Abstract
Background
Glutamatergic transmission in the amygdala is hypothesized as an important mediator of stimulus-reward associations contributing to drug-seeking behavior and relapse. Insight is, however, lacking regarding the amygdala glutamatergic system in human drug abusers.
Methods
We examined glutamate receptors and scaffolding proteins associated with the post-synaptic density (PSD) of excitatory synapses in the human post-mortem amygdala. mRNA or protein levels were studied in a multi-drug (7 heroin, 8 cocaine, 7 heroin/cocaine and 7 control) or predominant heroin (29 heroin and 15 control) population of subjects.
Results
The amygdala of drug abusers was characterized by a striking positive correlation (r > 0.8) between AMPA GluA1 and post-synaptic protein-95 (PSD-95) mRNA levels, which was not evident in controls. Structural equation multi-group analysis of protein correlations also identified the relationship between GluA1 and PSD-95 protein levels as the distinguishing feature of abusers. In line with the GluA1—PSD-95 implications of enhanced synaptic plasticity, Homer 1b/c protein expression was significantly increased in both heroin and cocaine users as was its binding partner dynamin-3, localized to the endocytic zone. Furthermore, there was a positive relationship between Homer 1b/c and dynamin-3 in drug abusers that reflected an increase in the direct physical coupling between the proteins. A noted age-related decline of Homer 1b/c—dynamin-3 interactions, as well as GluA1 levels, was blunted in abusers.
Conclusions
Impairment of key components of the amygdala PSD and coupling to the endocytic zone, critical for the regulation of glutamate receptor cycling, may underlie heightened synaptic plasticity in human drug abusers.
Keywords: addiction, PSD-95, dynamin-3, post mortem, synaptic plasticity, glutamate
Introduction
Drug addiction is a chronic disorder characterized by craving, compulsive drug use despite adverse consequences and high rates of relapse. The neurobiological mechanism underlying addiction disorders is at least partly related to long-lasting memories of the drug experience that are linked to glutamate-dependent plasticity in mesocorticolimbic brain circuits that mediate reward, goal-directed behavior, emotional expression, and cognition. Various lines of evidence suggest that glutamatergic plasticity in forebrain regions such as the nucleus accumbens, prefrontal cortex and amygdala is critical for maintaining drug-seeking and drug-taking behaviors (1, 2).
Glutamatergic signal transduction is mediated by glutamate receptors (GluRs), such as ionotropic α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), N-methyl-D-aspartic acid, (NMDA) and metabotropic glutamate receptors (mGluR), that are specifically targeted and clustered at the post-synaptic membrane by various scaffolding and adaptor proteins (3). Post-synaptic density protein 95 (PSD-95) is a prominent scaffolding protein in the PSD that complexes with NMDA and AMPA [via stargazin (TARP)] receptors (4). In addition to being required for AMPA and NMDA receptor stabilization, signaling, activity and trafficking (5–9), PSD-95 is also required for the control of AMPA glutamate receptor subunit (GluA)1 incorporation during experience-driven synaptic plasticity (6), which is crucial for drug-induced strengthening of excitatory synapses (10). Several studies evaluating gene deletion or transgenic mouse models support the notion of AMPA, NMDA receptors and PSD-95 as important contributors to glutamate plasticity and the development and persistence of addiction (11, 12).
The perisynaptic Group I mGluRs and Homer scaffolding proteins are also highly implicated in synaptic plasticity relevant for addiction disorders. Constitutively active C-terminal coiled-coil long isoforms of Homer (Homer 1–3) form complexes with Group I mGluRs (13) and regulate their downstream signaling, synaptic activity and surface clustering (3, 14, 15). Homer is also tightly linked to the NMDA receptor complex via interactions with a trimeric Shank–GKAP–PSD-95 complex (16–18), thereby providing a possibility to regulate NMDA receptor activity (19). A large body of evidence supports the role of Homer proteins in addiction disorders (20). For example, Homer1 and Homer2 knockout mice exhibit enhanced cocaine-induced place conditioning and cocaine-induced locomotor activity (21). Furthermore, over-expression of long Homer isoforms in the nucleus accumbens abolish cocaine-induced sensitization of locomotor hyperactivity and prevents development of glutamate abnormalities normally elicited by cocaine (22).
In striking contrast to the approach used to illuminate the neuropathology of many brain disorders, neurobiological studies of addiction have not been strongly anchored in investigations of the human brain. Although animal studies have provided valuable information as to the important contribution of excitatory plasticity in the actions of drugs of abuse especially in regard to drug-seeking behavior, limited information exists as to potential pathology of discrete glutamatergic molecular events in human abusers. To begin addressing this significant gap of knowledge, the current study examined the expression of GluRs and scaffolding proteins in the postmortem brain of human drug users. The study focused on the amygdaloid complex given the essential role of this structure in stimulus-reward association and emotional memory formation that are highly relevant to the chronic relapsing nature of addiction. In order to assess potential common neurobiological features of drugs of abuse, we examined heroin, cocaine and polysubstance heroin/cocaine users.
