Abstract
Background
The purpose of this study is to investigate the capabilities of a novel optical wide-field imaging technology known as Spatial Frequency Domain Imaging (SFDI) to quantitatively assess reconstructive tissue status.
Methods
Twenty two cutaneous pedicle flaps were created on eleven rats based on the inferior epigastric vessels. After baseline measurement, all flaps underwent vascular ischemia, induced by clamping the supporting vessels for two hours (either arterio-venous or selective venous occlusions) normal saline was injected to the control flap, and hypertonic hyperoncotic saline solution to the experimental flap. Flaps were monitored for two hours after reperfusion. The SFDI system was used for quantitative assessment of flap status over the duration of the experiment.
Results
All flaps demonstrated a significant decline in oxy-hemoglobin and tissue oxygen saturation in response to occlusion. Total hemoglobin and deoxy-hemoglobin were markedly increased in the selective venous occlusion group. After reperfusion and the solutions were administered, oxy-hemoglobin and tissue oxygen saturation in those flaps that survived gradually returned to the baseline levels. However, flaps for which oxy-hemoglobin and tissue oxygen saturation didn’t show any signs of recovery appeared to be compromised and eventually became necrotic within 24–48 hours in both occlusion groups.
Conclusion
SFDI technology provides a quantitative, objective method to assess tissue status. This study demonstrates the potential of this optical technology to assess tissue perfusion in a very precise and quantitative way, enabling wide-field visualization of physiological parameters. The results of this study suggest that SFDI may provide a means for prospectively identifying dysfunctional flaps well in advance of failure.
Introduction
Free flap transfer has become a routine procedure in many medical centers around the world. A flap is a volume of tissue that can be transferred and survives in its new location based on a new set of blood vessels. The critical aspect in all anatomic subsets of tissue transfer flap is the blood supply [1]. The ability to transfer cutaneous flap from one region to another has greatly facilitated the reconstruction of complex defects after trauma and cancer resection.
The advantages of skin flap transfer include stable wound coverage, improved aesthetic and functional outcomes, and minimal donor site morbidity. Since establishing free tissue transfer procedure in 1960s, the failure rate has been significantly reduced because of technical improvement, patient selection, and flap design. Success rates are estimated as high as 95–99% among experienced surgeons [2–4]. Nevertheless a recent survey of the literature indicates that 6–25% of skin flaps require a secondary surgical re-exploration due to complications [2, 5].
Complications occur for a variety of reasons including insufficiency of blood supply secondary to mechanical obstruction of the vessels, or ischemia-reperfusion (I/R) injury which occurs when blood flow is re-established to ischemic tissues [6]. Studies have demonstrated that venous thrombosis alone is more common than either arterial or combined arterial and venous thrombosis. Thrombosis typically occurs within the first 48 hours in 80% of patients [7].
Since complications related to flap transfer occur at a considerable rate, accurate assessment of flap status and the ability to determine whether or not it will survive is of paramount importance. When a compromised flap is recognized and managed within 6 hours post surgery, it has better than 50% salvage rate when taken back to the operating room [3, 8, 9]. Clinical exam is currently the most common practice for flap monitoring [10]. It is subjective and based on periodic observations of flap color, surface temperature, skin turgor, capillary refill time, and bleeding time after pinprick. Clinical evaluation has been, and remains, the standard of care with regard to post-operative surveillance. Not surprisingly, there have been considerable efforts to develop reliable objective methods to assess free tissue flap viability. These assessment methods can be divided into several categories including clinical assessment, chemical techniques and instrumental monitoring techniques [11].
Here we present a study in which we investigate the capabilities of a novel wide-field optical imaging technology to assess reconstructive tissue status. This device, which we have developed, is based on the principles of spatial frequency domain imaging (SFDI) [12, 13]. SFDI is a novel spectral imaging modality that relies on measurements, acquired by a Charge-Coupled Device (CCD) camera, using diffuse reflectance in the spatial frequency domain to deduce the absorption coefficient and the reduced scattering coefficient of tissue on a pixel by pixel basis.
Spatial Frequency Domain Imaging
The SFDI is a noncontact spectral imaging device that uses near infrared light spectrum (650–1100 nm) and is capable of rapid, wide-field measurements from which two-dimensional maps of the optical properties of a tissue can be generated [12, 14–17]. In this study the images were obtained at 670, 730, 790, 850, and 910 nm using two spatial frequencies (0/mm and 0.2/mm). This system was able to acquire data every 80 seconds throughout the experiment. Tissue optical properties (absorption coefficient and reduced scattering coefficient) were extracted from demodulated spectral reflectance images[17]. The technique is then able to create two-dimensional maps of chromophores (e.g. deoxygenated hemoglobin (Hb) and oxygenated hemoglobin (HbO2)) from the recovered absorption coefficients. From the determined concentration of Hb and HbO2 in micromoles per unit liter, the total amount of hemoglobin in the tissue (HbT) can be calculated, as well as tissue oxygen saturation (StO2). Relative percentage changes in these parameters were subsequently calculated to facilitate interpretation of the data. This technology is different from other tissue spectroscopy devices in several important ways. Foremost among these is that it is able to generate two-dimensional chromophore maps of the tissue region of interest, in a non-contact way without scanning or moving of an imaging probe. Other groups have demonstrated that near infrared tissue spectroscopy can detect and differentiate between arterial and venous occlusion, assuming that one knows where to place the measurement probe on the tissue, None of the technologies were wide-field imaging methods [18–21].
In the study that we describe here, we examine the performance of SFDI to quantitatively deduce changes in Hb, HbO2 HbT and StO2, in an epigastric pedicle flap model. We investigate the capability of SFDI device to assess the viability of flaps after venous and arterio-venous occlusion both acutely and over a period of 96 hours.
The work described here was carried out under University of California, Irvine, Institutional Animal Care and Use Committee (IACUC) approved protocol # 2006–2693.
Material and Methods
Twenty two cutaneous pedicle flaps were created on eleven male SASCO Sprague-Dawley rats, weighing between 350–450 gm were randomly assigned into two groups depend on the occlusion type:
Arterio-venous occlusion group (n=12 flaps)
Selective venous occlusion group (n=10 flaps)
Intact skin group (n=11, normal skin regions). It is an intact native region of skin, not surgically manipulated, and consists of tissue located between the flaps, that remained out of the surgical field.
After epilation and skin desinfection, a primary incision of about 5 cm on one side of the elliptically shaped flap design in the groin region was performed. The completed cutaneous pedicle flap measured about 5×3 cm, based on the inferior epigastric vessels. All branches were ligated using 9–0 nylon sutures until reaching the main femoral vessels. The femoral vessels were freed from the surrounding tissue and dissected in both proximal and distal directions for about 10 mm on both sides, as shown in Figure 1. All branches to the muscles and inguinal fat pad were ligated and cut, creating a pedicle skin flap, based on the inferior epigastric vessels. The femoral nerve was cut and part of the adventitia of the femoral vessels was removed to disrupt the perivascular plexus. For this reason, the hyperemia response was controlled only by local accumulation of metabolic products to stimulate blood flow following ischemia especially after elimination of neural control, in order to mimic free tissue transfer flap. A 27G cannula was inserted into the superficial femoral artery after it was freed from the surrounding tissue to control flap perfusion (Figure 2). After the cannula was secured in its position, it was tested by injection 0.5 ml of normal saline. This process was repeated on the contralateral side. Finally the pedicle tissue transfer flaps were sutured in back in their native positions using nylon 5-0 suture, creating two symmetrical pedicle flaps on each groin. After bilateral pedicle flaps were created, the sides were randomized to either the control or the experimental flap.
