is a major component of protective anti-VSV immunity31,32 and increased susceptibility of MyD88−/− mice to VSV infection correlated with impaired recruitment of immune cells to the site of infection.33
The goal of the present study was to identify key components of the innate immune system which sense intratumoral VSV and subsequently mediate antitumor therapy in the B16ova model. We show here that therapy with VSV is dependent upon signaling through MyD88 in host cells. MyD88 signaling was closely associated with a type I IFN response that inhibited virus replication within the tumor, protected the host from toxic viral dissemination, and also, critically, mediated therapy with VSV. Therefore, the innate immune response to oncolytic viruses can be simultaneously protherapeutic, antioncolytic, and systemically protective.
Results
Therapeutic efficacy of VSV depends upon MyD88 signaling
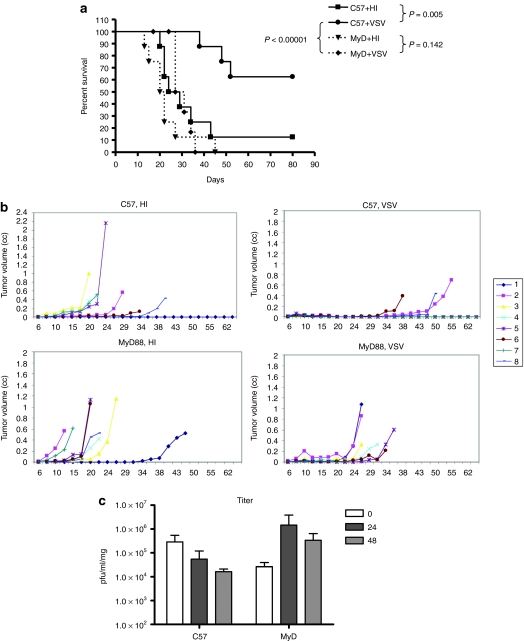
As we have shown previously, intratumoral injections of VSV significantly prolonged survival of C57BL/6 mice (P = 0.005 compared to control-treated mice) (Figure 1a). To investigate the role of innate immune responses in VSV therapy, we used mice lacking MyD88 signaling capability. Tumor-bearing MyD88−/− mice treated with VSV had no significant survival compared to mice treated with heat-inactivated (HI) virus (Figure 1a). In addition, survival of VSV-treated B16ova-bearing C57BL/6 mice was significantly greater than that of tumor-bearing VSV-treated MyD88−/− mice (P < 0.0001) (Figure 1a). Decreased survival of tumor-bearing MyD88−/− mice treated with VSV, compared to similarly treated C57BL/6 mice, was due to an inability of the virus to effect reductions in tumor size rather than any virus related toxicity (Figure 1b).
Figure 1.
VSV therapy depends upon MyD88 signaling. (a,b) C57BL/6 or MyD88−/− mice (n = 8/group) bearing 7 day established B16ova tumors were injected intratumorally with 5 × 108 pfu of VSV or heat-inactivated (HI) VSV on days 7, 9, 11, 13, 15, and 17 after tumor implantation. Survival (tumor <1.0 cm in any diameter) with time is shown. (c) C57BL/6 or MyD88−/− mice (n = 3 /group) bearing B16ova tumors were injected intratumorally with 5 × 108 pfu of VSV or HI-VSV. At the time points shown, tumors were harvested for viral titer determination. HI, heat-inactivated; MyD88, myeloid differentiation primary response gene 88; pfu, plaque-forming unit; VSV, vesicular stomatitis virus.
An intact antiviral innate immune response is likely to inhibit viral replication and spread within an injected tumor. As we have observed previously, viral titers rapidly contracted in injected B16ova tumors in C57BL/6 mice (in contrast to robust replication in B16ova cells in vitro)10 (Figure 1c). However, the reverse was true in B16ova tumors injected with VSV in MyD88−/− mice, where a clear viral burst was observed, indicating effective viral replication within the tumor with time (Figure 1c). Therefore, even when increased intratumoral viral replication was induced by loss of MyD88 signaling in host innate immune cells, antitumor therapy was lost.
VSV therapy is not dependent upon an adaptive immune response
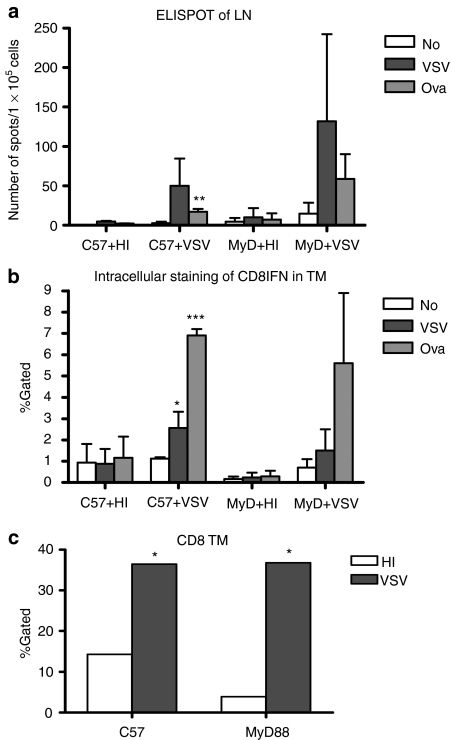
We could not detect significant differences in the magnitude of either virus antigen-specific (P = 0.238), or tumor antigen-specific (P = 0.553), T cell responses in tumor-bearing MyD88−/− mice compared to C57BL/6 mice in either the tumor-draining lymph nodes (TDLN) (Figure 2a) or in the tumor itself (Figure 2b) 7 days following intratumoral injection of virus. Similarly, infiltration of virus-injected tumors with CD8+ T cells was not different in MyD88−/− mice compared to C57BL/6 mice 7 days following treatment (Figure 2c). We also did not observe significant changes in the development of anti-VSV adaptive T cell responses when C57BL/6 or MyD88−/− mice were challenged with VSV in the absence of tumors (data not shown). Therefore, it is predominantly the innate, rather than adaptive, immune response that mediates virotherapy in this model.