Methods and Materials
Human brains
A total of 73 postmortem brain specimens were obtained from two separate resources of known drug abusers and respective controls that were separately evaluated in Study I (n = 29) and Study II (n = 44). Study I, defined here as a multidrug population, represents three groups of drug abuse subjects — heroin, cocaine, heroin-cocaine. Study II represents a group of heroin abusers. The subjects had a documented history of abuse, but no posthumous DSM-IV dependence diagnosis was assigned as it was not possible to fully characterize the behavioral pattern of the subject's drug use during life.
Multiple drug abuse population (Study 1)
Post-mortem brain specimens from documented heroin, cocaine, and heroin-cocaine polysubstance users as well as normal control subjects were collected within approximately 24h after death as part of the routine autopsy process under a protocol approved by Wayne State University's Human Investigation Committee. Cause and manner of death were determined after medicolegal examination by the Medical Examiner. The general characteristics for the Study I subjects (N=29; n = 7–8/group) are described in Table 1 and Supplement 1.
Table 1.
Demographic data on the multiple (heroin, cocaine, heroin-cocaine) drug abuse population (Study I).
| Group | Control | Heroin | Heroin-cocaine | Cocaine |
|---|---|---|---|---|
| Number | N = 7 | N = 8 | N = 7 | N = 7 |
| Age (yr) | 46 ± 8 | 41 ± 10 | 45 ± 7 | 45 ± 9 |
| Race B/W | 3/4 | 4/4 | 3/4 | 5/2 |
| Gender | M = 7, F = 0 | M = 7, F = 1 | M = 5, F = 2 | M = 6, F = 1 |
| Storage time (days) | 1066 ± 241 | 1136 ± 164 | 1155 ± 222 | 1004 ± 346 |
| Brain pH | 6.78 ± 0.17 | 6.54 ± 0.16 | 6.64 ± 0.27 | 6.51 ± 0.36 |
| Ethanol (blood) | n = 4 | n = 3 | n = 2 | n = 0 |
| Morphine (μg/ml blood) | 0 | 0.35 ± 0.52 | 0.01 ± 0.02 | 0 |
| 6-MAM (μg/ml blood) | 0 | 0.011 ± 0.012 | 0.013 ± 0.033 | 0 |
| Cocaine (μg/ml blood) | 0 | 0 | 0.04 ± 0.08 | 0.23 ± 0.30 |
| Benzo. (μg/ml blood) | 0 | 0 | 0.32 ± 0.40 | 1.87 ± 2.00 |
| Cause of death | ASCVD (n = 4), GSW (n= 1), stab wound (n = 1), drowning (n= 1). | Heroin intoxication/abuse (n = 7) cardiomyopathy (n= 1) | Heroin and cocaine intoxication/abuse (n =7) | Cocaine intoxication/abuse (n = 4) GSW (n = 1), ASCVD (n = 1), acute myocardial infarction (n= 1) |
Values presented as mean ± SD. ASCVD, atherosclerotic cardiovascular disease; Benzo, benzoylecgonine; B, Black; C, Caucasian; F, female; GSW, gun shot wound; M, male; 6-MAM, 6-Monoacetylmorphine; yr, year. Subjects are grouped based on their history of drug abuse and positive blood levels of the drug(s) at the time of death (see Supplemental Information).
Heroin abuse population (Study II)
Brain specimens from a large population of predominant heroin abusers were also studied. These specimens, including control samples, were collected at the Department of Forensic Medicine at Semmelweis University, Hungary, as well as from the National Institute of Forensic Medicine, Karolinska Institutet, Stockholm, Sweden. The specimens were collected under the guidelines approved by the local Human Ethical Committees. The demographics and general characteristics for control (n = 15) and heroin (n = 29) subjects (Study II) are described in Table 2 and Supplement 1.
Table 2.
Demographic data on the heroin abuse population (Study II).
| Group | Control | Heroin |
|---|---|---|
| Number | N = 15 | N = 29 |
| Age (yr) | 37 ± 12 | 27 ± 5 |
| Race | Caucasian | Caucasian |
| Gender | M = 12, F = 3 | M = 24, F = 5 |
| Storage time (days) | 3778 ± 656 | 3702 ± 679 |
| Brain pH | 6.72 ± 0.23 | 6.54 ± 0.21 |
| Ethanol (blood) | n = 1 | n = 4 |
| Morphine (μg/ml blood) | 0 | 0.37 ± 0.47 |
| 6MAM (μg/ml blood) | 0 | 0.002 ± 0.010* |
| Cause of death | Electric shock (n=2), pulmonary emboli (n = 1), myocardial infarct (n = 8), viral infection (n=1), sudden death (n = 2), AMI (n=1) | Heroin intoxication |
Values presented as mean ± SD. AMI, acute myocardial infarction; F, female; M, male; 6-MAM, 6-Monoacetylmorphine; PMI, postmortem interval; yr, year.