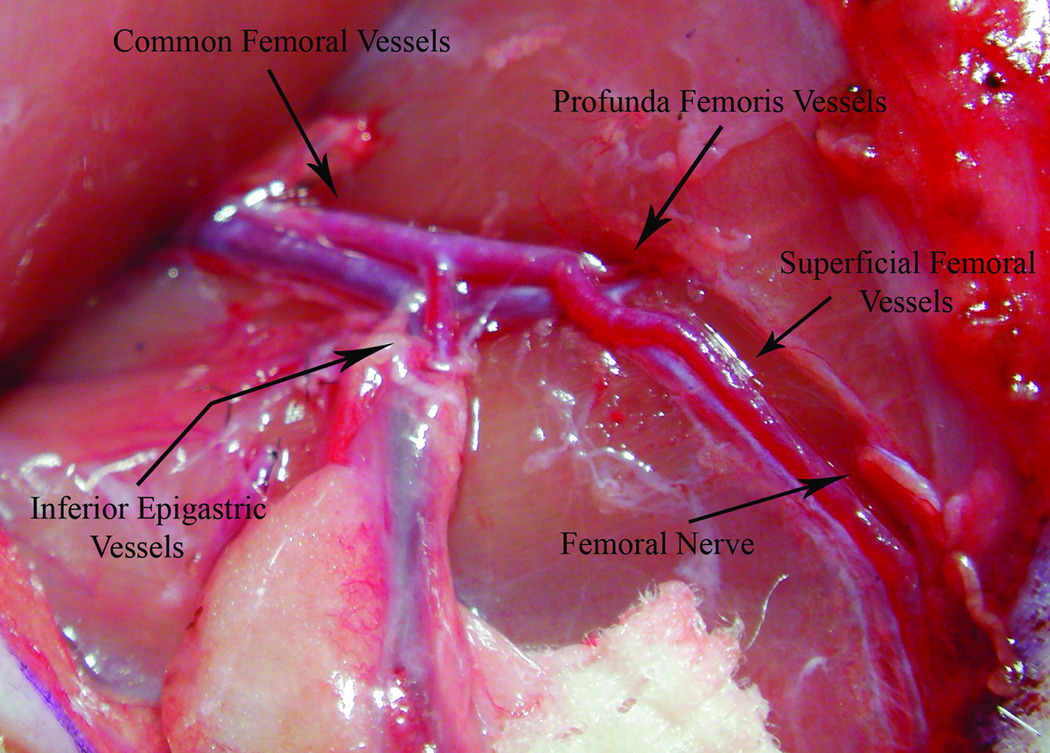
Figure 1.
The common femoral artery and vein were freed from the surrounding tissue. The superficial femoral artery was prepared for cannulation as well.
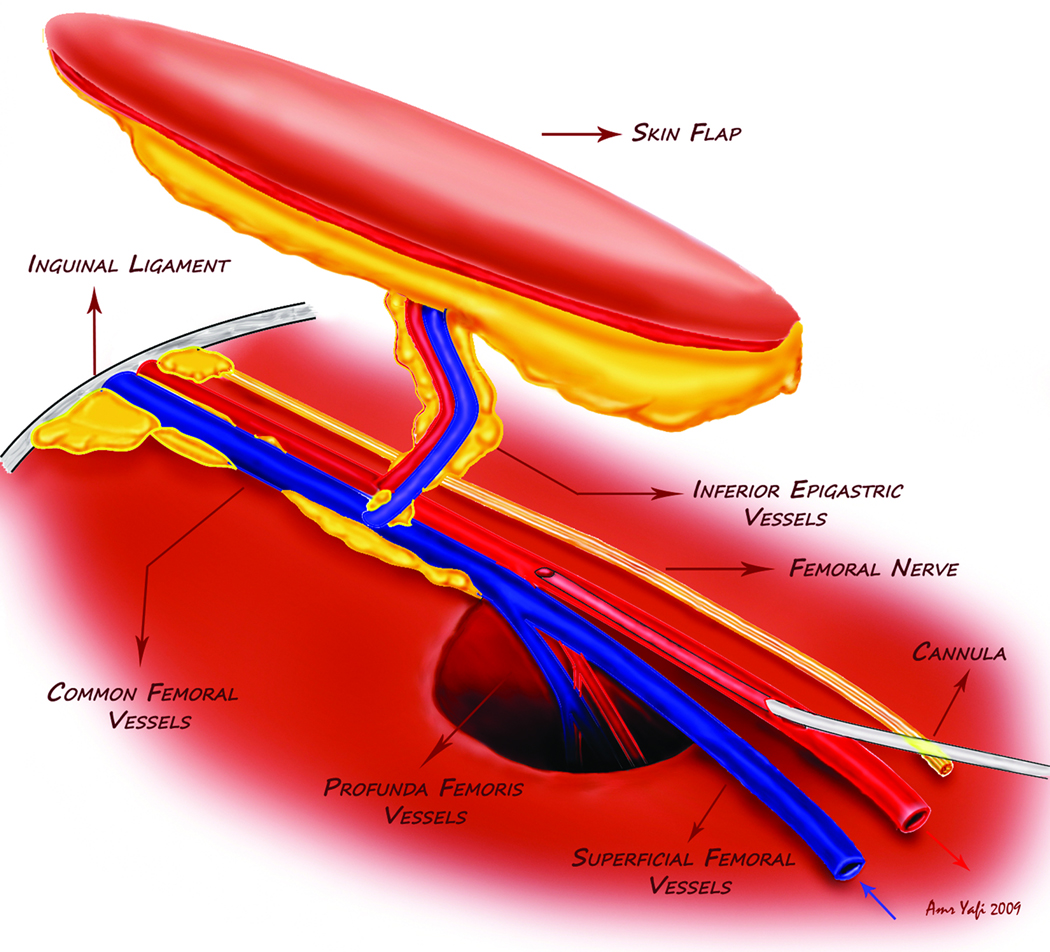
Figure 2.
Illustration of the flap design and the position of the cannula.
The average surgery time required for both flaps with the cannulas placement in the arterio-venous occlusion group was 4 hours and 10 minutes versus 5 hours and 1 minute for the selective venous occlusion group. Each flap required 2 hrs on average to complete, the time interval between starting the first flap and beginning the second flaps was approximately ~2 hours.
SFDI images were obtained over the entire duration of the experiment including a 15 minute baseline period on the both flaps simultaneously. Afterward clamps were applied on the both pedicles to induce either venous or arterio-venous occlusion. After 2 hours of ischemia the clamps were released and re-perfusion was allowed. Once the clamps were removed from the experimental flap, 1 ml of the hypertonic hyperoncotic saline solution (HHS) (0.5 ml 7.5% hypertonic saline + 0.5 ml hyperoncotic 6% Dextrane70) was administered locally to the flap via the inferior epigastric artery using a 27G cannula in order to augment cellular injury and induce necrosis. In addition, 1 ml of Normal Saline solution (NS) was administered to the control flap, using a dual syringe pump (Harvard Apparatus model 11 plus) at a rate of 0.15 ml/min. SFDI Images again acquired for an additional 2 hours. In addition to SFDI images, reference photographs were obtained using a consumer grade digital camera (FujiFilm, FinePix F30) mounted in a fixed position with respect to the experimental set-up every 10 minutes over the duration of data acquisition. This was done in order to provide a record of “clinical impression” that could later be compared to SFDI images. The ischemia and reperfusion periods were approximately ~ 2:02 ± 00:02 and 2:15 ± 00:15 hours respectively for both occlusion groups. The animal was again imaged using SFDI at 24, 48, 72 and 96 hours post-operatively.