Figure 2.
MyD88 signaling does not overtly affect adaptive immune responses. Tumor-bearing C57BL/6 or MyD88−/− mice (n = 3/group) were intratumorally injected with 5 × 108 pfu of HI-VSV or VSV on day 7 after tumor implantation. (a) Tumor-draining lymph nodes (LN) and (b) tumors (TM) were harvested for ELISPOT assay and intracellular staining for IFN-γ, respectively. (c) Tumor infiltrating CD8+ T cells were analyzed by flow cytometry. P values; *P < 0.05, **P < 0.01, ***P < 0.001. ELISPOT, enzyme-linked immunosorbent spot; HI, heat-inactivated; MyD88, myeloid differentiation primary response gene 88; VSV, vesicular stomatitis virus.
MyD88-dependent VSV therapy does not depend on TLR4, TLR7, or IL-1R signaling in isolation
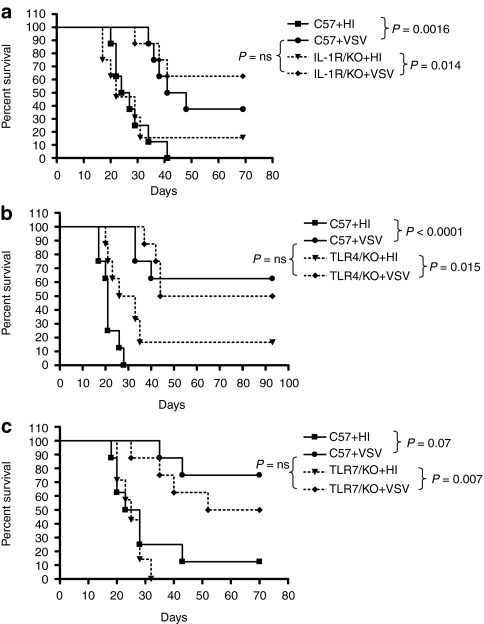
MyD88 activation is a downstream effector of signaling through IL-1, IL-18, and most TLR, and innate immunity to VSV can be activated through TLR4 and TLR7.31,34 However, intratumoral injections of VSV retained significant therapy against established B16ova tumors in mice genetically deficient for TLR4, TLR7, and IL-1 receptor (IL-1R) compared to injection of HI-virus (P = 0.0014 for IL-1R−/− mice (Figure 3a); P = 0.015 for TLR4−/− mice (Figure 3b) and P = 0.007 for TLR7−/−mice (Figure 3c)). Similarly, the therapy afforded by VSV injection into B16ova tumors in each of the IL-1R−/−, TLR4−/−, and TLR7−/− mice was very similar to that observed in C57BL/6 mice (Figure 3a–c).
Figure 3.
VSV-mediated therapy is not dependent upon IL-1R, TLR4, or TLR7 signaling in isolation. (a) C57BL/6 and IL-1R−/−, (b) TLR4−/−, or (c) TLR7−/− mice (n = 8/group) bearing 7 day established subcutaneous B16ova tumors were injected intratumorally at days 7, 9, 11, 13, 15, and 17 with 5 × 108 pfu of HI-VSV or VSV. Survival of mice with time is shown. IL, interleukin; ns, not significant; pfu, plaque-forming unit; VSV, vesicular stomatitis virus; TLR, toll-like receptor.
Therefore, these data suggest either that signaling through IL-1, TLR4, or TLR7 has no role in VSV-mediated therapy of B16va tumors, or that MyD88-dependent innate immune signaling through TLR4, TLR7, or IL-1R alone does not play a dominant role, in isolation of other pathways, in VSV-mediated therapy of B16ova tumors.