Only one subject positive for 6-MAM (0.05lμg/ml) Subjects are grouped based on their history of predominant heroin abuse and acute heroin intoxication as the cause of death (see Supplemental Information).
In situ hybridization histochemistry
Riboprobes for GluA1, Homer 1 and PSD-95 (Supplement 1) were generated by in vitro transcription using SP6 or T7 polymerase and [35S]-αUTP (Amersham Biosciences). In situ hybridization was performed on 20μm-thick cryosections from the freshly frozen amygdala samples as previously described (23, 24)(information provided in Supplement 1). Briefly, brain sections were incubated with 270 ml (20×103 CPM/ml) overnight at 55° C and following post-hybridization washes were exposed to Kodak Biomax MR film for 5–15 days.
Image analysis
Optical density values were measured using Scion Image (NIH, MD) from digitalized images with a resolution of 300 dpi and converted to DPM (disintegrations per minute)/mg by reference to co-exposed C14 standards (American Radiolabeled Chemicals, St. Louis, MO). Measurements were taken within discrete amygdala subnuclei (Fig. 1) according to published sources of the human amygdala (25, 26). DPM/mg values from duplicate slides were averaged.
Figure 1.
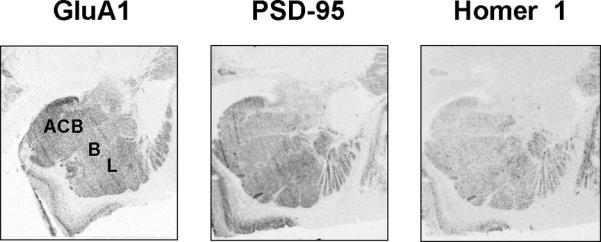
Representative autoradiograms of coronal cryosections hybridized with GluA1, PSD-95 and Homer 1 antisense riboprobes in the human amygdala from the multi drug abuse population (Study I). ACB, accessory basal nucleus; B, basal nucleus; L, lateral nucleus.
Western Blot Analysis
Detailed information is provided in Supplement 1. Briefly, solubilized protein (10–60 μg per lane) was subjected to electrophoresis, transferred to nitrocellulose membranes and stained with Memcode Reversible Protein Stain Kit (Thermo Fisher Scientific). The membranes were blocked in blocking buffer and incubated at 4°C overnight with primary antibodies. Rabbit polyclonal antibodies were used against Homer 1, PSD-95 [1:5000;1:2000; Synaptic Systems GmbH (SYSY) Goettingen, Germany], or mGluR5 and GluA1 (1:200;1:1000 Millipore (Upstate), Billerica, MA) or dynamin-3 (1:1000; Abcam). In addition mouse monoclonal antibodies were used against GluN1 (114 011, 1:1000, SYSY) and GAPDH (MAB374, 1:60,000 Millipore (Upstate)). Membranes were incubated with goat anti-rabbit or goat anti-mouse IRDye 680 or IRDye 800 secondary antibodies (LI-COR, Lincoln, NE, USA). Each protein was analyzed as a single or a double band based on predicted molecular size 45 (Homer 1), 95 (PSD95), 130 (mGluR5), 106 (GluA1), 110 (GluN1-analysed as a double band), 100 (dynamin-3) and 35 (GAPDH) kDa. GAPDH and/or Memcode optical density were used to control for total protein content. Membranes were developed with the LI-COR infrared imaging system (LI-COR) and images quantified using average integrated intensity values.
Immunoprecipitation
Detailed information is provided in the Supplement 1. Briefly, Homer 1b/c and control mouse IgG (Santa Cruz) antibodies were crosslinked to Dynabeads (Invitrogen) using Bis(Sulfosuccinimidyl) suberate (Thermo Scientific) and incubated with solubilized protein (500 μg). The protein complexes were separated by SDS-PAGE, transferred to nitrocellulose membranes which were blocked and then probed with either 1 µg/ml Homer 1b/c or Dynamin 3 (Abcam) antibodies overnight at 4°C. The blots were developed and analyzed as described above.
Statistical Analysis
Statistical analysis is described in detail in Supplement 1. Briefly, General linear stepwise regression analysis was used to evaluate statistical group in relation to the potential influence of various variables: age, brain pH, sex, blood ethanol and brain freezer storage time. Variables with a significant association with group were included in the final statistical model as covariates. To compare the correlation structure between heroin and control brains a structural equation multi-group analysis was performed. Two models were estimated, one in which the correlations were constrained to be the same across the two groups and one were the correlation parameters were unconstrained. Akaike's information criteria (AIC) and chi-square test (likelihood ratio test) was used to identify the best model fit. If the correlations were found to be invariant across groups, a Z-statistic (critical ratio test) was used for pairwise comparisons between single correlation estimates. Significance was set at P < 0.05 and trends considered for P < 0.10. Statistical evaluations were carried out using JMP, Statistica and SPSS software packages.