Prior to engaging in the full study protocol, we tested the primary (vascular occlusion) and secondary interventions (solutions administration) by themselves and found that neither one, taken alone, was sufficient to induce fatal insult. We chose to use a 2 hour occlusion period based on the experience of co-authors Scholz and Evans [22]. In that study, which looked at the ability of orthogonal polarization spectroscopy to resolve changes in microcirculatory flow in response to different reperfusion fluids, the time interval between flap harvest and reperfusion, was around 2 hours. We also performed 3 hour occlusions on 2 animals (4 flaps). We experimentally determined that a 3 hour occlusion period was too long and would induce irreversible ischemia regardless of the treatment received (either NS or HHS). All of these flaps failed after 24–48 hours. Additionally, we carried out a small investigation using 4 animals (8 flaps) to ensure that the effects of NS and HHS alone would not be sufficient to critically injure the flaps. All flaps were monitored for 2 hours without any primary intervention (vascular occlusion) then 4 flaps were injected with HHS and another 4 flaps with NS and monitored for additional 2 hours. All of these flaps survived 96 hours post-op, regardless of the solution injected. Thus neither primary intervention nor secondary intervention alone should not critically injure the flaps.
Statistical Analysis
The variability of vital signs (heart rate, respiratory rate, and arterial oxygen saturation) between groups was statistically analyzed by using Student paired t-test (GraphPad Prism version 5.00, GraphPad software, San Diego, California). A one-way ANOVA was used to compare the chromophore baseline values of control flaps and experimental flaps to intact skin. A Dunnett’s post-test analysis was used for direct comparison of baseline chromophore values between all the flaps in the three groups. Variables between survived, compromised flaps, and intact skin region were statistically analyzed using two-ways ANOVA. A Bonferroni post-test analysis was used to compare the data point differences at given time along the curves. This analysis allows direct comparison of the data in a paired fashion. A value of p <0.05 is considered significant. Data are present as mean of percent change± Standard Error of the Mean.
Results
The dynamics of heart rate, respiratory rate, and arterial blood oxygen saturation level were similar for all animals over the duration of the imaging session, without any statistically significant differences among groups (Student paired t-test). The mean heart and respiratory rates varied between 212–317 BPM (p=0.1076), and 32–49 (p=0.3256) per minute respectively. The mean arterial oxygen saturation ranged between 98–99.9 % (p=0.4574).
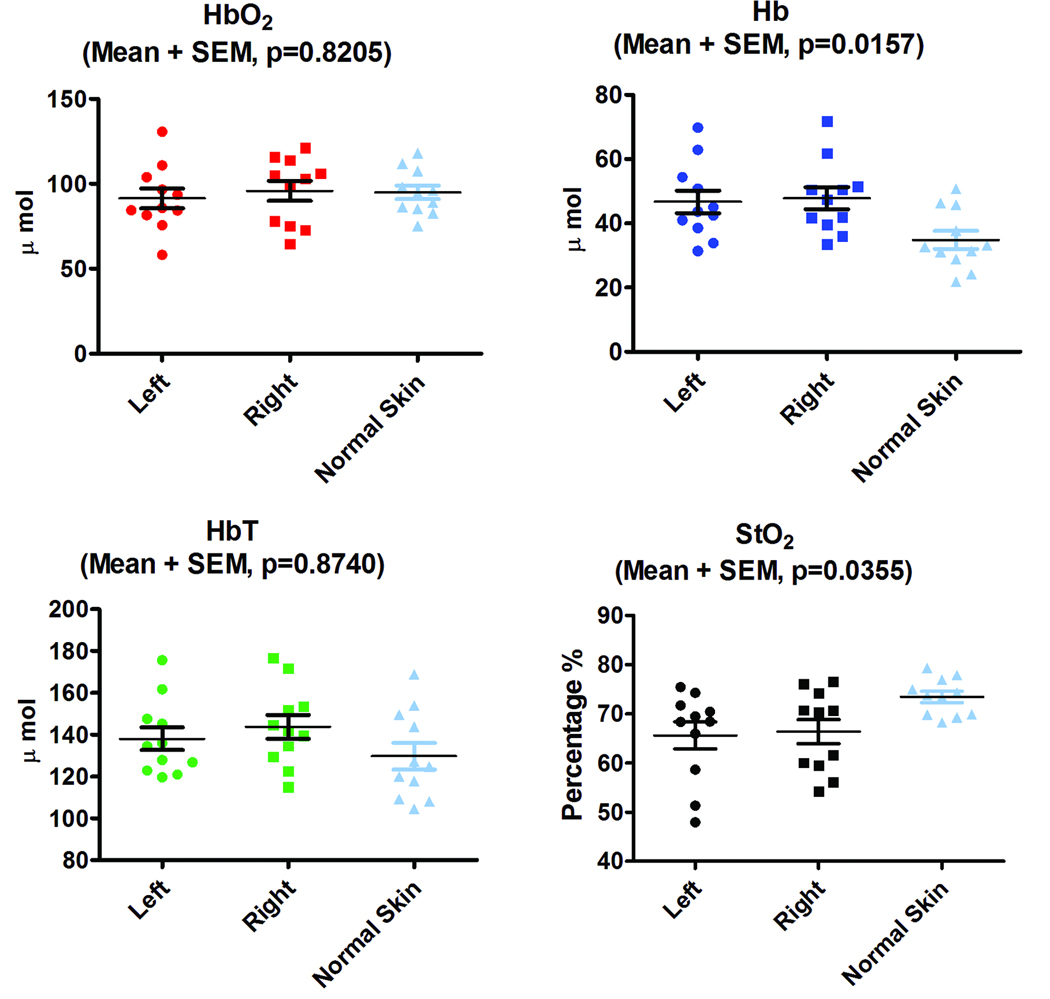
The baseline values of HbO2, Hb, HbT, and StO2 revealed no statistical significant differences based on Student paired t-test (p= 0.2138, p=0.3878, p=0.2215, and p=0.3666 respectively) between right and left skin flaps among all animals in all groups. When the cutaneous flaps were compared to adjacent intact skin there were a significant statistical difference in Hb (p=0.0157) and StO2 (p=0.0355) based on one-way ANOVA analysis however there was no significant difference in HbO2 (p=0.8205) and HbT (p=0.8740) as depicted in Figure 3.
Figure 3.
Chromophore plots for left and right side flaps as well as for the region of intact skin that lies between the flaps. There are no statistical differences in HbO2 (a), and HbT (c) but StO2 (d) and Hb (b) show statistically significant differences between flaps and intact skin based on one-way ANOVA analysis. There are no statistical significant differences in chromophore baseline values between right and left flaps among all animal based on Student paired t-test analysis(HbO2 p= 0.2138 ,Hb p= 0.3878, HbT p=0.2215, and StO2 p=0.3666).