MyD88 signaling mediates VSV-induced cytokines and immune infiltration
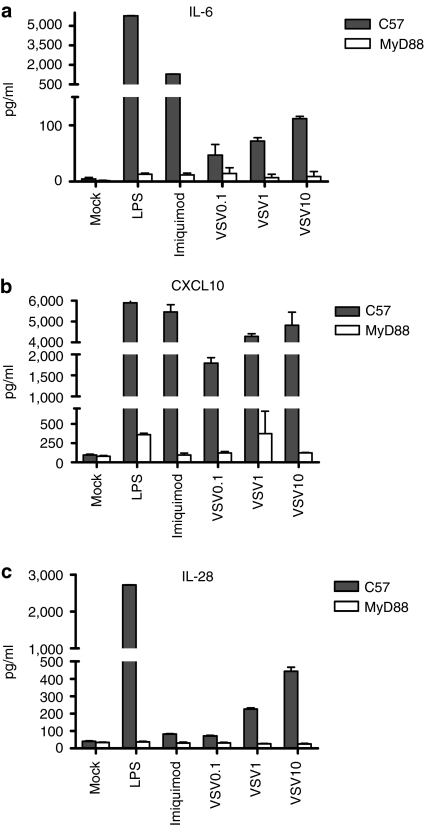
To identify candidate effector cytokines induced by MyD88-mediated signaling which may be critical for antitumor therapy, we used an in vitro assay of VSV infection of isolated bone-marrow cells.19 As expected, multiple cytokines were induced upon infection of C57-derived bone-marrow cultures by VSV (data not shown). However, of these, secretion of the type III IFN IL-28, IL-6, and CXCL10 were significantly decreased from bone marrow of MyD88−/− mice compared to C57BL/6 mice (Figure 4a–c).
Figure 4.
Effects of MyD88 signaling on cytokine expression. Bone-marrow cells from C57BL/6 or MyD88−/− mice were left untreated, pulsed with the TLR agonists LPS or imiquimod, or infected with VSV at MOI 0.1, 1, or 10. Fourty-eight hours later, supernatants were assayed for (a) IL-6, (b) CXCL10, and (c) IL-28 by ELISA. All treatments of C57 bone-marrow cells gave significantly higher levels of cytokine secretion compared to MyD88−/− bone-marrow cells. P value <0.001. ELISA, enzyme-linked immunosorbent assay; IL, interleukin; MOI, multiplicity of infection; MyD88, myeloid differentiation primary response gene 88; TLR, toll-like receptor; VSV, vesicular stomatitis virus.
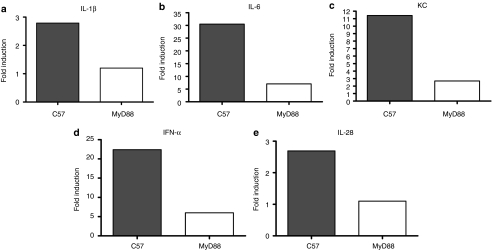
To correlate these in vitro data with the in vivo model, significant decreases in expression of IL-1β, IL-6, KC, IFN-α, and IL-28 were observed in tumors excised from MyD88−/− mice compared to tumors from C57BL/6 mice (Figure 5a–e). In contrast, other cytokines such as IL-2 or IL-12, which are usually associated with induction of type I adaptive immunity, were not detectable in this assay. There were several cytokines from the cytokine multiplex analysis, which showed no statistically significant difference of intratumoral levels following injection of VSV, compared to HI-VSV, in C57BL/6 mice. There were also several cytokines which did not show any significant difference in intratumoral levels following intratumoral injection of VSV, compared to HI-VSV, in MyD88−/− mice. However, Figure 5 represents those cytokines for which there was statistically significant induction (relative to HI-VSV control) by VSV in C57BL/6 mice and for which that induction was significantly decreased in MyD88−/− mice.
Figure 5.
Intratumoral VSV induces cytokine expression in vivo. Seven day established tumors in C57BL/6 or MyD88−/− mice (n = 3/group) were intratumorally injected with 5 × 108 pfu of HI-VSV or VSV. Twenty-four hours later, tumors were harvested and analyzed for cytokine expression using a multiplex assay. Results for (a) IL-1β, (b) IL-6, (c) KC, (d) IFN-α, and (e) IL-28 (fold induction over control-injected tumors) are shown. The cytokines in this figure represent those cytokines for which there was (i) a statistically significant difference in levels induced intratumorally by VSV, compared to HI-VSV, in C57BL/6 mice, but for which (ii) the difference in levels induced intratumorally by VSV, compared to HI-VSV, upon injection in MyD88−/− mice was not significant (three mice per group, per mouse strain). The fold induction in cytokine level between the mean level of cytokine induced by VSV treatment, compared to HI-VSV, in C57BL/6 mice (filled bar), compared to the fold induction in cytokine between the mean level of cytokine induced by VSV treatment, compared to HI-VSV, in MyD88−/− mice (open bar) was then plotted to generate the figure. HI, heat-inactivated; IL, interleukin; IFN, interferon; MyD88, myeloid differentiation primary response gene 88; pfu, plaque-forming unit; VSV, vesicular stomatitis virus.
