Abstract
Purpose
Concerns regarding long-term toxicities have led some to withhold radiation therapy (RT) for the treatment of stage I and II Hodgkin's disease (HD). This study was undertaken to assess the utilization of RT in HD and its impact on overall survival (OS) and secondary malignancies.
Materials
This was a study from the Surveillance, Epidemiology, and End Results database that included patients who were 20 years and older who had been diagnosed with stage I or II HD diagnosed from 1988–2006. OS was estimated by the Kaplan-Meier method, and Cox multivariable Regression model was used to analyze trends.
Results
A total of 12,247 patients were selected and 51.5% received RT. The median follow up for this cohort was 4.9 years, with 21% of the cohort with > 10 years of follow-up. In 1988–1991, 62.9% received RT whereas in 2004–2006 only 43.7% received RT (p < 0.001). Among this cohort the 5 year OS was 76% for patients who did not receive RT and 87% for those that did receive RT (p < 0.001). The hazard ratio adjusted for other variables in regression model showed that patients who did not receive RT (HR – 1.72, 95% CI – 1.72–2.02) was associated with significantly worse survival when compared to patients who received RT.
The actuarial rate of developing a second malignancy was 14.6% vs 15.0% at 15 years for patients who received RT vs. those with no RT (p = 0.089).
Conclusions
This is one of the largest studies to examine the role of RT in stage I and II HD and revealed a survival benefit with the addition of RT with no increase in secondary malignancies compared to patients who did not receive radiation therapy. Furthermore, this nationwide study revealed an over 20% absolute decrease in the utilization of RT from 1988–2006.
Keywords: Hodgkin’s, Radiation, second malignancy
Introduction
The treatment of early stage Hodgkin's disease (HD) has been a success story in oncology, where an incurable disease became curable in the 1960’s with the use of external beam radiation therapy. Since that time, chemotherapy has similarly demonstrated significant improvements in outcomes in patients with advanced disease as well as early stages. The results have been so impressive that co-operative groups changed the treatment paradigm from improving survival to maintaining the same survival and reducing morbidity by trying to minimize radiotherapy and chemotherapy. In the early 1990's several publications revealed significant long term complications associated with a combined modality approach involving full-dose chemotherapy and extended field radiation therapy (1–4).
This led to the investigation of two treatment strategies: 1. Utilization of combined modality therapy but with a reduction in the irradiated volume, radiation dose, the number of chemotherapy cycles, and the number of chemotherapy agents or 2. Utilize chemotherapy alone with additional cycles and eliminate radiation from the treatment paradigm. Proponents of chemotherapy alone strategies believed an improved overall survival would be seen due to the reduction in late radiation related mortality. Furthermore, any increase in relapses seen in chemotherapy alone strategies would not affect survival because these patients could be salvaged with additional chemotherapy and stem-cell transplant (5).
These two approaches were then tested in several prospective phase III trials (6–10). All 5 trials showed a significant improvement in disease control with the addition of radiation therapy, however this did not translate into a benefit in overall survival in stages I and II. These trials were limited by low patient numbers and limited follow-up which may have precluded them from demonstrating any significant survival benefit or any detriment from delayed radiation morbidity.
We undertook this study to determine in a large population based cohort if radiation therapy is associated with a survival benefit in stage I and II HD. Furthermore, we wanted to examine overall trends in the utilization of radiation therapy in early stage HD and the impact of radiation therapy on the second malignancy rate compared to patients who did not receive radiation therapy.
Methods and Materials
The Surveillance, Epidemiology, and End Results (SEER) database of the National Cancer Institute covers 26% of the US population and collects incidence and survival data from 17 population based cancer registries. The database contains information on primary tumor site, age, gender, histology, stage at diagnosis, first course of treatment, follow-up, and cause of death.
Data and Study Population
Eligible patients had histologically confirmed HD. Lymphocyte-predominant Hodgkin lymphoma was excluded from this analysis. We restricted the analysis to patients aged 20 years and older who were diagnosed between 1988–2006. Patients with extent of disease codes that corresponded to the current American Joint Committee on Cancer stages I and II were included. Patient who presented with stage III or IV, or unknown stage were excluded. Patients were classified into two groups based on whether they underwent radiation therapy as part of their initial treatment. The final sample size included 12,467 patients.
Overall Survival (OS) was the primary study endpoint. OS was defined as the time from diagnosis to the date of death from any cause. Cause specific survival (CSS) was also estimated and defined from the time of diagnosis to the date of death from HD. Exposure variables included categorical variables for type of treatment received. Plausible risk factors included in the statistical analysis included age, sex, radiation therapy, histology, year of diagnosis, stage (I vs II), extranodal involvement, B symptoms, and city population. Information regarding the utilization of chemotherapy, local control, performance status, and specific radiation therapy technique (dose, fractionation, beam energy) was not available in the SEER database.
Statistical Analysis
Estimates of OS and CSS were calculated using the Kaplan-Meier method. The log rank test was used to estimate whether there were differences in OS and CSS experience among these patients. All statistical tests were two-sided, and done at the 0.05 level of significance.
