Abstract
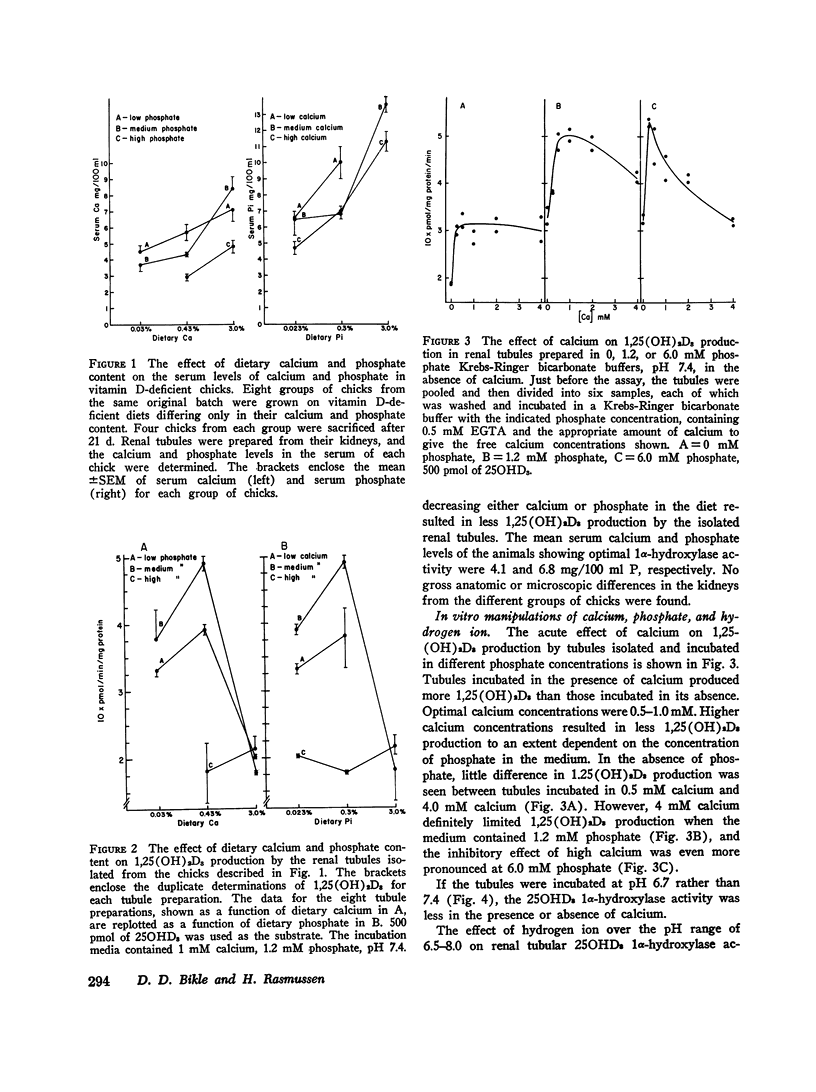
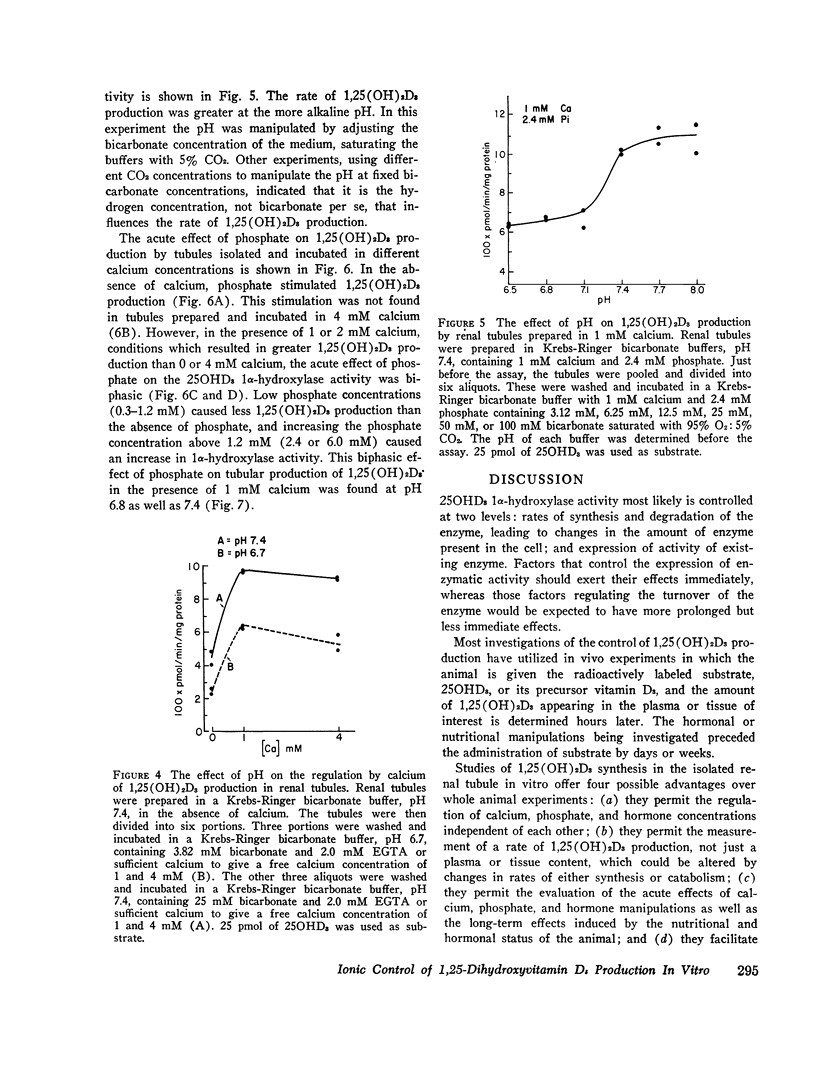
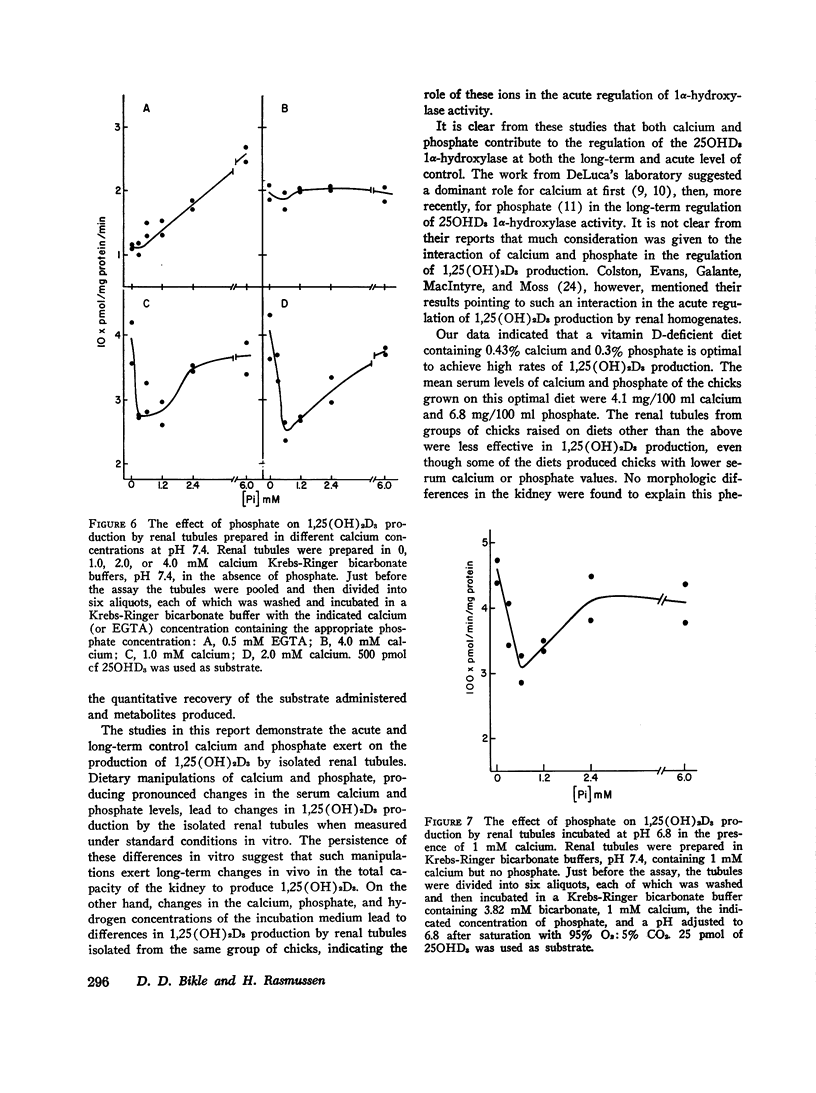
Isolated renal tubules prepared from vitamin D-deficient chicks catalyze the 1 alpha-hydroxylation of 25-hydroxyvitamin D3 (250HD3) in vitro. The effect of calcium and phosphate on the rate of synthesis of the product, 1, 25-dihydroxyvitamin D3 (1,25(OH)2D3), was studied at two levels: the long-term effects of various dietary calcium and phosphate contents on the ability of the tubules to produce 1, 25 (OH)2D3, and the acute effects of different calcium and phosphate concentrations in the incubation medium on the rate of synthesis of 1,25(OH)2D3 by the tubules. Manipulation of dietary calcium and phosphate sufficient to produce marked changes in the concentration of calcium and phosphate in the serum led to altered rates of 1,25(OH)2D3 synthesis by the isolated renal tubules. The renal tubules isolated from chicks raised on a vitamin D-deficient diet containing 0.43% calcium and 0.3% P as inorganic phosphate showed the highest rate of synthesis of 1,25(OH)2D3. Diets containing more or less of either calcium or phosphate produced chicks whose renal tubules had a slower rate of 1,25(OH)2D3 production. The calcium, phosphate, and hydrogen ion content of the incubation medium were manipulated to determine the possible factors concerned with the immediate regulation of 1,25(OH)2D3 production. A calcium concentration of 0.5-1.0 mM was necessary for optimal enzymatic activity. Concentrations of calcium greater than this optimal concentration inhibited 1,25(OH)2D3 production if phosphate was also present, and this inhibition was more pronounced as the phosphate concentration was increased. The stimulation of 1,25(OH)2D3 production by calcium was less at pH 6.7 than at 7.4. Raising the phosphate concentration from 0 to 6 mM in the absence of calcium also stimulated the rate of synthesis of 1,25(OH)2D3. This stimulatory effect was blocked by 4 mM calcium. However, at 1-2 mM calciu, phosphate had a biphasic influence on 1,25(OH)2D3 production; extracellular concentrations of phosphate from 0.6 to 1.2 mM resulted in less 1,25(OH)2D3 production than higher or lower phosphate concentrations. This biphasic effect was seen both at pH 7.4 and 6.8.