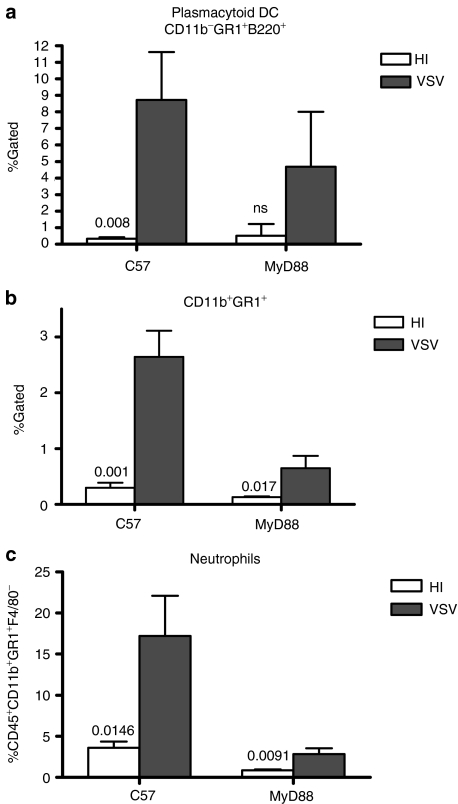
Consistent with the changes in cytokine levels in vivo, C57BL/6 mice contained significantly increased levels of both myeloid-derived suppressor cell-like CD11b+Gr1+F4/80+ cells, as well as pDC (CD11b−Gr1+B220+) in the TDLN following treatment of B16ova tumors with VSV compared to treatment with HI-virus (P = 0.001 and P = 0.008, respectively) (Figure 6a,b). However, infiltration of the TDLN with CD11b+Gr1+ cells was significantly reduced in MyD88−/− mice treated intra-tumorally with VSV (P = 0.003) (Figure 6b). With respect to the tumor itself, VSV treatment induced significant infiltration of neutrophils in C57BL/6 mice (P = 0.0146 compared to treatment with HI-VSV) (Figure 6c). In contrast, although neutrophil infiltration into B16ova tumors growing in MyD88−/− mice treated with VSV was significantly different to that in tumors treated with HI-VSV (P = 0.0091), the levels of infiltration into VSV-treated tumors in MyD88−/− mice were significantly lower than those in C57BL/6 mice (P = 0.00125) (Figure 6c).
Figure 6.
Effects of MyD88 signaling on tumor infiltration following VSV treatment. C57BL/6 or MyD88−/− mice (n = 3/group) bearing B16ova tumors were injected intratumorally with 5 × 108 pfu of HI-VSV (open columns) or VSV (filled columns). Draining lymph nodes were harvested 48 hours after VSV injection and analyzed by flow cytometry for (a) CD11b−GR1+B220+ or (b) CD11b+GR1+ cells. (c) Tumors harvested 24 hours following injection were analyzed for neutrophils (CD45+CD11b+GR1+F4/80−). HI, heat-inactivated; ns, nonsignificant; MyD88, myeloid differentiation primary response gene 88; pfu, plaque-forming unit; VSV, vesicular stomatitis virus.
MyD88-mediated IL-6 signaling is not important for VSV therapy
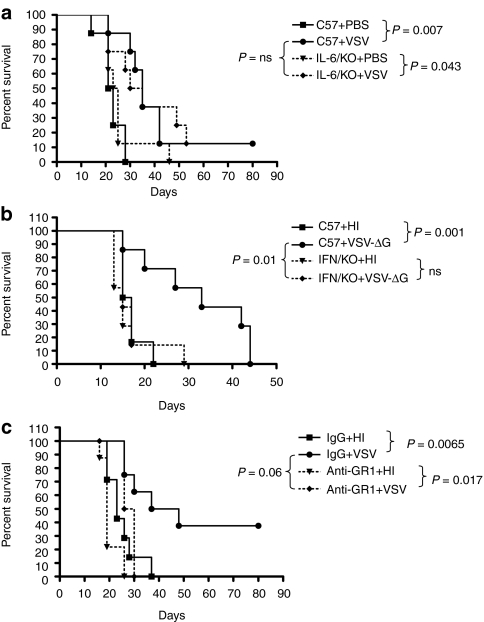
We hypothesized that the loss of the ability of MyD88−/−-derived viral sensor cells to secrete one or more of the cytokines identified in Figures 4 and 5 in response to VSV would contribute to the loss of VSV-mediated antitumor therapy in MyD88−/− mice (Figure 1). Consistent with this hypothesis, we have shown that IL-28 is indeed an important mediator of VSV therapy in the B16ova model.19 In contrast, there was no significant difference between the efficacy of VSV against B16ova tumors grown in C57BL/6 or IL-6-deficient mice (Figure 7a). This result suggests either that IL-6 is not a critical mediator of the innate response to virus that mediates tumor regressions in this model, or that a role for IL-6 is not revealed by these experiments due to redundancy in the multiple innate immune pathways involved in the response to VSV in vivo.
Figure 7.
VSV therapy is mediated through IFNα/β signaling. (a) C57BL/6 or IL6−/− mice (n = 8/group) bearing s.c. B16ova tumors were intratumorally treated with 5 × 108 pfu HI-VSV or VSV at days 7, 9, 11, 13, 15, and 17. Survival with time is shown. (b) C57BL/6 or IFNα/βR−/− mice (n = 8/group) bearing s.c. B16ova tumors were intratumorally treated with 5 × 107 pfu HI-VSV or VSV-ΔG at days 7, 9, 11, 13, 15, and 17. Survival with time is shown. (c) Mice were depleted of GR1+ cells (0.2 mg anti-GR1 Ab) or mock depleted (IgG control) (antibodies administered intraperitoneally 5.5 days after tumor implantation and every other day). Viruses or PBS were injected intratumorally on days 7, 9, 11, and 13 after tumor implantation. Tumor sizes in mice which had developed a tumor by the time of injection were monitored every other day and survival was plotted using a Kaplan–Meier survival curve. HI, heat-inactivated; IFN, interferon; IgG, immunoglobulin; PBS, phosphate-buffered saline; pfu, plaque-forming unit; VSV, vesicular stomatitis virus.