Results
GluA1 and PSD-95 mRNA expression are strongly correlated in the amygdala of drug abusers
mRNA expression levels were evaluated in the amygdaloid complex (focused on the lateral, accessory basal and basal nuclei) of the Study I multi-drug population that consisted of subjects with heroin, cocaine or polysubstance heroin-cocaine use as well as normal controls (Table 1). No significant group differences were detected in the mRNA expression levels of GluA1, PSD-95 or Homer 1 in the amygdala subregions studied (Fig. 1, Table 3). However, a very strong positive correlation was observed between GluA1 and PSD-95 in the drug users that was absent in control subjects (Table 4). Although the pattern was consistent throughout the amygdala subnuclei, all substance abuse groups showed the most significant alterations in the lateral nucleus. For example, the correlation between GluA1 and PSD-95 in the lateral amygdala for heroin subjects was r=0.95 (p=0.01), cocaine r=0.94 (p=0.005) and heroin-cocaine r=0.94 (p=0.002; (Figure S1 in Supplement 1). However, no significant positive correlation was observed between GluA1 and PSD-95 in the control group in any subnuclei (e.g., lateral nucleus, r=0.60, p=0.20; Figure S1A in Supplement 1) and even a negative trend was observed in the basal nucleus (r= −0.74, p=0.09).
Table 3.
GluA1, PSD –95 and Homer 1 mRNA expression levels in distinct subregions of the amygdala of control, heroin, cocaine and heroin-cocaine subjects users (Study I).
| Subregion | Control group | Heroin group | Cocaine group | Heroin-cocaine group | p - value | |
|---|---|---|---|---|---|---|
| GluAl | ACB | 201.15 ± 10.99 | 191.50 ± 14.74 | 189.91 ± 11.90 | 191.26 ± 8.86 | p = 0.890 |
| Bmc | 171.58 ± 7.55 | 163.40 ± 13.26 | 171.58 ± 9.87 | 168.76 ± 10.97 | p = 0.944 | |
| Bpc | 216.96 ± 15.08 | 221.54 ± 21.85 | 197.84 ± 20.28 | 214.13 ± 12.77 | p = 0.356 | |
| Lateral | 190.89 ± 14.86 | 191.57 ± 19.21 | 186.24 ± 16.93 | 188.21 ± 21.33 | p = 0.694 | |
| PSD-95 | ACB | 491.22 ± 18.05 | 434.68 ± 32.82 | 445.09 ± 33.78 | 445.53 ± 21.62 | p = 0.707 |
| Bmc | 485.75 ± 26.91 | 421.85 ± 32.86 | 434.77 ± 38.55 | 457.47 ± 30.11 | p = 0.936 | |
| Lateral | 502.21 ± 57.47 | 403.88 ± 33.92 | 390.79 ± 52.68 | 439.24 ± 36.56 | p = 0.510 | |
| Homer 1 | ACB | 145.55 ± 5.56 | 143.23 ± 14.03 | 141.79 ± 10.09 | 137.93 ± 10.52 | p = 0.833 |
| Bmc | 151.75 ± 3.11 | 141.96 ± 13.43 | 145.23 ± 11.85 | 144.56 ± 12.55 | p = 0.699 | |
| Bpc | 142.71 ± 7.53 | 154.26 ± 4.93 | 154.83 ± 19.22 | 145.49 ± 13.08 | p = 0.904 | |
| Lateral | 146.59 ± 7.66 | 139.62 ± 13.57 | 143.07 ± 15.85 | 142.44 ± 15.34 | p = 0.378 |
mRNA expression levels are presented as DPM per milligram (mean ± SEM). ACB, accessory basal nucleus; Bmc, basal nucleus magnocellular division; Bpc, basal nucleus parvicellular division. (n=5−7).
Table 4.
GluA1 and PSD-95 correlations in distinct amygdala subregions of control, heroin, cocaine and heroin-cocaine users (Study I).
| Subregion | Control group | Heroin group | Cocaine group | Heroin-cocaine group |
|---|---|---|---|---|
| ACB | r = 0.17, p = 0.75 | r = 0.92, p = 0.03* | r = 0.77, p = 0.07 | r = 0.42, p = 0.35 |
| Basal | r = −0.74, p = 0.09 | r = 0.88, p = 0.05 | r = 0.71, p = 0.11 | r = 0.81, p = 0.03* |
| Lateral | r = 0.60 p = 0.20 | r = 0.95, p = 0.01* | r = 0.94, p = 0.005* | r = 0.94, p = 0.002* |
ACB, accessory basal nucleus; Basal, basal nucleus; Lateral; lateral nucleus.
p < 0.05. (n=5−7).
Blood morphine levels were not significantly correlated with the mRNA expression levels, but blood cocaine concentrations in the cocaine group were significantly correlated with amygdala levels of GluA1 (basal parvocellular division, r=0.9723, p=0.0011; lateral r=0.7745, p=0.0409) and Homer (lateral nucleus, r=0.8867, p=0.0078). No association to cocaine and cocaine metabolite levels was evident in the heroin-cocaine subjects which could be due to their lower blood cocaine and benzyloecognine concentrations as compared to the cocaine only group (Table 1).