Arterio-Venous Occlusion Group
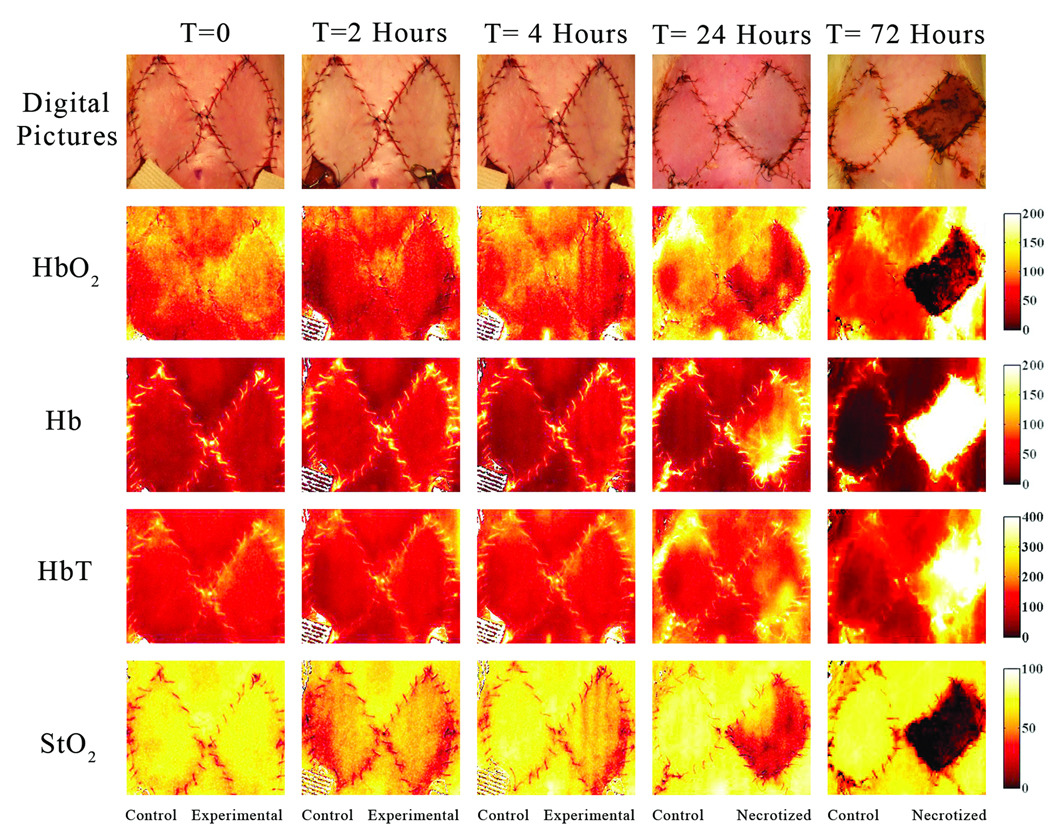
In this group, 12 flaps were created (6 treated with NS and 6 with HHS). By the 4th post-operative day, 8 of them survived while 4 of these flaps failed. SFDI images registered microcirculation changes over the entire time of data acquisition including, baseline, ischemia, and reperfusion periods. Figure 4 depicts quantitative functional images of different parameters at several time points. In this occlusion group, the quantitative images indicate changes chromophore concentration and distribution over time, including the baseline (T= 0), the end ischemia period (T= 2), after 2 hours of reperfusion (T= 4), 24, and 72 hours post-op. SFDI data for the flap that survived indicated that chromophore concentrations recovered (trended toward presurgical baseline values) after blood flow was reestablished. The StO2 and HbO2 concentration values actually overshot baseline values in compensatory fashion whereas these parameters didn’t show any considerable changes in the flap that was compromised and eventually necrotized within 24–48 hours.
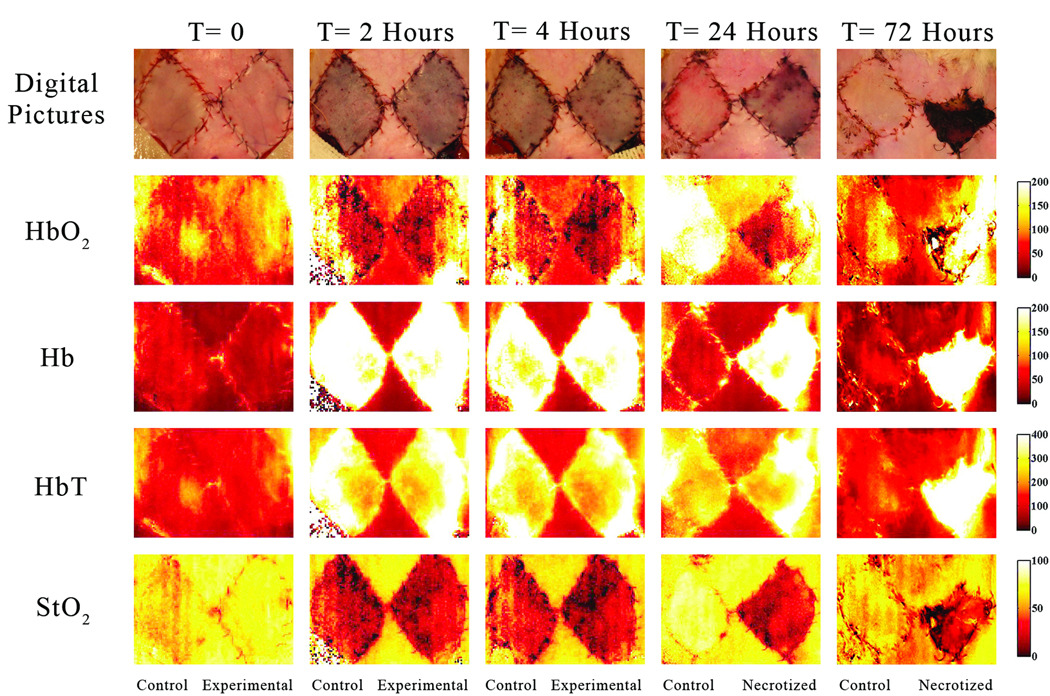
Figure 4.
These images have been acquired at t= 0 (baseline), 2 (two hours after arterio-venous occlusion), 4 (two hours after blood reperfusion and saline administration), 24 and 72 hours after the pedicle flap was raised in the arterio-venous occlusion group. The flap on the left in each image represents the control flap that was treated with normal saline after two hours of ischemia and ultimately survived. The right flap in each image represents the experimental flap that was treated with hypertonic-hyperoncotic saline after two hours of ischemia and eventually became necrotic within 24 to 48 hours. The gradient changes in concentration of oxy, deoxy-hemoglobin, total hemoglobin and tissue oxygen saturation are noticeable between different time points when comparing both flaps and using adjacent intact skin as reference. The StO2 chromophore map at t=24 hrs reveals high tissue oxygen saturation in the upper left quadrant of the right flap in comparison to the rest of the flap. The Hb chromophore map indicates a low concentration of deoxy-hemoglobin at the same location as well. In other words this region still has sufficient blood supply.
In order to quantitatively compare data between animals and flaps, the relative percent change of each chromophore value was calculated in three different regions of interest, including control flap, experimental flap and intact skin. The percent change of HbO2 and StO2 depicted in Figures 5a and b, demonstrated a sharp decline after arterio-venous occlusion when compared to adjacent intact skin. This reduction in HbO2 and StO2 values became statistically different after 4 minutes of vascular occlusion in both survived and compromised flaps groups.