Type I IFN responses mediate both antiviral protection and antitumor therapy
GR1+ pDC, major mediators of type I IFN responses to viral infection,35,36 were significantly increased in TDLN of C57BL/6 mice treated with VSV compared to the TDLN of MyD88−/− mice in which VSV has no therapeutic effects. Therefore, we hypothesized that type I IFNs may also be the principal mediators of MyD88-mediated VSV immune therapy of B16ova tumors.
When B16ova tumors growing in IFNα/βR knockout (KO) mice were injected with VSV, mice rapidly developed fatal toxicity, associated with dissemination of virus to the brain, confirming the role of IFNα/β in protection of normal cells from viral infection. Therefore, to address the role of IFNα/β in VSV-mediated therapy of B16ova tumors, we used a virus in which the VSV-G glycoprotein is deleted from the viral genome (VSV-ΔG). We have shown that this VSV-ΔG, which can infect target cells, triggers an antiviral innate type I response but cannot replicate,10 is as effective as wild type, fully replication competent VSV in generating antitumor therapy in the B16ova model10 (Figure 7b). Thus, intratumoral injection of VSV-ΔG significantly prolonged survival of C57BL/6 mice compared to injection with HI-virus (P = 0.001)10 (Figure 7b). In contrast, this therapy was lost completely following injection of B16ova tumors with VSV-ΔG in IFNα/βR KO mice (Figure 7b).
Similarly, the profile of cytokine secretion from bone marrow-derived cells of IFNα/βR KO mice infected with VSV largely mirrored that from MyD88−/− mice. Thus, bone-marrow cells from neither MyD88−/− (Figure 4) nor IFNα/βR−/− (data not shown) mice were able to secrete IL-6, CXCL10, or IL-28 in response to VSV at levels approaching those observed from C57BL/6-derived bone marrow (data not shown).
Finally, upon depletion of GR1+ cells from C57BL/6 mice, which would include both pDC and neutrophils, VSV-mediated therapy of B16ova tumors was completely lost in the experiment of Figure 7c. It is important to stress that repeats of this depletion experiment did not always generate statistically significant loss of therapy in the GR1-depleted mice, compared to the control-treated groups, although the trend was always the same. We believe that this is due both to technical difficulties in achieving complete depletion within the tumor using the RB6-8C5 antibody and because the GR1 marker is also expressed on a proportion of CD8+ T cells which positively mediate therapy in this model.15 Nonetheless, these data, taken together with those from Figure 7b, suggest that type I IFN, probably derived from GR1+ pDC, play a critical role in mediating immune-based therapy of B16ova tumors, possibly through the activity of GR1+ neutrophils, following intratumoral injection of VSV.
Discussion
We show here that innate immune signaling through the MyD88 adaptor protein in host cells is critical for effective antitumor therapy following intratumoral injection of VSV into B16ova tumors. These conclusions are consistent with our previous findings that virotherapy with VSV in this model does not depend upon viral replication and is predominantly mediated through host immune reactivity to an immunogenic virus at the tumor site.10,12,15,16,19
Whereas viral replication in B16ova tumors in vivo was severely restricted in C57BL/6 mice, we observed a viral burst characteristic of ongoing replication in MyD88−/− mice (Figure 1). However, even when innate immune reactivity to virus was at least partially inhibited by loss of MyD88 signaling in host cells, with a concomitant increase in levels of intratumoral viral replication, antitumor therapy was completely lost (Figure 1). We also observed that absence of MyD88 signaling in vivo did not significantly impair either virus-specific or tumor-specific adaptive immunity, at least at day 7, following injection of virus (Figure 2), although we did not assess the effects of MyD88 on development adaptive responses over longer periods of time.37 Since antitumor therapy was lost in MyD88−/− mice, these data suggest that it is the innate, rather than adaptive, immune response that mediates virotherapy in this model.
Overall, these data indicate that the antitumor activity of antiviral immune effectors predominate in vivo over the therapeutic effects of viral replication—which is extremely limited10 in this model (Figure 1c). We believe that the increased viral burst seen in B16 tumors from MyD88−/− injected mice, relative to that in C57BL/6 mice, will also be associated with a shift in the balance from immune-mediated tumor cell destruction to virus-induced tumor necrosis, even though the increased levels of viral replication were insufficient to induce antitumor therapy in MyD88−/− mice. However, more detailed immunohistochemical experiments will be required to confirm this hypothesis.