Correlation structure analysis reveals that GluA1—PSD-95 protein expression in the lateral amygdala differentiates heroin abusers from control subjects
In consideration of the gene expression findings we wanted to explore glutamatergic measures at the protein level in the drug users. However, the greater variability of the protein measurements in the postmortem material as compared to the mRNA analysis, in combination with the small sample size, precluded a broad assessment of protein measures in the multi-drug abuse population of Study I. In order to obtain further insight into the potential dysregulation of glutamatergic function in the drug abusers, we thus focused on a large population of heroin abusers in Study II (Table 2) in which abundant amygdala tissue was available to allow detection of multiple proteins of interest. Given that the lateral nucleus is the major receptive subnucleus of the amygdala for glutamatergic input from the cerebral cortex and thalamus, western blot analysis was carried out in this subnucleus to measure protein levels of GluA1, PSD-95, Homer 1b/c as well mGluR5 and NMDA glutamate receptor subunit (GluN1) 1 (Fig. 2A). Furthermore, in order to address possible differences in the organization of the glutamatergic protein network, we used a structural equation multi-group analysis model to determine whether the correlation structure relationship of the glutamatergic markers differed between heroin abusers and controls. The structural equation model was based on published data regarding the known biological organization/connectivity of the glutamatergic markers in the PSD. As such, the model evaluated correlations between, e.g., GluA1—PSD-95, PSD-95—Homer 1, mGluR5—Homer 1 and GluN1—PSD-95 (4, 13, 16, 17, 27, 28). Specifically, the structural equation multi-group analysis model tested whether equal or different correlation structures between heroin abusers and controls best described the data set. The analysis revealed that heroin abusers and control subjects differ in the correlation pattern of the glutamatergic markers evaluated within the lateral amygdala (AIC values, unconstrained model: 52.96, structural residual model: 58.11, X2, p=0.006). Further analysis revealed that alterations in the correlation structure were due to a difference in the relationship between GluA1 and PSD-95 (zeta = 2.31). Independent Pearsons correlation also showed that GluA1—PSD-95 correlated positively in heroin abusers (r=0.534 p=0.003), but not in controls (r=0.029, p=0.925).
Figure 2.
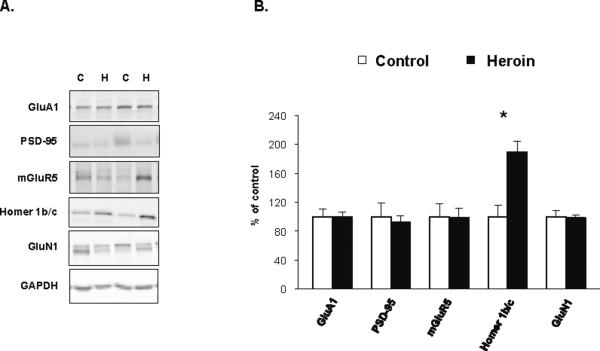
Protein levels of glutamatergic markers in the lateral amygdala of human heroin and control subjects from the heroin abuse population (Study II). A. Representative WB images of GluA1 (106 kD), PSD-95 (95 kD), mGluR5 (130 kD), Homer 1b/c (45 kD), GluN1 (110) kD and GAPDH (38 kD) in two control and two heroin subjects. B. Comparison of the immunoreactivities between human heroin (n=27–28) and control subjects (n=8–13). Protein levels are presented as percent of mean control values (mean ± SEM). *, p < 0.05 as compared to control.
Homer 1b/c protein expression is upregulated in the lateral amygdala of drug users and linked to disturbance of dynamin-3
Evaluation of the general protein levels between heroin and control subjects revealed that of the markers studied (Fig. 2), only Homer 1b/c protein expression differed significantly between the heroin and control groups, with a nearly 2-fold increase detected in heroin subjects (F1,34=11.132; p=0.002; Fig. 2B). No significant associations were observed between the heroin metabolites and protein levels of Homer 1 b/c or the other glutamatergic markers. Homer 1 b/c acts as a scaffold to maintain mGluRs, localized to the perisynaptic region, in the vicinity of the PSD (29, 30). A strong positive correlation was present between mGluR5 and Homer 1b/c levels in control subjects (r=0.831, p=0.004), but this relationship was absent in heroin abusers (r=0.013, p=0.949).
The upregulation of Homer 1b/c in heroin abusers raised the question as to whether such impairment was mimicked in other drug groups. Although we were unable to conduct a broad assessment of protein levels in the small multi-drug population of Study I, it was possible to focus on Homer 1 b/c. Statistical analysis showed an overall group difference in Homer 1b/c (F3,22=3.42, p=0.034) with significantly higher levels in the cocaine (p=0.007) and heroin-cocaine (p=0.018) subjects compared to controls, with a trend noted for the heroin group (p=0.08; Fig. 3).