The multivariable Cox regression model was used to determine whether variables were independent predictors of OS and CSS. Hazard ratios (HR) and the corresponding 95% confidence intervals (CI) were constructed in models adjusted for all listed covariates of interest. Data were analyzed using SAS (Version 9.3, SAS Institute, Cary, NC).
Results
The median follow up for this cohort was 4.9 years, with 21% of the cohort with > 10 years of follow-up. Among the 12,247 patients included in this study, 51.5% received radiation therapy as a primary component of their treatment. The categorical variables of age, sex, SEER registry, year of diagnosis, stage, extranodal involvement, B symptoms, histology, and city population were significant predictors of the administration of radiation therapy (Table 1). Patients with stage II disease, extranodal involvement, B symptoms, and lymphocyte rich or nodular sclerosis histology were more likely to receive radiation therapy.
Table 1.
Predictors of Radiation Therapy Use
| No. of Patients* |
% Who Received Radiation Therapy† |
% Who Did Not Receive Radiation Therapy† |
P-value‡ | |
|---|---|---|---|---|
| Overall | 12,447 | 51.5 | 48.5 | |
| Patient characteristics | ||||
| Age in years | <.001 | |||
| 20–44 years | 8077 | 55.3 | 44.7 | |
| 45–59 years | 2031 | 47.7 | 52.3 | |
| 60–74 years | 1398 | 43.0 | 57.0 | |
| 75+ years | 941 | 39.6 | 60.4 | |
| Sex | <.001 | |||
| Male | 6289 | 49.6 | 50.5 | |
| Female | 6158 | 53.4 | 46.6 | |
| Location of SEER registry | <.001 | |||
| San Francisco | 1059 | 69.6 | 30.4 | |
| Connecticut | 1404 | 42.8 | 57.2 | |
| Detroit | 1222 | 52.6 | 47.4 | |
| Hawaii | 213 | 55.9 | 44.1 | |
| Iowa | 803 | 62.3 | 37.7 | |
| New Mexico | 336 | 47.3 | 52.7 | |
| Seattle | 1064 | 67.3 | 32.7 | |
| Utah | 494 | 56.7 | 43.3 | |
| Atlanta | 691 | 39.9 | 60.1 | |
| San Jose | 450 | 68.4 | 31.6 | |
| Los Angeles | 1429 | 42.1 | 57.9 | |