Although VSV can activate innate immunity through activation of TLR4 and TLR7,31,34 the experiments of Figure 3 indicate that TLR4 alone, TLR7 alone, or IL-1 signaling alone was not sufficient to generate the antitumor immune therapy induced by VSV. It may be that TLR activation through multiple signaling pathways, which collectively culminate through MyD88, induce threshold levels of innate immune reactivity, which lead to tumor clearance. In this scenario, redundancy of the multiple signaling pathways activated by VSV, which are only collectively defective in MyD88−/− mice, may compensate for the absence of any single TLR pathway in the TLR deficient mice that we studied. In addition, in our experiments here, only the host-derived cells are defective for MyD88 or specific TLR signaling pathways. The B16ova tumor cells may themselves respond to VSV through TLR activation and may secrete inflammatory cytokines, which induce immune clearance of tumor cells. Studies are underway to knockdown-specific TLR pathways, as well as MyD88 in the tumor cells to resolve these confounding factors. In addition, MyD88 activation is a downstream effector of signaling through IL-18. Because previous reports suggested that IL-18 is not a key mediator of the host response to VSV infection,38,39 we have not yet studied the effects of IL-18 in VSV-mediated therapy of B16ova tumors. However, it may be that IL-18, or other molecules, which have minimal role in mediating the response to VSV infection in other tissues, are important in the context of intratumoral VSV infection. Therefore, studies to test the role of IL-18 are currently underway.
To identify critical host effector cytokines and cells which mediate the antitumor effects in response to VSV infection of B16ova tumors, we observed significant decreased expression of several cytokines—including IL-1β, CXCL10, IL-6, KC, IFN-α, and IL-28—in bone marrow-derived cells, as well as tumors from MyD88−/− mice compared to C57BL/6 mice (Figures 4 and 5). Consistent with Figures 4 and 5, we recently reported that induction of IL-28 from bone-marrow cells in C57BL/6 mice is, indeed, an important mediator of VSV therapy in the B16ova model.19 However, despite IL-6 being consistently down regulated in tumors and in bone-marrow cells, following VSV infection, anti-B16ova therapy by VSV was retained in IL-6−/− mice (Figure 7). Once again, it may be that animals deficient for a single cytokine may not reveal the true role of that molecule in an immune response, which involves multiple, possibly compensatory, pathways. These studies also suggest various additional effectors—such as CXCL10 and KC—which are induced by VSV in C57BL/6, but not in MyD88−/− KO, mice may mediate immune-based tumor cell clearance in response to innate activation by VSV. Previously, we have shown that natural killer cells are a major mediator of VSV-induced therapy of B16ova tumors.15,19 We have also shown that induction of IL-28 by intratumoral injection of VSV mediates sensitivity of B16ova tumors to natural killer cell killing.19 Moreover, we show here that induction of IL-28 in VSV-injected tumors is significantly reduced in MyD88−/− mice (Figure 5). Therefore, loss of innate immune signaling, through molecules such as IL-28 amongst others, to natural killer cells and other immune effectors, is likely to be a major mechanism by which the antitumor activity of VSV is lost in MyD88−/− mice.
The elevated levels of GR1+ cells in the TDLN of C57BL/6 mice treated intratumorally with VSV were significantly reduced in MyD88−/− mice (Figure 6). pDC are principal mediators of type I IFN secretion in response to viral infection,35,36 and we also observed significant loss of IFN-α in B16ova tumors injected in vivo with VSV from MyD88−/− mice compared with C57BL/6 tumor-bearing mice treated with VSV (Figure 5). Therefore, we hypothesized that, in addition to their potent antiviral activity, type I IFNs may also be the principal mediators of MyD88-dependent, VSV immune therapy of B16ova tumors. Injection of replication competent VSV into B16ova tumors in IFNα/βR KO mice led to rapid, fatal toxicity because of virus dissemination to the brain—consistent with the role of type I IFN in controlling the spread of VSV in normal cells in vivo. However, using a replication defective, single cycle VSV, which has equal therapeutic efficacy to wild-type VSV in the B16ova/C57BL/6 model,10 it was clear that an intact type I IFN response in the host was required for the antitumor therapy of VSV (Figure 7b).
Therefore, the type I IFN response to VSV in this model serves several conflicting roles as they pertain to the success of VSV-mediated oncolytic virotherapy. Finally, we show here that the type I IFN response is also essential for antitumor efficacy, both through its own direct antitumor effects40,41,42,43,44 as well as through stimulation of other antitumor immune effectors in vivo. In this respect, neutrophils were recruited at high levels to VSV-infected tumors in C57BL/6 mice, but much less so in MyD88−/− mice (Figure 6), consistent with previous studies on the importance of MyD88 for neutrophil recruitment to sites of pathogen infection.45 Our data are consistent with those of Breitbach and colleagues, who reported that VSV-induced neutrophil infiltration into tumors was associated with rapid vascular shutdown.7 In those studies, the effects of neutrophil depletion on therapy were not reported. In our model, depletion of GR1+ cells was accompanied by loss of VSV-mediated therapy (Figure 7), although this did not consistently reach significance across experiments.