Figure 3.
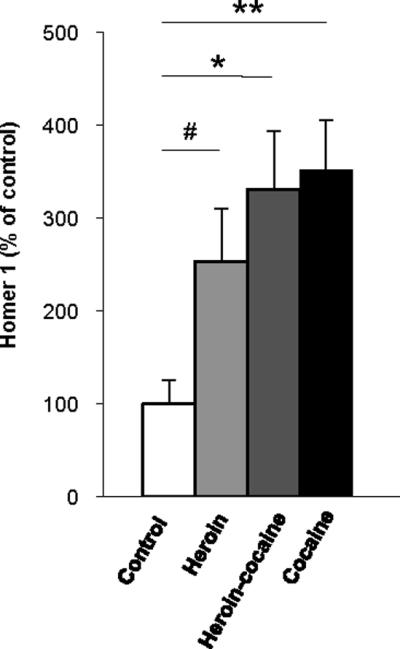
Homer 1b/c immunoreactivities in the lateral amygdala of heroin (n=7), cocaine (n=7), heroin-cocaine (n=5) and control (n=5) groups from the multi drug abuse population (Study I). Protein levels are presented as percent of mean control values (mean ± SEM). **, p < 0.01, *, p < 0.05 as compared to control.
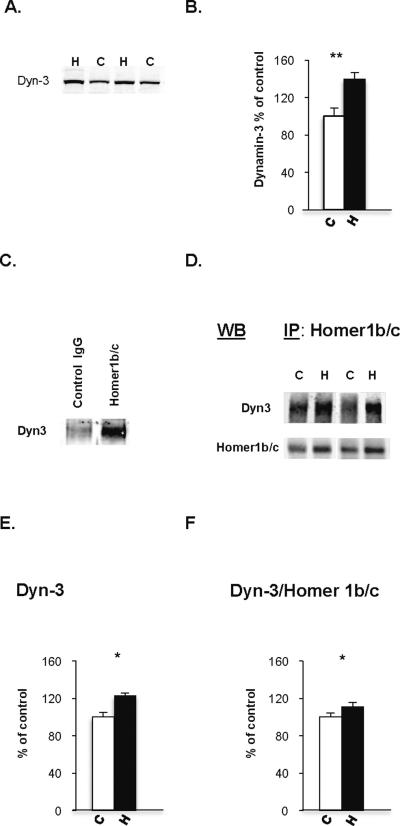
Homer has been recently shown to bind to the endocytic protein dynamin-3, localized to dendritic spines, which physically links the endocytic zone to the PSD, and regulates AMPA recycling and synaptic strength (31, 32). Given the disturbance of Homer 1 b/c observed in heroin abusers, we reasoned that alterations in synaptic plasticity implicated in addiction could be related to the interaction between Homer and dynamin-3, not only the relationship between GluA1 and PSD-95. As such, we examined protein expression levels of dynamin-3 and observed a 40% increase in the lateral nucleus of heroin abusers compared to control subjects (F1, 33=7.32, p=0.011; Fig. 4). Moreover, dynamin-3 levels were positively correlated with Homer 1 b/c (r=0.52, p=0.005) in the heroin abusers, but no such association was evident in control subjects (r=−0.066, p=0.846). Considering that no study has yet to date examined the direct relationship of Homer and dynamin-3 in neuropsychiatric populations, we next sought to assess whether the correlative data reflected true disturbance of the physical interactions between the proteins. We thus carried out immunoprecipitation of the lateral amygdala samples with Homer 1b/c followed by Western blot with dynamin-3 (Fig. 4C–D). As evident in figure 4E, there was a significant increase of dynamin-3 after Homer 1b/c immunoprecipitation in heroin abusers compared to controls (p=0.007). The apparent potentiation of dynamin-3- Homer physical interaction could just reflect the general enhancement of Homer 1b/c levels in the amygdala of heroin abusers. As such, we normalized the dynamin-3 immunoprecipitated protein levels in relation to the amount of Homer 1b/c measured by Western blot in the co-immunoprecipitated samples and noted a significant increase in heroin abusers as compared to controls (p=0.0287). Thus, there was an increase in the functional interaction between Homer 1b/c and dynamin-3 and, in addition, in the absolute amount of dynamin-3 linked to Homer 1b/c in heroin subjects. Although neither Homer 1b/c nor dynamin-3 levels alone were significantly associated with blood opiate concentrations, there was a significant positive correlation (r=0.492, p=0.019) between Homer 1 b/c—dynamin-3 interaction and blood morphine levels in heroin abusers.
Figure 4.