| California (other than SF/SJ/LA) | 1384 | 49.0 | 51.0 | |
| Kentucky | 440 | 42.7 | 57.3 | |
| Louisiana | 442 | 45.3 | 54.8 | |
| New Jersey | 1016 | 39.3 | 60.7 | |
| Year of diagnosis | <.001 | |||
| 1988–1991 | 1413 | 62.9 | 37.1 | |
| 1992–1994 | 1488 | 56.2 | 43.8 | |
| 1995–1997 | 1542 | 53.3 | 46.7 | |
| 1998–2000 | 2008 | 54.5 | 45.5 | |
| 2001–2003 | 2996 | 48.5 | 51.5 | |
| 2004–2006 | 3000 | 43.7 | 56.3 | |
| Tumor characteristics | ||||
| Stage | .03 | |||
| I | 4569 | 50.2 | 49.8 | |
| II | 7877 | 52.2 | 47.8 | |
| Involvement | .05 | |||
| No Extranodal Involvement | 930 | 48.4 | 51.6 | |
| Extranodal Involvement | 11517 | 51.7 | 48.3 | |
| B Symptoms | <.001 | |||
| Yes | 3244 | 44.6 | 55.4 | |
| No | 9203 | 53.9 | 46.1 | |
| Histology | <.001 | |||
| Lymphocyte Rich | 526 | 63.5 | 36.5 | |
| Mixed Cellularity | 1954 | 48.9 | 51.1 | |
| Lymphocyte Depleted | 135 | 33.3 | 66.7 | |
| Nodular Sclerosis | 8416 | 53.5 | 46.5 | |
| Hodgkin Lymphoma NOS | 1416 | 40.3 | 59.8 | |
| Metro Counties | 0.003 | |||
| Areas of 1 million population or more | 8105 | 51.3 | 48.8 | |
| Areas of 250,000 to 1 million | 2458 | 49.8 | 50.2 | |
| Areas of fewer than 250,000 population | 764 | 58.5 | 41.5 | |
| Nonmetro counties | ||||
| Urban population of 20,000 or more, adjacent to a metro area | 309 | 55.0 | 45.0 | |
| Urban population of 20,000 or more, not adjacent to a metro area | 178 | 48.9 | 51.1 | |
| Urban population of 2,500 to 19,999, adjacent to a metro area | 283 | 47.7 | 52.3 | |
| Urban population of 2,500 to 19,999, not adjacent to a metro area | 240 | 52.1 | 47.9 | |
| Completely rural or less than 2,500 urban population | 108 | 57.4 | 42.6 | |
| Unknown/not official USDA Rural-Urban Continuum code | 2 | 50.0 | 50.0 |
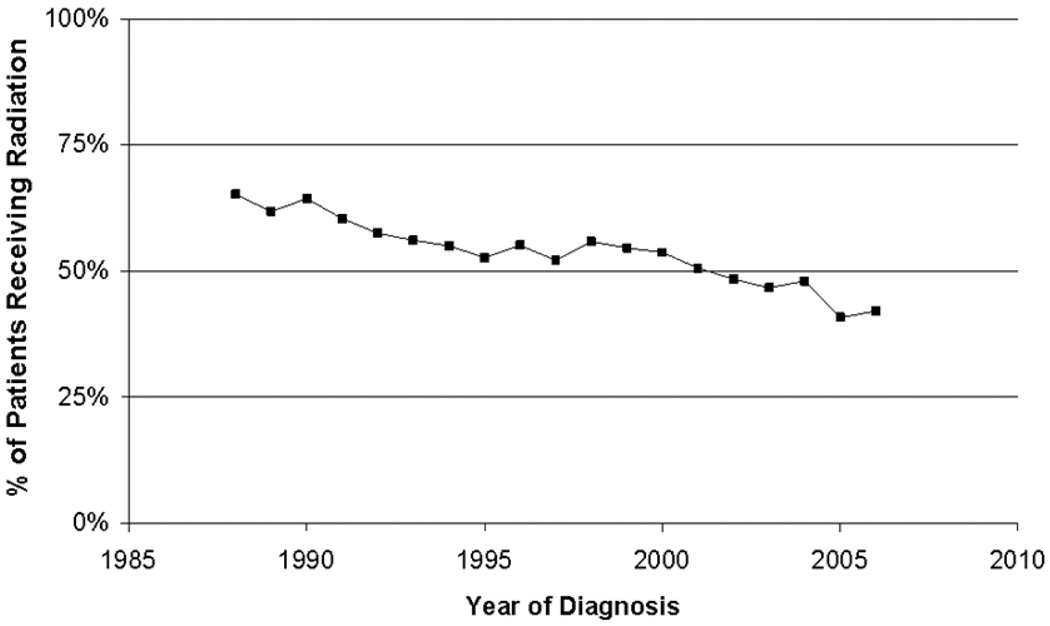