Our data here are significant because they indicate that antitumor therapy in patients is likely to be contributed by a balance between direct viral replication/oncolysis and antiviral immune reactivity. This balance needs to be carefully considered when using immune suppression to enhance oncolytic virus delivery/replication/spread in vivo.12,20,43,44 If only suboptimal levels of innate immune inhibition are achieved in vivo, viral replication/oncolysis in the tumor may be only partially increased. However, under such conditions, immune-mediated therapy in response to the immunogenic virus may still be significantly impaired, thereby decreasing therapeutic outcome. This is what we observed in MyD88−/− mice, where increased viral replication was still insufficient to compensate for the loss of therapeutic benefits of the defective antiviral immune response (Figure 1). Alternatively, it may be possible to suppress the antiviral innate immune response sufficiently to allow for significantly increased, therapeutic levels of viral replication through the tumor. If this can be achieved, however, it will be important to restrict this immune suppression locally to the tumor site. If not, there is a risk of increased toxicity to the patient through viral release from the tumor, accompanied by unrestrained replication in normal tissues—as we observed with the lethality of VSV-treated IFNα/βr KO mice.
In summary, we show here, for the first time to our knowledge that antitumor therapy of oncolytic VSV in the B16ova immune-competent model is dependent upon signaling through MyD88 in host immune cells, which form part of the antiviral innate immune response. MyD88 immune signaling, in response to VSV, was also closely associated with a type I IFN response, which inhibited virus replication within the tumor, protected the host from uncontrolled viral spread from the tumor, and mediated antitumor therapy. Therefore, the innate immune response to oncolytic viruses can be, simultaneously, protherapeutic, antioncolytic, and systemically protective. These paradoxically conflicting roles need to be carefully considered in future strategies designed to improve the efficacy of oncolytic virotherapy in patients.
Materials and Methods
Cell lines and viruses. Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells transduced with a complementary DNA encoding the chicken ovalbumin gene.46 Cells were grown in Dulbecco's modified Eagle's medium (Life Technologies, Rockville, MD) supplemented with 10% (vol/vol) FCS (Life Technologies), -glutamine (Life Technologies) and 5 mg/ml of G418 to select for retention of the ova gene. All cell lines were monitored routinely and found to be free of mycoplasma infection.
VSV-GFP and VSV-ova (Indiana serotype) were generated by cloning the complementary DNA for the green fluorescence protein or chicken ovalbumin into the plasmid pVSV-XN2 as described previously.26 Concentration and purification of viruses were done by sucrose gradient centrifugation. Virus titers were measured by standard plaque assays of serially diluted samples on BHK-21 cells.26 VSV-GFP is referred to as VSV.
In vivo studies. All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57BL/6 mice, TLR4, TLR7, and IL-1R KO mice (TLR4−/−, TLR7−/−, and IL-1R−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks of age. IFNα/βR KO mice, previously backcrossed onto the C57BL/6 background, were a kind gift from Dr Cattaneo, Mayo Clinic (Rochester, MN). MyD88 KO mice (MyD88−/−) were a kind gift from Dr Pease, Mayo Clinic. To establish subcutaneous tumors, 5 × 105 B16ova tumor cells in 100 µl of phosphate-buffered saline were injected into the flank of mice. Viral injections (50 µl) were administered intratumorally at days 7, 9, 11, 13, 15, and 17 after tumor establishment. Animals were examined daily and tumor sizes were measured thrice-weekly using calipers. Animals were euthanized when tumor size was >1.0 × 1.0 cm in two perpendicular directions. For in vivo depletion of GR1+ cells, 0.2 mg of anti-GR1 antibody, RB6-8C5 (BioXcell, West Lebanon, NH), or IgG control (ChromPure Rat IgG; Jackson ImmunoResearch, West Grove, PA), was administered intraperitoneally on days 5.5, 8, 10, 12, 15, 18, and 23 after tumor implantation.
Enzyme-linked immunosorbent spot assay for IFN-γ secretion. TDLN were harvested and 1 × 105 cells/well were plated in 96-well plates in triplicate. Cells were stimulated for 48 hours at 37 °C with peptides at 5 µg/ml. Peptide-specific, IFN-γ positive spots were detected according to the manufacturer's protocol (Mabtech, Cincinnati, OH) and were quantified by computer-assisted image analyzer. The synthetic, H-2Kb–restricted peptides ova SIINFEKL and VSV N protein-derived RGYVYQGL were synthesized at the Mayo Foundation Core Facility.
Flow cytometry and IFN-γ intracellular staining assay. TDLN and tumors were recovered from mice and dissociated in vitro to achieve single-cell suspensions. Cells were washed, resuspended in phosphate-buffered saline containing 0.1% bovine serum albumin (wash buffer), and incubated with directly conjugated primary antibodies for 30 minutes at 4 °C. Cells were then washed and resuspended in 500 µl phosphate-buffered saline containing 4% formaldehyde. Cells were analyzed by flow cytometry using Flowjo software (Flowjo, Ashland, OR). Anti-CD11b FITC, anti-B220 PE, anti-F4/80 APC, anti-GR1 PE-Cy7, anti-CD45 Per-CP, and their respective isotype controls were purchased from BD Pharmingen (San Jose, CA).
For intracellular staining, single-cell suspensions were prepared from TDLN (three mice per group) harvested 7 days after viral injection. IFN-γ production, in response to antigen, was measured by incubation with peptides (5 µg/ml) in the presence of Golgi Plug reagent for 4 hours. Cells were then stained, fixed, and permeabilized for intracellular staining using a Cytofix/Cytoperm kit from BD Biosciences according to the manufacturer's instructions. All antibodies were obtained from BD Biosciences (San Diego, CA).