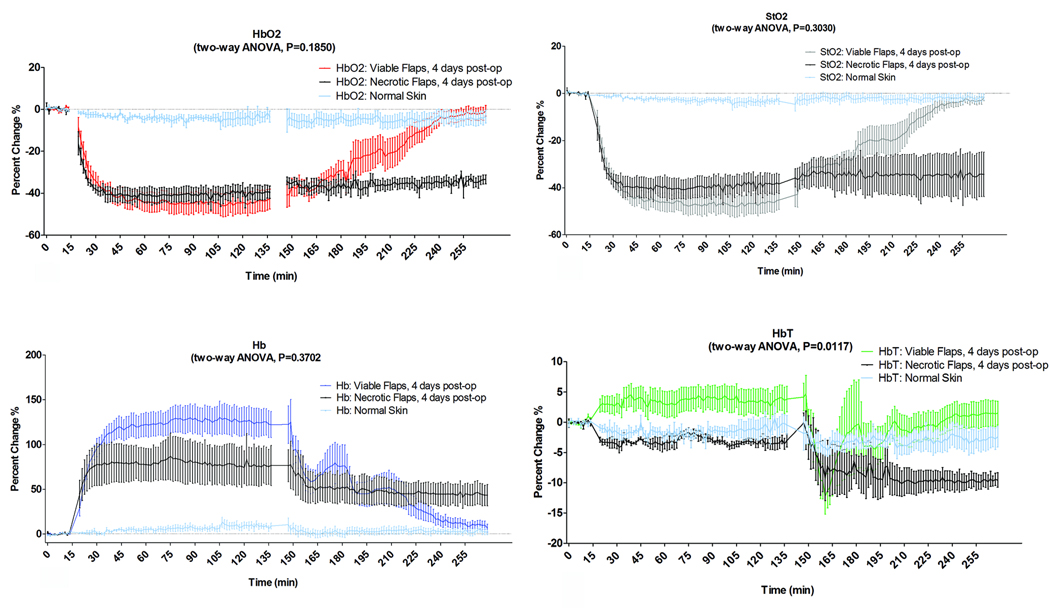
Figure 5.
These plots demonstrate the percentage change of each parameter in the arterio-venous occlusion group. We compared the group of flaps that survived to the group of flaps that eventually became necrotic within 24–48 hours post-op and to adjacent intact skin as reference of un-manipulated skin surgically during the three phases of data acquisition (baseline, ischemia, and reperfusion with saline administration). The data was analyzed based on two-way ANOVA analysis and reported as the mean +SEM. (a) HbO2, (b) StO2, (c) Hb, (d) HbT.
In the occlusion phase, The values of HbO2 (a), StO2 (b) and Hb level (c) reached significant difference after 4–4.5 minutes of vascular occlusion in both groups survived and compromised flaps based on two-way ANOVA analysis (HbO2, StO2 and Hb p < 0.0001) when compared to adjacent intact skin the HbO2 and StO2.
In the reperfusion phase, the statistical analysis showed that the HbO2 StO2 and Hb variations between compromised and survived flaps didn’t become significantly different. (p=0.1850, p=0.3030, p=0.3702 respectively), however StO2 showed statistical significant difference at minute 81 after reperfusion based on Bonferroni post-test analysis (p>0.05).
The HbT level (d) did not fluctuate much over the entire duration of the experiment, but statistical analysis indicates a significant difference between flaps that survive and that eventually became necrotic based two-way ANOVA (p=0.0117).
In the group of flaps that survived, we observed a gradual increase in HbO2 and StO2 levels which began after 18 minutes of reperfusion. Statistically, the HbO2 and StO2 levels, in the group of flaps that survived, completely recovered after 42 and 46.5 minutes respectively after reperfusion, when compared to the intact skin group. On the other hand, there was no recovery of HbO2 and StO2 levels in the group of flaps that ultimately revealed themselves to be critically compromised. The HbO2 and StO2 variations between critically compromised flaps and those that survived were analyzed using two- way ANOVA but didn’t show any statistically significant difference. On the other hand a Bonferroni post-test analysis showed significant difference at minute 81 after reperfusion.
Figure 5c illustrates a sharp increase in Hb values following complete pedicle occlusion in all flaps, becoming significantly different from adjacent intact skin after 4.5 minutes. The Hb values of the flaps that survived decreased steadily after 42 minutes following reperfusion. However, the flaps that eventually became necrotic displayed no significant changes in Hb for this set of samples. Over the entire duration of the experiment, the HbT level did not fluctuate much, as shown in Figure 5d.
Table 1 shows the mean value of each parameter after 24, 48, 72, and 96 hours in micromoles per unit liter. The data analysis indicates that there was a statistically significant difference in StO2 concentration when compared the intact skin group and the flaps that survived to those flaps that were critically compromised.
Table 1.
shows the mean values of the chormophore concentrations, in micromoles per unit liter, at 24, 48, 72, and 96 hours in the arterio-venous occlusion group. The data is presented as the mean of the absolute value for each parameter ± standard error of mean as well as the clinical status. The statistical analysis indicates that tissue StO2 was significantly different when we compared the viable flaps and the intact skin group to those that eventually became necrotic starting in the first 24 hours post–op, (p<0.0001). Also the HbO2 and Hb concentrations became statistically different on the second day post-op (48 hours), (p=0.0011, P=0.0042 respectively). Whereas HbT concentration became significantly different on the third day (72 hours) post-op, (p=0.0483). There weren’t any statistically significant differences in HbO2, StO2 Hb and HbT concentrations when we compared the viable flaps to the adjacent intact skin group. The data was analyzed based on two-way ANOVA analysis and are reported as mean ± standard error of the mean.
| Arterial-Venous Occlusion Group | |||||
|---|---|---|---|---|---|
| HbO2 | StO2 | Hb | HbT | ||
| 24 Hours |
Survived | 104.6 ± 6.89 | 80.47 ± 0.68 | 25.08 ± 1.29 | 129.6 ± 7.91 |
| Compromised | 75.44 ± 5.25 | 52.26 ± 5.89 | 124.4 ± 16.37 | 147.1 ± 14.96 | |
| Normal Skin | 92.64 ± 3.87 | 74.99 ± 1.61 | 31.13 ± 3.46 | 123.8 ± 6.45 | |
| 48 Hours |
Survived | 122.5 ± 11.82 | 79.86 ± 1.52 | 30.31 ± 2.94 | 152.8 ± 13.64 |
| Compromised | 60.91 ± 11.96 | 32.99 ± 8.76 | 124.4 ± 34.30 | 185.3 ± 34.61 | |
| Normal Skin | 105.5 ± 9.31 | 73.78 ± 1.12 | 37.02 ± 3.49 | 142.6 ± 12.35 | |
| 72 Hours |
Survived | 111.2 ± 7.74 | 78.75 ± 1.38 | 30.72 ± 4.60 | 142 ± 12.28 |
| Compromised | 45.07 ± 16.67 | 25.01 ± 12.19 | 183.6 ± 67.87 | 228.6 ± 54.77 | |
| Normal Skin | 101.5 ± 5.74 | 72.76 ± 3.04 | 38.34 ± 5.87 | 139.8 ± 9.12 | |
| 96 Hours |
Survived | 107.9 ± 6.67 | 79.12 ± 1.35 | 28.09 ± 2.08 | 136 ± 7.25 |
| Compromised | 61.36 ± 17.32 | 26.44 ± 12.38 | 209.4 ± 70.35 | 270.8 ± 60.17 | |
| Normal Skin | 97.27 ± 4.17 | 74.7 ± 2.76 | 33.11 ± 4.35 | 130.4 ± 5.81 | |
Selective Venous Occlusion Group
There were 10 flaps (5 animals) in this group (5 treated with NS and 5 with HHS). Four flaps were critcically compromised and six survived. Figure 6 displays the SFDI images at several time points (T=0, 2, 4, 24, and 72 hours). The variations in HbO2, Hb, HbT, and StO2 concentrations during baseline, ischemia, and reperfusion periods reflect changes in microcirculation of the flaps. In particular, during the ischemia period, we observed blood pooling inside the control and experimental flaps, which was indicated by high HbT and Hb concentrations at T=2 hrs, following by complete recovery of the survived flap after 24 –48 hours.