When examining year of diagnosis, there was a significant change in the utilization of radiation therapy over the years. Between 1988–1991, 62.9% of the cohort received radiation therapy whereas from 2004–2006 only 43.7% received radiation therapy (p < 0.001) (Figure 1).
Figure 1.
Percentage of Hodgkin's Patients Receiving Radiation Between 1988–2006, by Year of Diagnosis
Univariate Analysis
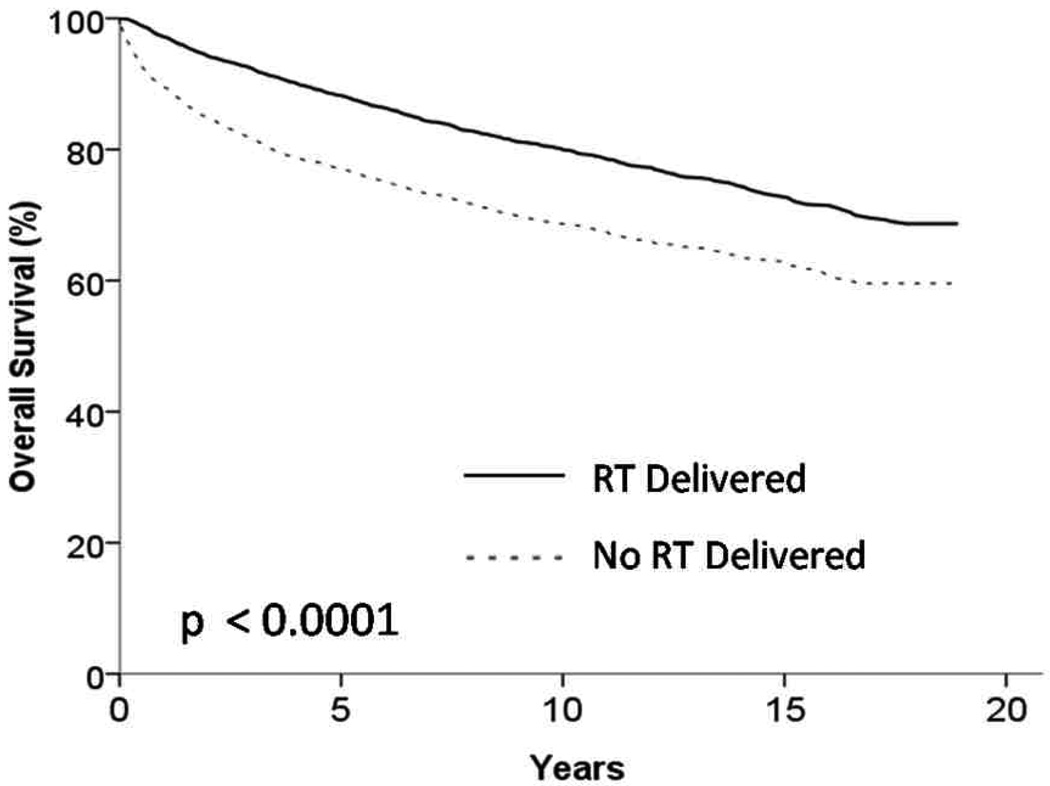
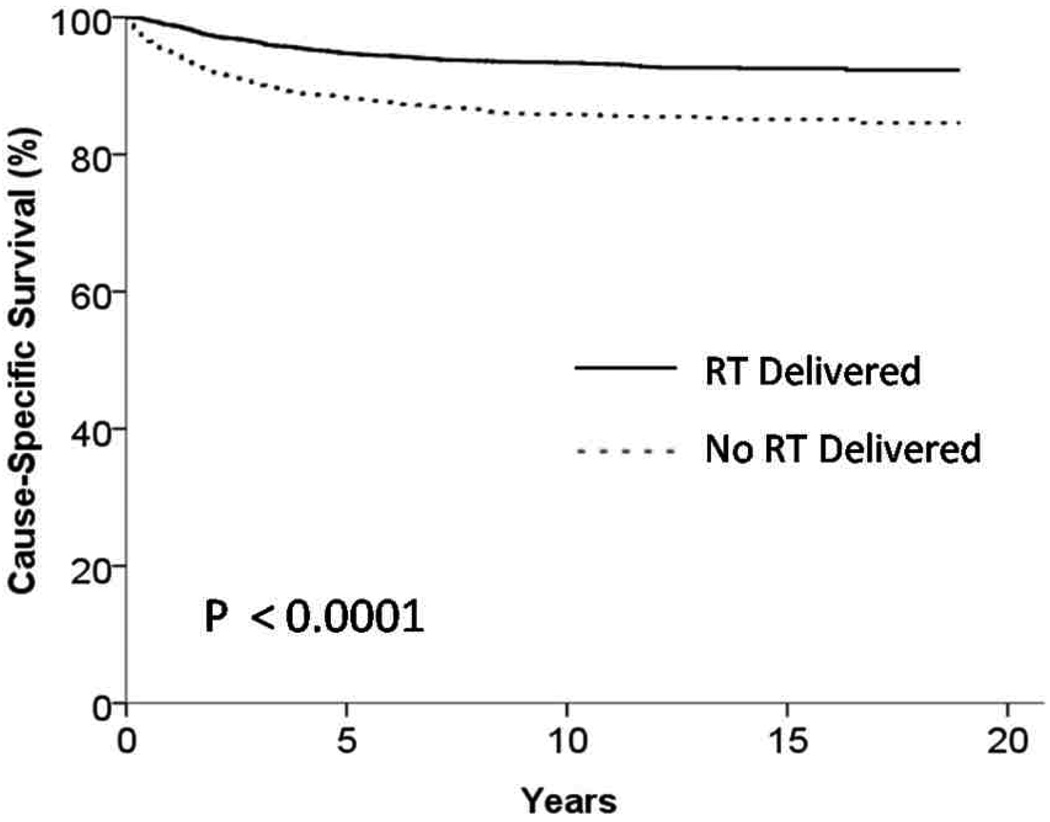
Among patients with stage I and II HD the 5 year OS was 76% for patients who did not receive radiation therapy and 87% for those that did receive radiation therapy (p < 0.001) (Figure 2). In patients with stage I and II HD the 5 year CSS was 88% for patients who did not receive radiation therapy and 94% for those that did receive radiation therapy (p < 0.001) (Figure 3). In order to control for bias that would have precluded patients from receiving any therapy we also examined patients who survived a minimum of one year and in this cohort the 5 year OS was 85% for patients who did not receive radiation therapy and 90% for those that did receive radiation therapy (p < 0.001).
Figure 2.
Kaplan Meier Curve showing overall survival of patients receiving and not receiving radiation therapy (RT) (p <0.001)
Figure 3.
Kaplan Meier Curve showing cause specific survival of patients receiving and not receiving radiation therapy (RT) (p < 0.001)
Multivariate Analysis
Among all patients, radiation therapy was associated with significantly improved overall survival, even after adjustment for patient and tumor characteristics (Table 2). Other variables adversely affecting survival on multivariable analysis included older age, female sex, earlier year of diagnosis, no extranodal involvement, B symptoms, and lymphocyte depleted histology. Patients with stage I and II tumors when analyzed separately were both found to benefit from the addition of radiation therapy after adjusting for known patient and tumor characteristics.
Table 2.