Bone-marrow activation. Bone-marrow cells were removed from the femurs and tibias of female C57BL/6, MyD88−/−, or IFNα/βR KO mice by flushing with RPMI 1640 and passed through a 100-µm filter to prepare single-cell suspensions. 1 × 106 bone-marrow cells were plated for 6 hours at 37 °C in a 10% CO2 incubator. Cells were then infected with different multiplicity of infection of VSV or pulsed with lipopolysaccharide (Sigma-Aldrich) or imiquimod (InvivoGen, San Diego, CA) for 48 hours. Supernatants were harvested for enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assay and cytokine multiplex analysis. Cell-free supernatants, or tumor lysates, were tested for IL-28 (PBL Interferonsource, Piscataway, NJ), IL-6 and IL-1β (BD OptEIA, San Diego, CA), and CXCL10/IP-10 (R&D Systems) as directed in the manufacturer's instructions.
For cytokine multiplex analysis, tumor lysates harvested 24 hours after viral injection were normalized by protein concentration and 20 cytokine multiplex (Procarta, Cytokine Assay Kit; Affymetrix, Fremont, CA) were performed and analyzed according to manufacturer's instructions.
Statistics. Survival data from the animal studies were analyzed by log-rank test using GraphPad Prism 4 (GraphPad Software, La Jolla, CA). In vitro experiments were analyzed using JMP Software (SAS Institute, Cary, NC). Statistical significance was determined at the level of P < 0.05.15
Acknowledgments
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard M. Schulze Family Foundation, the Mayo Foundation, and by NIH grants CA107082, CA130878, and CA132734.
REFERENCES
- Parato KA, Senger D, Forsyth PA., and, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Aghi M., and, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Stanford MM., and, McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7:1415–1425. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol Ther. 2007;15:1686–1693. doi: 10.1038/sj.mt.6300215. [DOI] [PubMed] [Google Scholar]
- Friedman A, Tian JP, Fulci G, Chiocca EA., and, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote D, Cattaneo R., and, Fielding AK. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003;63:6463–6468. [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120:1551–1560. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf F, Diallo JS, McCart JA, Thorne S, Falls T, Stanford M, et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol Ther. 2010;18:888–895. doi: 10.1038/mt.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J, et al. Interference of CD40L-mediated Tumor Immunotherapy by Oncolytic VSV. Human Gene Therapy. 2010;21:439–450. doi: 10.1089/hum.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL., and, Fraser NW. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8:543–551. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- Toda M, Rabkin SD, Kojima H., and, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J, et al. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF., and, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wagner RR., and, Rose JK.1996Rhabdoviridae: The Viruses and Their Replication. Field Virology. vol. 37, Philadelphia. pp1121–1135.
- Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- Balachandran S., and, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D., and, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ebert O., and, Woo SL. Treatment of multi-focal colorectal carcinoma metastatic to the liver of immune-competent and syngeneic rats by hepatic artery infusion of oncolytic vesicular stomatitis virus. Int J Cancer. 2005;114:659–664. doi: 10.1002/ijc.20772. [DOI] [PubMed] [Google Scholar]
- Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J, et al. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., and, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., and, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kurt-Jones EA, Fitzgerald KA, Wang JP, Cerny AM, Chan M, et al. Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J Immunol. 2007;178:5173–5181. doi: 10.4049/jimmunol.178.8.5173. [DOI] [PubMed] [Google Scholar]
- Lang KS, Navarini AA, Recher M, Lang PA, Heikenwalder M, Stecher B, et al. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur J Immunol. 2007;37:2434–2440. doi: 10.1002/eji.200737310. [DOI] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Barchet W, Cella M., and, Colonna M. Plasmacytoid dendritic cells–virus experts of innate immunity. Semin Immunol. 2005;17:253–261. doi: 10.1016/j.smim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, et al. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JL, Ireland DD., and, Reiss CS. The role of interleukin-18 in vesicular stomatitis virus infection of the CNS. Viral Immunol. 2001;14:181–191. doi: 10.1089/088282401750234556. [DOI] [PubMed] [Google Scholar]
- Zhou S, Kurt-Jones EA, Fitzgerald KA, Wang JP, Cerny AM, Chan M, et al. Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J Immunol. 2007;178:5173–5181. doi: 10.4049/jimmunol.178.8.5173. [DOI] [PubMed] [Google Scholar]
- Goldstein D., and, Laszlo J. The role of interferon in cancer therapy: a current perspective. CA Cancer J Clin. 1988;38:258–277. doi: 10.3322/canjclin.38.5.258. [DOI] [PubMed] [Google Scholar]
- Jonasch E., and, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002;29 3 Suppl 7:18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- Miller CG., and, Fraser NW. Requirement of an integrated immune response for successful neuroattenuated HSV-1 therapy in an intracranial metastatic melanoma model. Mol Ther. 2003;7:741–747. doi: 10.1016/S1525-0016(03)00120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA., and, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]