Protein expression levels of Dynamin-3 and Homer 1b/c in the lateral amygdala of subjects from the heroin abuse population (Study II). A. Representative Western blot (WB) images of dynamin-3 (~100kD) in two heroin and two control subjects. B. Dynamin-3 immunoreactivity in heroin (n=26) and control (n=11) subjects. C. Examination of the Dynamin 3—Homer 1b/c protein complex (control, n= 11; heroin, n= 24). Immunoprecipitations (IPs) were performed with an antibody directed against Homer 1b/c and the precipitated protein was probed Dynamin 3. A nonspecific mouse IgG Ab failed to immunoprecipitate dynamin-3. D. Representative WB of Homer 1b/c immunoprecipitate probed with dynamin-3 and Homer 1b/c antibody. E, F. A comparison of the dynamin-3 (E) and dynamin-3/Homer 1b/c (F) immunoreactivities was performed after co-immuno precipitation of Homer 1b/c. Protein levels are presented as percent of mean control values (mean ± SEM). *, p < 0.05, **, p = 0.01 as compared to control.
Interestingly, the results demonstrated that the physical interaction between Homer 1 b/c and dynamin-3 was reduced with increasing age, but this observation was most pronounced in control subjects (r=−0.859, p=0.003; heroin subjects, r=−0.394, p=0.06). A significant correlation was also observed in relation to dynamin-3 levels and age in controls (not heroin subjects) (r=0.762, p=0.028), but it was a positive relationship to age. There was no correlation to age in regard to Homer 1b/c levels in the lateral amygdala. Of the other glutamateric proteins studied, only GluA1 levels were significantly correlated to age, and this was only apparent in control subjects (r=−0.578, p < 0.05).
Discussion
The present study reveals disruption of key components of the PSD and coupling to the endocytic zone in the amygdala of human heroin, cocaine and polysubstance heroin-cocaine users that strongly imply disturbances in the regulation of synaptic plasticity.
Regardless of the nature of the illicit drug abused, there was a strong positive correlation between GluA1 and PSD-95 mRNA expression levels that was not observed in control subjects. Similarly, correlation structure analysis of the network of proteins related to glutamatergic neurotransmission revealed that the GluA1—PSD-95 relationship specifically distinguished heroin abusers from controls. These findings are intriguing given that PSD-95 induces GluA1 delivery into synapses, which is coupled to the strengthening of excitatory synapses during experience-driven learning (6). In addition, trafficking of GluA1 into the active synaptic site is consistently observed in relation to drug-seeking behavior in animal models (33–36).
Most of the information garnered to date about amygdala dysfunction of synaptic plasticity derive from studies of fear conditioning that is widely used to examine associative emotional memory formation (37), which is of critical importance in the etiology of addiction. As such, similar neurobiological mechanisms are likely to play a significant role in both fear conditioning and the development of addiction disorders (38, 39). It is well documented that fear conditioning induces strengthening of excitatory synapses within the lateral and basal amygdala nuclei and requires trafficking of GluA1 into synapses (40–42). Increased GluA1 in the plasma membrane has been reported following fear conditioning although the total amount of GluA1 mRNA and protein levels are unchanged (43). The GluA1 and PSD-95 correlation observed in our study was also not accompanied by alteration in the total GluA1 or PSD-95 mRNA or protein levels. It is therefore possible that functional rearrangement of GluA1 subunits is masked when measuring total levels of GluA1 similar to that observed in morphine-exposed rats (44). It would therefore be tempting to speculate that the strong coupling between GluA1 and PSD-95 in drug abusers represent an induction of synaptic GluA1 that leads to strengthening of synaptic connectivity and increased responsiveness of the amygdala during, for example, relapse.
In addition to the strong association evident between GluA1 and PSD-95, the relationship detected between Homer and dynamin-3 in heroin abusers would also strongly suggest enhanced availability of AMPA receptors at glutamatergic synapses and thus potentiated synaptic transmission. Homer is concentrated to the PSD and together with dynamin-3 is localized to the lateral spine membrane with a distribution that spans the PSD and endocytic zone (32, 45). In vitro studies have demonstrated that the physical link between dynamin-3 and Homer positions the endocytic zone near the PSD to maintain cycling AMPA receptors at the synapse (31, 32). Those investigations provide clear evidence that synapses lacking a PSD directly linked to the endocytic zone results in depletion of AMPA receptors at the synapse thereby leading to a reduction of excitatory synaptic transmission. Our data demonstrating that the physical coupling between Homer 1b/c and dynamin-3 was positively correlated to blood morphine levels would be in line with an upregulation of AMPA transmission upon drug intake.