Hazard Ratios and 95% Confidence Intervals for Cox Proportional Hazards Models of Overall Mortality, Stratified by Stage
| All Patients | Stage I | Stage II | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Multivariable | Multivariable | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Radiation therapy* | ||||||||
| Radiation therapy | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| No radiation | 1.86 | 1.72, 2.02 | 1.53 | 1.41, 1.67 | 1.57 | 1.39, 1.77 | 1.50 | 1.33, 1.68 |
| Patient characteristics | ||||||||
| Age in years | ||||||||
| 20–44 years | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| 45–59 years | 2.94 | 2.60, 3.31 | 2.80 | 2.48, 3.16 | 3.20 | 2.64, 3.88 | 2.53 | 2.15, 2.98 |
| 60–74 years | 7.77 | 6.97, 8.66 | 7.14 | 6.38, 8.00 | 7.94 | 6.65, 9.49 | 6.72 | 5.77, 7.82 |
| 75+ years | 19.43 | 17.41, 21.69 | 18.34 | 16.32, 20.61 | 20.03 | 16.65, 24.09 | 17.86 | 15.30, 20.85 |
| Sex | ||||||||
| Male | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| Female | 0.76 | 0.70, 0.83 | 0.80 | 0.74, 0.87 | 0.77 | 0.68, 0.87 | 0.82 | 0.74, 0.92 |
| Year of diagnosis | ||||||||
| 1988–1991 | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| 1992–1994 | 0.95 | 0.83, 1.08 | 0.84 | 0.73, 0.95 | 0.72 | 0.59, 0.87 | 0.95 | 0.79, 1.14 |
| 1995–1997 | 0.96 | 0.84, 1.10 | 0.87 | 0.76, 0.99 | 0.80 | 0.65, 0.97 | 0.93 | 0.77, 1.12 |
| 1998–2000 | 0.86 | 0.74, 0.98 | 0.75 | 0.65, 0.87 | 0.81 | 0.66, 0.99 | 0.71 | 0.58, 0.86 |
| 2001–2003 | 0.85 | 0.74, 0.98 | 0.72 | 0.63, 0.83 | 0.71 | 0.58, 0.88 | 0.72 | 0.59, 0.87 |
| 2004–2006 | 0.99 | 0.83, 1.16 | 0.79 | 0.67, 0.94 | 0.81 | 0.62, 1.05 | 0.78 | 0.63, 0.97 |
| Tumor characteristics | ||||||||
| Stage | ||||||||
| I | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| II | 0.75 | 0.69, 0.81 | 1.06 | 0.97, 1.15 | -- | -- | -- | -- |
| Extranodal Involvement | ||||||||
| No Involvement | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| Extranodal Involvement | 0.67 | 0.58, 0.76 | 0.84 | 0.73, 0.96 | 0.82 | 0.65, 1.04 | 0.84 | 0.71, 1.01 |
| B Symptoms | ||||||||
| Yes | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| No | 0.77 | 0.70, 0.83 | 0.75 | 0.68, 0.82 | 0.82 | 0.70, 0.97 | 0.72 | 0.64, 0.80 |
| Histology | ||||||||
| Lymphocyte Rich | 1.0 | reference | 1.0 | reference | 1.0 | reference | 1.0 | reference |
| Mixed Cellularity | 1.58 | 1.30, 1.93 | 1.16 | 0.95, 1.41 | 1.26 | 0.97, 1.63 | 0.99 | 0.72, 1.37 |
| Lymphocyte Depleted | 3.66 | 2.74, 4.90 | 1.81 | 1.35, 2.43 | 2.15 | 1.39, 3.31 | 1.53 | 0.998, 2.34 |
| Nodular Sclerosis | 0.69 | 0.57, 0.84 | 1.03 | 0.85, 1.26 | 1.22 | 0.95, 1.57 | 0.83 | 0.61, 1.13 |
| Hodgkin Lymphoma NOS | 1.57 | 1.27, 1.94 | 1.31 | 1.06, 1.62 | 1.39 | 1.05, 1.84 | 1.15 | 0.82, 1.61 |
Data for metro and non-metro counties not shown.
Secondary Malignancies
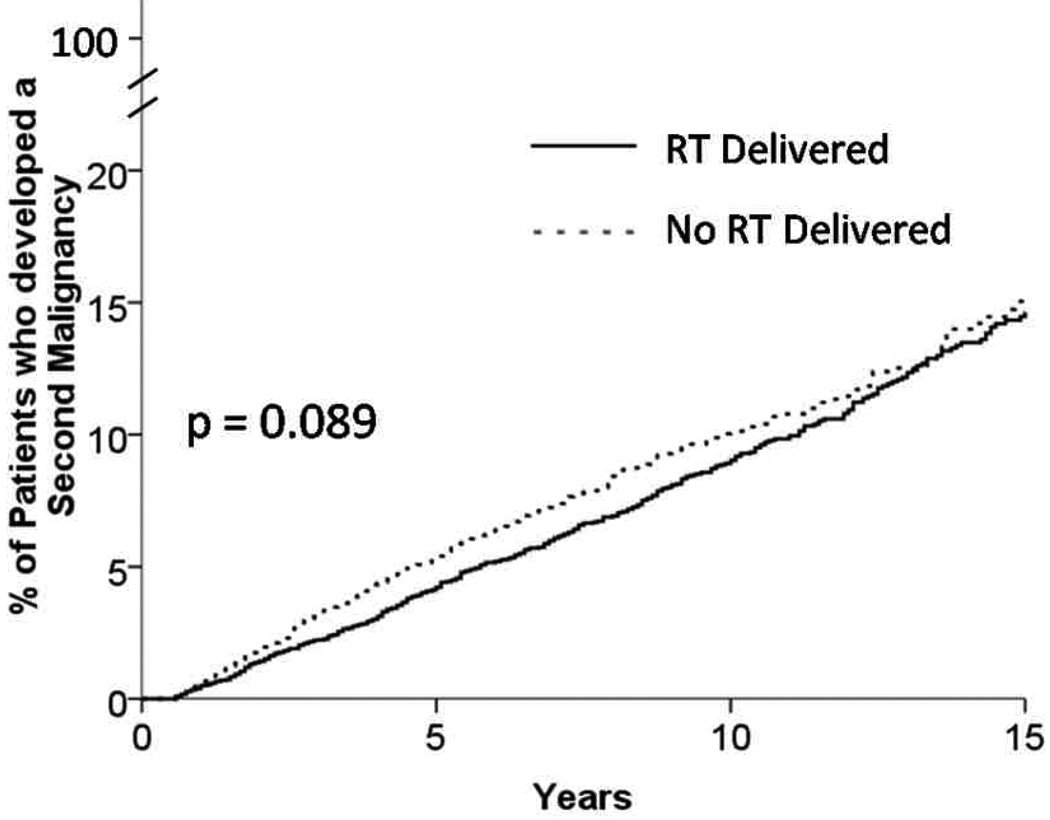
Among the entire cohort, 5.3% of the patients experienced a second malignancy at a median of 4.3 years. Patients who received radiation therapy were more likely to develop secondary solid malignancies while those that did not receive radiation had a higher incidence of developing a secondary leukemias. The actuarial rate of developing any second malignancy was 14.6% vs 15.0% at 15 years for patients who received radiation therapy vs. those that did not receive radiation therapy (Figure 4). In order to control for advancements in therapy which may have significantly changed the second malignancy rate in this cohort we also examined the rate of developing a second malignancy by treatment era. When examined by treatment era the actuarial rate of developing a second malignancy was 6.9% at 8 years if treated from 1998–2007, and 7.3% at 8 years if treated from 1988–1997 (p = 0.18).
Figure 4.
Kaplan Meier Curve showing incidence of patients who developed a second malignancy. (p – 0.089)
Discussion