Figure 6.
These images were acquired at t= 0 (baseline), 2 (two hours after selective venous occlusion), 4 (two hours after blood reperfusion and saline administration), 24 and 72 hours after the pedicle flap was raised in selective venous occlusion group. The flap on the left in each image represents a control flap that was treated with normal saline after two hours of ischemia and survived. The right flap in each image represents the experimental flap that was treated with hypertonic-hyperoncotic saline after two hours of ischemia and eventually became necrotic within 24–48 hours. The gradient changes in concentration of oxy, deoxy-hemoglobin, total hemoglobin and tissue oxygen saturation are noticeable between different time points when comparing both flaps and using adjacent intact skin as reference.
The relative percent change of chromophore concentrations were calculated in the same way as was done for the arterio-venous occlusion experiment. The levels of HbO2 in all the flaps in this group displayed a sharp drop in their values after transient increase following the application of the clamps, as depicted in Figure 7a. The StO2 level dropped dramatically after occlusion (Figure 7b) as well, becoming significantly different from the intact skin group after ~4 minutes. Figure 7c and d shows dramatic changes in the Hb and HbT levels after the clamps were applied. All flaps displayed a steep increase in Hb and HbT level within a few minutes after occlusion in comparison to adjacent intact skin group, becoming significantly different at 4.5 and 1.5 minutes after occlusion respectively, based on Bonferroni post-test analysis.
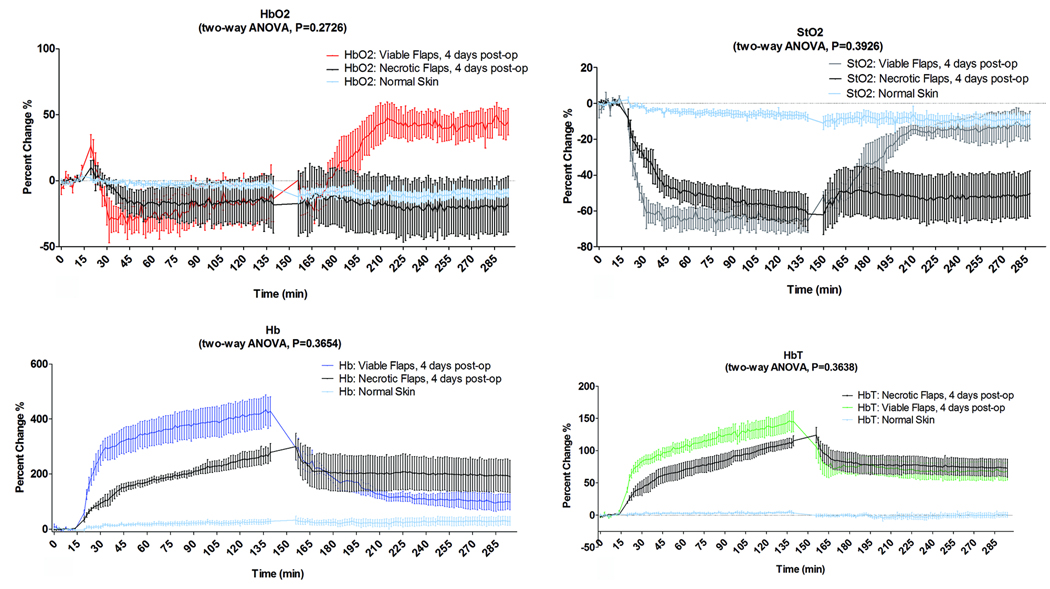
Figure 7.
These plots demonstrate the percentage change of each parameter in the selective venous occlusion group. We compared the flaps in the group that survived to the flaps group that eventually became necrotic after 24–48 hours post-op and to adjacent intact skin as reference during the three phases of data acquisition (baseline ischemia, and reperfusion with saline administration). The data was analyzed based on two-way ANOVA analysis and reported as the mean +SEM. (a) HbO2, (b) StO2, (c) Hb, (d)HbT.
In the occlusion phase, the decrease of HbO2 was not statistically significant (p=0.3561) and Bonferroni post-test didn’t show any significant differences at any time point when compared to adjacent intact skin. However The reduction of StO2 level became significantly different from intact skin group after ~4 minutes (p =0.0006). The increase of Hb and HbT levels increased became significantly different (p < 0.0001) at 4.5 and 1.5 minutes after occlusion respectively based on Bonferroni post-test analysis.
In the reperfusion phase, the flaps in the group that survived showed an increase in the StO2 level. This increase was statistically insignificant after 24 minutes based on Bonferroni post-test (p>0.05) when compared to the intact skin group. The changes in HbO2, StO2, Hb and HbT concentrations of survived and compromised flap groups after reperfusion didn’t become significantly different based on two-way ANOVA (p=0.2726, p=0.3926, p=0.3654 p=0.3638 respectively) when comparing the survived flaps to compromised flaps.
The flaps that survived showed rapid recovery after the clamps were released and the saline solutions were injected. The HbO2 level rebounded, eventually exceeding the baseline values in a compensatory fashion (Figure 7a). Conversely the StO2 level steadily increased, returning to near pre-ischemic level (baseline values). Statistically the StO2 increase was insignificant after 24 minutes based on Bonferroni post-test analysis when compared to intact skin group (Figure 7b). However the HbO2, StO2, Hb, and HbT levels in the critically compromised did not change appreciably after reperfusion. On the other hand, Hb level showed signs of recovery after 24 minutes following reperfusion period when compared to the intact skin group.
Table 2 shows the mean value of each chromophore after 24, 48, 72, and 96 hours in micromoles per unit liter. The data analysis indicates that there was a statistically significant difference in in StO2 and Hb concentration when compared the intact skin group and the flaps that survived to those flaps that were critically compromised.