While the recent use of heroin was associated with enhanced Homer 1b/c—dynamin-3 interaction, both PSD proteins were significantly increased irrespective of blood morphine levels suggesting persistent disturbance of the excitatory synapse than only a rapid dynamic modulation due to the immediate pharmacological action of the drug. Enhanced Homer 1 b/c protein in the lateral amygdala was also evident in cocaine users emphasizing the important upregulation of the scaffolding protein in association with both psychostimulant and opiate drugs. Despite the critical role of Homer 1b/c and dynamin-3 in synaptic plasticity, no data are currently available in regard to dynamin-3's potential role in addiction and only limited investigations have directly evaluated amygdala Homer regulation in relation to behavior. To date, studies have examined either transgenic animals with a global developmental knockout of Homer 1 or have focused on manipulating Homer specifically in the nucleus accumbens (21, 46, 47). Such studies, based on locomotor sensitization as the behavioral indicator of addiction sensitivity, have led to the speculation that overexpression of Homer reduces addiction vulnerability (21, 46, 47). However, no animal investigation has evaluated Homer regulation specifically in the amygdala, thus brain region-specific disturbances in the long Homer isoforms might underlie different components of the addiction phenotype. This is particularly relevant since it has been recently documented, for example, that Homer 1b/c is differentially altered in the nucleus accumbens (decreased) and prefrontal cortex (increased) after early drug withdrawal in animals that self-administered cocaine (48). Moreover, the prefrontal cortical increase of Homer 1b/c was only evident in animals that experienced daily, extended access to cocaine self-administration (48), an animal model mimicking loss of control over drug intake, compared to animals with only short access to the drug. Such findings implicate an important contribution of compulsive drug use to the cortical Homer 1b/c alterations. It remains to be studied whether allocortical amygdala Homer 1 b/c alterations are more comparable to those evident in the prefrontal cortex in contrast to that seen in the nucleus accumbens. The current results obtained by the direct investigation of human abusers provide a significant foundation to guide future animal studies in evaluating novel amygdala molecular targets as potential contributors to the short-term and long-term regulation of behaviors more reflective of the human addiction condition.
Another interesting observation of the present study was the association of glutamatergic markers with aging. In addition to demonstrating a decline of GluA1 with age that has been observed in the hippocampus in rats (49), the current findings document for the first time an age-related decline in regard to the interaction between dynamin-3 and Homer 1b/c. Such impairment of the PSD and the coupling to the endocytic zone would be consistent with the known reduction of synaptic plasticity and concomitant deficit in learning and memory as seen during normal aging (50, 51).
There are inherent limitations with post-mortem human brain studies with confounds such as drug use history, co-morbidity with psychiatric disorders, and lifestyle characteristics that are difficult to validate. In addition, studies of the post-mortem human brain only allow evaluation at a fixed time point; thus following the trajectory of neurobiological measures with behaviors over time is not feasible and it is impossible to know the state of these systems prior to drug use. Nevertheless it is critical to expand our understanding of what is a quintessential human condition by the direct study of the human brain and that can provide a foundation for future experimental animal models in which causal relationship to specific addiction-related behaviors can be determined.
In conclusion, our study reveals dynamic synchronization of PSD-95 and GluA1 in the amygdaloid complex of human drug abusers. The fact that enhanced GluA1-PSD-95 coupling, well established to reflect strengthening of excitatory synapses, was evident in heroin, cocaine, and polysubstance users is consistent with the hypothesis that potentiated glutamatergic long-term plasticity is a common feature of drug abuse. Upregulation of amygdala dynamin-3 and Homer 1b/c levels together with potentiation of their physical interaction suggests abnormality of the PSD and endocytic zone structural network in the amygdala of drug abusers. Such disturbances might be a fundamental component of the pathophysiology underlying addiction disorder.
Supplementary Material
Acknowledgements
We thank Mrs. Alexandra Tylec for technical assistance as well as Jacob Bergström (Department of Learning, Informatics, Management, and Ethics, Medical Statistics Division, Karolinska Institutet) for statistical guidance. This study was supported by National Institutes of Health Grant NIDA DA15446 (YLH) and DA006470 (MJB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Boeckers TM. The postsynaptic density. Cell Tissue Res. 2006;326:409–422. doi: 10.1007/s00441-006-0274-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 8.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 9.Xu W, Schluter OM, Steiner P, Czervionke BL, Sabatini B, Malenka RC. Molecular dissociation of the role of PSD-95 in regulating synaptic strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 11.Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 13.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, et al. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 14.Duncan RS, Hwang SY, Koulen P. Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med (Maywood) 2005;230:527–535. doi: 10.1177/153537020523000803. [DOI] [PubMed] [Google Scholar]
- 15.Kammermeier PJ. Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins. BMC Neurosci. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 17.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 18.Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- 19.Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 20.Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 24.Drakenberg K, Nikoshkov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, et al. Mu Opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci U S A. 2006;103:7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- 26.Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- 27.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 28.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, et al. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- 30.Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Helton TD, Blanpied TA, Racz B, Newpher TM, Weinberg RJ, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 35.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 41.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 42.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 43.Yeh SH, Mao SC, Lin HC, Gean PW. Synaptic expression of glutamate receptor after encoding of fear memory in the rat amygdala. Mol Pharmacol. 2006;69:299–308. doi: 10.1124/mol.105.017194. [DOI] [PubMed] [Google Scholar]
- 44.Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58:1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- 45.Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 46.Szumlinski KK, Abernathy KE, Oleson EB, Klugmann M, Lominac KD, He DY, et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology. 2006;31:768–777. doi: 10.1038/sj.npp.1300890. [DOI] [PubMed] [Google Scholar]
- 47.Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, et al. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging. 2008;29:1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.