This population based study examined whether radiation therapy affected survival outcomes of patients with HD. It found a statistically significant survival benefit for patients with stage I and II disease. Furthermore, among the cohort that did receive radiation therapy there was no increase in secondary malignancies compared to the cohort that did not receive radiation therapy.
There have been several modern randomized studies that have examined chemotherapy vs. chemotherapy plus radiation in Hodgkin's disease (7–11). EORTC H9-F was a three arm study in which patients with favorable stage disease who were complete responders to 6 cycles of EBVP II (epirubicin, bleomycin, vinblastine and prednisone) were randomized to 36Gy of involved field therapy, 20 Gy of involved field radiation therapy, or no radiation. An interim analysis revealed the four year event free survival to be 87% in the 36Gy arm, 84% in the 20Gy arm, and 70% in the no radiation arm (P < 0/001). The chemotherapy alone arm was subsequently closed due to a higher than expected number of relapses that met the proposed early stopping rule (6). The Grupo Argentino de Tratamiento de la Leucemia Aguda performed another trial where patients with stage I and II disease were treated with six cycles of CVPP (cyclophophamide, vinblastine, procarbazine, and prednisone) and then randomized to receive 30Gy of involved field radiation vs. no radiation therapy. With a median follow up of 84 months, the disease free survival was significantly different at 71% vs 62% for patients who radiation therapy vs. those that did not receive radiation (11). A Children's Cancer Group also examined patients with Hodgkin's disease who were complete responders to chemotherapy and randomized them to receive involved radiation therapy or no radiation therapy. An interim analysis revealed an unacceptable number of failures in the patients who did not receive radiation and the trial was stopped early (10).
Our study, with over 12,000 patients, is one of the largest cohorts of patients with Hodgkin's disease and revealed that radiation led to an improved overall and cause specific survival in both stage I and II. This is the first study to demonstrate a survival benefit in stage I and II Hodgkin's disease with the addition of radiation therapy. We hypothesize that the known improvement in local control from the addition of radiation therapy did translate into an improvement in survival. A Cochrane Review examined 9312 patients from 37 trials and revealed an improvement in chemoradiotherapy over chemotherapy alone (OR = 0.62, 95% CI, 0.44 to 0.88, p = 0.006). Furthermore, the second malignancy rate was not significantly increased for those that received chemoradiotherapy versus chemotherapy alone in early stage patients (12). Another meta-analysis included 1,245 patients from 5 randomized trials and revealed an improved hazard ratio of 0.40 (95% CI 0.27 – 0.59) for patients receiving chemoradiotherapy compared to chemotherapy alone (13). Our results are in accordance with these meta-analyses of early stage patients.
Concerns over long-term toxicities with radiation have led some investigators towards favoring chemotherapy alone strategies in favorable risk Hodgkin's disease (14). However, as mentioned above, all clinical trials investigating this approach have shown an increased rate of relapse in patients who do not receive radiation therapy. Our data was surprising that despite the lack of evidence for the omission of radiation therapy for stage I and II Hodgkin's disease, this nationwide study revealed an over 20% absolute decrease in the utilization of radiation therapy from 1988–2006. This large decrease may be a result of patients not receiving centralized care in a multi-disciplinary setting. It is paramount that all cases of early stage Hodgkin's lymphoma be considered for combined modality therapy (15). Treatment planning involving radiation oncologists and medical oncologists should be standard of care.