Table 2.
shows the mean values of the chormophore concentrations, in micromoles per unit liter at 24, 48, 72, and 96 hours in the selective venous occlusion group. The data is presented as the mean of absolute value for each parameter ± standard error of mean as well as the clinical status. The statistical analysis indicates that tissue StO2 and H) concentrations were significantly different on the first day post-op (24 hours), (p<0.0001) when we compared the viable flaps and adjacent intact skin group to the flaps that eventually become necrotic. In addition, the HbO2 and Hb concentrations became significantly different on the second day post-op (48 hours), (p=0.0292 and p<0.0001 respectively). There was no significant difference in HbO2, StO2 Hb and HbT concentrations when we compared the flaps that survived to adjacent intact skin. The data was analyzed based on two-way ANOVA analysis and is reported as the mean ± standard error of the mean.
| Selective Venous Occlusion Group | |||||
|---|---|---|---|---|---|
| HbO2 | StO2 | Hb | HbT | ||
| 24 Hours |
Survived | 156.5 ± 17.94 | 81.05 ± 2.17 | 37.05 ± 5.49 | 194.8 ± 19.74 |
| Compromised | 94.2 ± 6.74 | 38.17 ± 6.99 | 174.6 ± 42.56 | 268.8 ± 39.41 | |
| Normal Skin | 108.3 ± 8.60 | 74.14 ± 2.28 | 37.11 ± 2.97 | 145.4 ± 9.17 | |
| 48 Hours |
Survived | 149.9 ± 11.07 | 79.33 ± 2.82 | 38.57 ± 5.01 | 188.4 ± 11.28 |
| Compromised | 92.64 ± 29.61 | 21.3 ± 5.57 | 313.1 ± 12.85 | 405.7 ± 40.49 | |
| Normal Skin | 121.2 ± 6.88 | 75.21 ± 1.50 | 40.46 ± 5.60 | 161.7 ± 12.20 | |
| 72 Hours |
Survived | 131.3 ± 6.97 | 77.22 ± 2.87 | 38.38 ± 4.74 | 169.7 ± 5.49 |
| Compromised | 70.13 ± 63.21 | 10.33 ± 9.56 | 388 ± 29.73 | 458.2 ± 89.54 | |
| Normal Skin | 100.2 ± 5.20 | 90.75 ± 16.38 | 40.69 ± 5.43 | 140.9 ± 4.54 | |
| 96 Hours |
Survived | 134.7 ± 5.60 | 78.92 ± 2.76 | 36.17 ± 5.69 | 170.9 ± 8.27 |
| Compromised | 110.3 ± 137.24 | 8.917 ± 18.58 | 538.4 ± 128.30 | 648.7 ± 47.57 | |
| Normal Skin | 101 ± 6.81 | 71.12 ± 7.31 | 42.76 ± 13.26 | 143.8 ± 6.88 | |
Intact Skin Group
As expected, chromophores concentrations in the intact skin did not fluctuate much over the entire duration of experiment. (See Figures 5 and 7)
Discussion
Accurate assessment of tissue transfer flap status remains of paramount importance for the surgeon and the patient, especially during the postoperative period. When compromised flaps are identified and treated early, their survival rate is improved. Previous published studies of tissue transfer flap assessment reported evidence supporting the use of non-invasive optical technologies for reconstructive tissue status monitoring postoperatively such as near infrared spectroscopy [18, 19, 23–27]. Regardless of the differences in devices, data processing, and study design, each of these studies has emphasized the reliability of optical technologies as a means for flap viability assessment.
Our results demonstrate that the major advantage of SFDI over other technologies to date is its capability to rapidly generate two-dimensional spatially resolved maps of each parameter within the region of interest, as shown in Figures 4 and 6. The gradient changes in concentration of HbO2 Hb, HbT, and StO2 were readily apparent between different time points and when compared to adjacent intact skin as illustrated in Figures 4 and 6. An additional strength of this modality is that it confers the ability to assess viability of specific regions within a given flap as shown in Figure 4. This capability to visualize the integrity of the entire flap may be particularly relevant to intraoperative flap assessment and may have the potential to identify defects before the flap has left the operating room. This will be the subject of future work.
Tissue response to venous congestion and arterial and venous occlusion were easily identifiable via SFDI as is illustrated in Figures 5 and 6. Hemodynamic and tissue oxygenation changes in the flap microcirculation were clearly observed using SFDI during reperfusion and saline solution administration as well. The ability to quantitatively follow these changes has the potential to enable enhanced understanding of the hemodynamics associated with different types of ischemia, in addition to providing a means for identifying signs of flap recovery. The data obtained for the intact skin group did not fluctuate much even when the animal was moved in and out of the imaging field of view in order to apply and remove micro-clamps. This stability in hemodynamic parameters for this region of tissue provides a nice reference point, indicating that artifacts introduced into the data because the subject was moved in and out of the field of view of the camera were minimal.
Based on the statistical analysis, the indications of flap recovery after occlusive insult occurred soon after blood flow was reestablished, and was apparent through dramatic hemodynamic changes especially in HbO2 and StO2. In the arterio-venous occlusion group the HbO2 concentration surged upward in flaps that survived and this occurred within~45 minutes of reperfusion. We observed the same pattern of changes in the selective venous occlusion group within ~24 minutes of reperfusion. These parameters showed no such changes after re-establishment of blood flow in the flaps that were eventually identified as failed. In this study we have demonstrated that SFDI is capable of detecting micromolar concentration and the changes in HbO2 Hb, HbT, and StO2 caused by circulatory failure prior to clinical identifiable signs. This technology has the ability to visualize the entire flap which may be particularly valuable in the clinical practice, in terms of identifying potential problems with a flap before it leaves the operating room.
Most of the flaps that received HHS failed (6/11) and the majority of the flaps that survived received normal saline (8/11). However the HHS solution we chose in this study was selected according to previously published worked by co-Authors from our Aesthetic and Plastic Surgery Institute [22]. It seems that because of subtle differences between their experiment (for which actual free flaps were created) and the study described here, that the Na concentration was high enough, in combination with the effects of occlusive insult, to induce fatal insult in the majority of the flaps that received HHS.
Finally, it should be said that there are some aspects of SFDI measurements that require particular care. The first of these is that it requires systemic calibration to produce accurate results. If any of the subsystems of the instrument, such as alterations in camera focus, projector lens, light source, or room illumination, are changed without subsequent capture of a new calibration, the subsequent calculation of HbO2, Hb, HbT and StO2 may no longer accurately reflect the amounts present within the flap. Second, note that the subjects used in this investigation have minimal pigmentation. The melanin present in very highly pigmented skin has the potential to obscure accurate determination of oxy and deoxy-hemoglobin concentrations. There are strategies for obviating this challenge with appropriate modeling that accounts for pigmentation this is an area of research within Dr. Durkin’s lab. It should be said that the technology is still under development and efforts to reduce complexity and improve robustness continue.
Conclusions
The results of the study presented here indicate that within the context of a preclinical investigation, SFDI can be used to provide spatially resolved maps of chromophores in pedicle flaps and can be used to record local tissue hemodynamics in response to occlusion and reperfusion. Furthermore, SFDI can be used as a straightforward means for recording the status of tissue for longitudinal studies of tissue response to perturbation (up to 96 hrs in this study). Taken as a whole, these results indicate that SFDI may be useful for early identification of critically injured flaps that within the context of this study eventually became necrotic.
Additional studies are planned under IRB approved protocol, to evaluate the performance of SFDI in assessment of flaps in the clinic. SFDI may have the potential to provide the surgeon with an early, reliable, and objective tool to identify vascular compromised flap shortly after reestablishing blood flow, allowing more time for intervention in order to save the flap and reduce the costs. This technology has multiple potential clinical implications that are currently under investigation including burn severity assessment and skin cancer detection as well as providing a way to follow response of tumors grown in animal models to different chemotherapeutic agents. The next generation device, which is being developed under an NIH SBIR grant, is more compact, easier to handle and has dramatically faster data acquisition than the device which we used in this study.
Acknowledgments
Sources of Funds:
The authors gratefully acknowledge funding provided by:
NIH, Impact of hypertonic-hyperoncotic saline solutions on ischemia-reperfusion injury in free flaps using Modulated Imaging (1R03EB009451-01)
NIH NCRR Biomedical Technology Research Center (LAMMP: 5P-41RR01192).