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Murphy E. W., Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 synthesis in isolated chick renal mitochondria. The role of calcium as influenced by inorganic phosphate and hydrogen-ion. J Clin Invest. 1975 Feb;55(2):299–304. doi: 10.1172/JCI107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. A comparison of the metabolism of 25-hydroxyvitamin D3 by chick renal tubules, homogenates and mitochondria. Biochim Biophys Acta. 1974 Oct 8;362(3):439–447. doi: 10.1016/0304-4165(74)90139-1. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. The metabolism of 25-hydroxycholecalciferol by isolated renal tubules in vitro as studied by a new chromatographic technique. Biochim Biophys Acta. 1974 Oct 8;362(3):425–438. doi: 10.1016/0304-4165(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Galante L., MacIntyre I., Moss D. W. Regulation of vitamin D metabolism: factors influencing the rate of formation of 1,25-dihydroxycholecalciferol by kidney homogenates. Biochem J. 1973 Jul;134(3):817–820. [PMC free article] [PubMed] [Google Scholar]
- DeLuca H. F. Recent advances in the metabolism and function of vitamin D. Fed Proc. 1969 Sep-Oct;28(5):1678–1689. [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Galante L., Colston K. W., MacAuley S. J., MacIntyre I. Effect of calcitonin on vitamin D metabolism. Nature. 1972 Aug 4;238(5362):271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- Galante L., Colston K., MacAuley S., MacIntyre I. Effect of parathyroid extract on vitamin-D metabolism. Lancet. 1972 May 6;1(7758):985–988. doi: 10.1016/s0140-6736(72)91156-7. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972 Jun 9;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Kodicek E., Lawson D. E., Wilson P. W. Biological activity of a polar metabolite of vitamin D. Nature. 1970 Nov 21;228(5273):763–764. doi: 10.1038/228763a0. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Rasmussen H. Ioni control of renal gluconeogenesis. I. The interrelated effect of calcium and hydrogen ions. Biochim Biophys Acta. 1973 Jun 20;313(1):17–31. doi: 10.1016/0304-4165(73)90185-2. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Rasmussen H. Ionic control of renal gluconeogenesis. IV. Effect of extracellular phosphate concentration. Biochim Biophys Acta. 1973 Jun 20;313(1):59–71. doi: 10.1016/0304-4165(73)90188-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Omdahl J. L., Gray R. W., Boyle I. T., Knutson J., DeLuca H. F. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat New Biol. 1972 May 10;237(71):63–64. doi: 10.1038/newbio237063a0. [DOI] [PubMed] [Google Scholar]
- REILLEY C. N. Methods of detecting and controlling metal ion levels. Fed Proc. 1961 Sep;2:22–32. [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D., Goodman D. B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J Clin Invest. 1972 Sep;51(9):2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain S. A. In vitro metabolism of 25-hydroxycholecalciferol by chick intestinal and renal cell preparations. Identification of a metabolic product as 1,25-dihydroxycholecalciferol and delineation of its metabolic fate in intestinal cells. J Biol Chem. 1972 Jul 10;247(13):4393–4403. [PubMed] [Google Scholar]
- Shain S. A. The in vitro metabolism of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol by chick renal tubules. Effect of actinomycin D, puromycin, calcium, and parathyroid hormone. J Biol Chem. 1972 Jul 10;247(13):4404–4413. [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Wong R. G., Norman A. W. Studies on calciferol metabolism. IV. Subcellular localization of 1,25-dihydroxy-vitamin D 3 in intestinal mucosa and correlation with increased calcium transport. J Biol Chem. 1972 Sep 10;247(17):5511–5519. [PubMed] [Google Scholar]
- Wong R. G., Myrtle J. F., Tsai H. C., Norman A. W. Studies on calciferol metabolism. V. The occurrence and biological activity of 1,25-dihydroxy-vitamin D 3 in bone. J Biol Chem. 1972 Sep 25;247(18):5728–5735. [PubMed] [Google Scholar]