Second malignancies are the most serious late complication following successful treatment of Hodgkin's lymphoma (1, 3, 16–18). There have been conflicting reports regarding whether the addition of radiation therapy to chemotherapy leads to a significant increase in the incidence of second malignancies compared to chemotherapy along strategies (18–25). Our study revealed that the actuarial risk of developing a second malignancy was equivalent between patients that received radiation vs. those that did not receive radiation. There was a higher number of secondary leukemias in the group that did not receive radiation therapy, which may be due to this group having received more cycles of chemotherapy. Also, older chemotherapy regimens such as MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) which utilized higher doses of alkylating agents may also explain the increased incidence of leukemia observed in this cohort (18, 19, 26). There have been several advancements in the treatment of Hodgkin's disease over the past twenty years including the omission of alkylating agents from multi-agent chemotherapy and the use of involved field radiation therapy. Furthermore, we demonstrated in Figure 1 that there was a significant decrease in the utilization of radiation therapy over the past 20 years. However, it was interesting to note that despite these changes there was no significant difference in the actuarial rate of second malignancies between those patients treated in an earlier treatment era from 1988–1997 and from 1998–2007.
This study was limited primarily based on information availability in the SEER database (27). First, no information on radiotherapy technique (total dose, fraction size, beam energy) was available. Second, we could not determine which patients had unfavorable risk factors such as bulky disease, 3 or more involved nodal regions or an elevated ESR, however we did adjust for all available patient and tumor characteristics. We also cannot comment on what kinds of chemotherapy patients received. It is possible that patients treated in earlier years received less efficacious chemotherapy regimens. However, in spite of these limitations we were able to show a benefit to radiation therapy after adjusting for known patient and tumor characteristics. Furthermore, in an attempt to exclude patients whose performance status or other factors may have limited them from receiving a form of definitive therapy we examined patients who survived greater than a year. In this cohort, of one year survivors we were also able to demonstrate a survival benefit towards radiation therapy.
Conclusions
This is one of the largest studies to examine the role of radiation therapy in the initial management for patients with stage I and II Hodgkin's disease. Our analysis revealed a survival benefit with the addition of radiation therapy with no increase in secondary malignancies. Over the same time period, the utilization of the radiation in stage I and II Hodgkin's disease has decreased by over 20%.
Acknowledgments
Acknowledgement of Research Support: Shayna Rich's work on this study was supported by training grant T32 AG000262 from the National Institute on Aging, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No author indicated a conflict of interest
References
- 1.van Leeuwen FE, Klokman WJ, Hagenbeek A, Noyon R, van den Belt-Dusebout AW, van Kerkhoff EH, et al. Second cancer risk following hodgkin's disease: A 20-year follow-up study. J Clin Oncol. 1994 Feb;12(2):312–325. doi: 10.1200/JCO.1994.12.2.312. [DOI] [PubMed] [Google Scholar]
- 2.Tucker MA, Coleman CN, Cox RS, Varghese A, Rosenberg SA. Risk of second cancers after treatment for hodgkin's disease. N Engl J Med. 1988 Jan 14;318(2):76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow AJ, Douglas AJ, Hudson GV, Hudson BV, Bennett MH, MacLennan KA. Risk of second primary cancers after hodgkin's disease by type of treatment: Analysis of 2846 patients in the british national lymphoma investigation. BMJ. 1992 May 2;304(6835):1137–1143. doi: 10.1136/bmj.304.6835.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry-Amar M. Second cancer after the treatment for hodgkin's disease: A report from the international database on hodgkin's disease. Ann Oncol. 1992 Sep;3 Suppl 4:117–128. doi: 10.1093/annonc/3.suppl_4.s117. [DOI] [PubMed] [Google Scholar]
- 5.Yahalom J. Role of radiation therapy in hodgkin's lymphoma. Cancer J. 2009 Mar–Apr;15(2):155–160. doi: 10.1097/PPO.0b013e3181a1437a. [DOI] [PubMed] [Google Scholar]
- 6.Noordijk EM, Thomas J, Ferme C, van 't Veer MB, Brice P, Divine M, et al. First results of the EORTC-GELA H9 randomized trials: The H9-F trial (comparing 3 radiation dose levels) and H9-U trial (comparing 3 chemotherapy schemes) in patients with favorable or unfavorable early stage hodgkin's lymphoma (HL) ASCO Meeting Abstracts. 2005 Jun 1;23(16):6505. Available from: http://meeting.ascopubs.org/cgi/content/abstract/23/16_suppl/6505. [Google Scholar]