Military Photomedicine Program, AFOSR Grant # FA9550-08-1-0384
The Beckman Foundation.
Hazem H. Chehabi, MD, the director of Newport Diagnostic Center for providing a Research Fellowship for Dr. Yafi.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation:
It presented in Plastic Surgery Research Council, San Francesco, California on May 25th, 2010
Financial Disclosure:
There is no financial interest or commercial association for Amr Yafi, M.D., Thomas S Vetter, M.D., Michael R Pharaon, M.D., Thomas Scholz, M.D., Sarin Patel, Rolf B Saager, Ph.D., and Gregory R.D. Evans, M.D., that might pose or create a conflict of interest with the information presented in this article.
David J Cuccia, Ph.D. and Anthony J. Durkin, Ph.D. have a financial interest in Modulated Imaging, Inc. and Introspective Medical, Inc. (Irvine, CA), which developed the Spatial Frequency Domain Imaging, however the device used here was developed here was constructed outside of the realm of these entities and was built and constructed as part of the NIH/NCRR funded Laser and Medical Microbeam Program (LAMMP).
References
- 1.Brunicardi F, DA, Billiar Timothy, Dunn David, Hunter John, Pollock Raphael E. Schwartz's Principles of Surgery. 8th ed. New York: McGraw-Hill, Medical Pub. Division; 2005. c2005 1950. [Google Scholar]
- 2.J NF. Intraoperative and postoperative monitoring of microsurgical free tissue transfers. Clin Plast Surg. 1992;19:783–797. [PubMed] [Google Scholar]
- 3.Khouri RK, CB, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. doi: 10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kroll SS, SM, Reece GP, et al. Choice of flap and incidence of free flap success. Plast Reconstr Surg. 1996;98:459–463. doi: 10.1097/00006534-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Yuen JC, Feng Z. Monitoring free flaps using the laser Doppler flowmeter: Five-year experience. Plast. Reconstr. Surg. 2000;105:55–61. doi: 10.1097/00006534-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Siemionow M, Arslan E. Ischemia/reperfusion injury: a review in relation to free tissue transfers. Microsurgery. 2004;24(6):468–475. doi: 10.1002/micr.20060. [DOI] [PubMed] [Google Scholar]
- 7.Kroll SS, et al. Timing of pedicle thrombosis and flap loss after free-tissue transfer. Plast Reconstr Surg. 1996;98(7):1230–1233. doi: 10.1097/00006534-199612000-00017. [DOI] [PubMed] [Google Scholar]
- 8.PC N. Monitoring techniques for the detection of flow failure in the postoperative period. Microsurgery. 1993;14:162–164. doi: 10.1002/micr.1920140306. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo DA, JC The role of emergent exploration in freetissue transfer: a review of 150 consecutive cases. Plast Reconstr Surg. 1990;86:492–498. [PubMed] [Google Scholar]
- 10.Whitaker IS, Oliver DW, Ganchi PA. Postoperative monitoring of microvascular tissue transfers: current practice in the United kingdom and Ireland. Plast Reconstr Surg. 2003;111(6):2118–2119. doi: 10.1097/01.PRS.0000057070.74385.AF. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker IS, ROSK, Oliver DW, Ganchi PA, Gulati V, Malata CM. Current techniques in the post-operative monitoring of microvascular free-tissue transfers. Eur J Plast Surg. 2005;27:315–321. [Google Scholar]
- 12.Cuccia DJ, et al. Quantitation and mapping of tissue optical properties using modulated imaging. J Biomed Opt. 2009;14(2):024012. doi: 10.1117/1.3088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuccia DJ, et al. Modulated imaging: quantitative analysis and tomography of turbid media in the spatial-frequency domain. Opt Lett. 2005;30(11):1354–1356. doi: 10.1364/ol.30.001354. [DOI] [PubMed] [Google Scholar]
- 14.Cuccia DJ, et al. A new method for quantitative, depth-resolved imaging of tissue fluorescence using spatially modulated illumination. Society of Molecular Imaging Annual Meeting; St. Louis, MO: 2004. [Google Scholar]
- 15.Cuccia DJ, et al. Modulated Imaging: Quantitative Analysis and Tomography of Turbid Media in the Spatial Frequency Domain. Opt. Lett. 2005;30(11):1354–1356. doi: 10.1364/ol.30.001354. [DOI] [PubMed] [Google Scholar]
- 16.Ayers FR, et al. Wide-field spatial mapping of in vivo tattoo skin optical properties using modulated imaging. Lasers Surg Med. 2009;41(6):442–453. doi: 10.1002/lsm.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioux S, et al. Three-dimensional surface profile intensity correction for spatially modulated imaging. J Biomed Opt. 2009;14(3):034045. doi: 10.1117/1.3156840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MS, et al. Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg. 1995;48(1):14–22. doi: 10.1016/0007-1226(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 19.Thorniley MS, et al. The use of near-infrared spectroscopy for assessing flap viability during reconstructive surgery. Br J Plast Surg. 1998;51(3):218–226. doi: 10.1054/bjps.1997.0145. [DOI] [PubMed] [Google Scholar]
- 20.Cohn SM. Near-infrared spectroscopy: potential clinical benefits in surgery. J Am Coll Surg. 2007;205(2):322–332. doi: 10.1016/j.jamcollsurg.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Hampson NB, Piantadosi CA. Near infrared monitoring of human skeletal muscle oxygenation during forearm ischemia. J Appl Physiol. 1988;64(6):2449–2457. doi: 10.1152/jappl.1988.64.6.2449. [DOI] [PubMed] [Google Scholar]
- 22.Scholz T, Evans GR. Impact of hypertonic and hyperoncotic saline solutions on ischemia-reperfusion injury in free flaps. Plast Reconstr Surg. 2008;122(1):85–94. doi: 10.1097/PRS.0b013e31817743a1. [DOI] [PubMed] [Google Scholar]
- 23.Hayden RE, et al. Oxygenation and blood volume changes in flaps according to near-infrared spectrophotometry. Arch Otolaryngol Head Neck Surg. 1996;122(12):1347–1351. doi: 10.1001/archotol.1996.01890240055012. [DOI] [PubMed] [Google Scholar]
- 24.Stranc MF, et al. Assessment of tissue viability using near-infrared spectroscopy. Br J Plast Surg. 1998;51(3):210–217. doi: 10.1054/bjps.1997.0088. [DOI] [PubMed] [Google Scholar]
- 25.Scheufler O, Exner K, Andresen R. Investigation of TRAM flap oxygenation and perfusion by near-infrared reflection spectroscopy and color-coded duplex sonography. Plast Reconstr Surg. 2004;113(1):141–152. doi: 10.1097/01.PRS.0000095940.96294.A5. discussion 153-5. [DOI] [PubMed] [Google Scholar]
- 26.Payette JR, et al. Assessment of skin flaps using optically based methods for measuring blood flow and oxygenation. Plast Reconstr Surg. 2005;115(2):539–546. doi: 10.1097/01.prs.0000148415.54546.ca. [DOI] [PubMed] [Google Scholar]
- 27.Holzle F, et al. Free flap monitoring using simultaneous noninvasive laser Doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34(1):25–33. doi: 10.1016/j.jcms.2005.07.010. [DOI] [PubMed] [Google Scholar]