- 7.Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, et al. Consolidation radiation after complete remission in hodgkin's disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: Is there a need? J Clin Oncol. 2004 Jan 1;22(1):62–68. doi: 10.1200/JCO.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Straus DJ, Portlock CS, Qin J, Myers J, Zelenetz AD, Moskowitz C, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky hodgkin disease. Blood. 2004 Dec 1;104(12):3483–3489. doi: 10.1182/blood-2004-04-1311. [DOI] [PubMed] [Google Scholar]
- 9.Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Bezjak A, Wells WA, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage hodgkin's lymphoma: National cancer institute of canada clinical trials group and the eastern cooperative oncology group. J Clin Oncol. 2005 Jul 20;23(21):4634–4642. doi: 10.1200/JCO.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 10.Nachman JB, Sposto R, Herzog P, Gilchrist GS, Wolden SL, Thomson J, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002 Sep 15;20(18):3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovsky S, Maschio M, Santarelli MT, Muriel FS, Corrado C, Garcia I, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I–II hodgkin's disease. J Natl Cancer Inst. 1988 Nov 16;80(18):1466–1473. doi: 10.1093/jnci/80.18.1466. [DOI] [PubMed] [Google Scholar]
- 12.Franklin JG, Paus MD, Pluetschow A, Specht L. Chemotherapy, radiotherapy and combined modality for hodgkin's disease, with emphasis on second cancer risk. Cochrane Database Syst Rev. 2005 Oct 19;(4) doi: 10.1002/14651858.CD003187.pub2. (4):CD003187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst C, Rehan FA, Brillant C, Bohlius J, Skoetz N, Schulz H, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage hodgkin's lymphoma: A systematic review. Haematologica. 2010 Mar;95(3):494–500. doi: 10.3324/haematol.2009.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longo DL. Radiation therapy in the treatment of hodgkin's disease--do you see what I see? J Natl Cancer Inst. 2003 Jul 2;95(13):928–929. doi: 10.1093/jnci/95.13.928. [DOI] [PubMed] [Google Scholar]
- 15.Girinsky T, Ghalibafian M. Radiotherapy of hodgkin lymphoma: Indications, new fields, and techniques. Semin Radiat Oncol. 2007 Jul;17(3):206–222. doi: 10.1016/j.semradonc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Canellos GP, Arseneau JC, DeVita VT, Whang-Peng J, Johnson RE. Second malignancies complicating hodgkin's disease in remission. Lancet. 1975 Apr 26;1(7913):947–949. doi: 10.1016/s0140-6736(75)92007-3. [DOI] [PubMed] [Google Scholar]
- 17.Constine LS, Tarbell N, Hudson MM, Schwartz C, Fisher SG, Muhs AG, et al. Subsequent malignancies in children treated for hodgkin's disease: Associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008 Sep 1;72(1):24–33. doi: 10.1016/j.ijrobp.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow AJ, Barber JA, Horwich A, Cunningham D, Milan S, Omar RZ. Second malignancy in patients with hodgkin's disease treated at the royal marsden hospital. Br J Cancer. 1997;75(1):116–123. doi: 10.1038/bjc.1997.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, et al. Breast cancer and other second neoplasms after childhood hodgkin's disease. N Engl J Med. 1996 Mar 21;334(12):745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 20.Brusamolino E, Anselmo AP, Klersy C, Santoro M, Orlandi E, Pagnucco G, et al. The risk of acute leukemia in patients treated for hodgkin's disease is significantly higher after combined modality programs than after chemotherapy alone and is correlated with the extent of radiotherapy and type and duration of chemotherapy: A case-control study. Haematologica. 1998 Sep;83(9):812–823. [PubMed] [Google Scholar]
- 21.Franklin J, Pluetschow A, Paus M, Specht L, Anselmo AP, Aviles A, et al. Second malignancy risk associated with treatment of hodgkin's lymphoma: Meta-analysis of the randomised trials. Ann Oncol. 2006 Dec;17(12):1749–1760. doi: 10.1093/annonc/mdl302. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, et al. Long-term solid cancer risk among 5-year survivors of hodgkin's lymphoma. J Clin Oncol. 2007 Apr 20;25(12):1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 23.Mauch PM, Canellos GP, Rosenthal DS, Hellman S. Reduction of fatal complications from combined modality therapy in hodgkin's disease. J Clin Oncol. 1985 Apr;3(4):501–505. doi: 10.1200/JCO.1985.3.4.501. [DOI] [PubMed] [Google Scholar]
- 24.Ng AK, Bernardo MV, Weller E, Backstrand K, Silver B, Marcus KC, et al. Second malignancy after hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood. 2002 Sep 15;100(6):1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 25.Tester WJ, Kinsella TJ, Waller B, Makuch RW, Kelley PA, Glatstein E, et al. Second malignant neoplasms complicating hodgkin's disease: The national cancer institute experience. J Clin Oncol. 1984 Jul;2(7):762–769. doi: 10.1200/JCO.1984.2.7.762. [DOI] [PubMed] [Google Scholar]
- 26.Kaldor JM, Day NE, Clarke EA, Van Leeuwen FE, Henry-Amar M, Fiorentino MV, et al. Leukemia following hodgkin's disease. N Engl J Med. 1990 Jan 4;322(1):7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- 27.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: Applications and limitations in oncology research. Oncology. 2009;23(3) [PubMed] [Google Scholar]