Abstract
Context:
The effects of fatigue on impact loading during running are unclear, with some authors reporting increased impact forces and others reporting decreased forces.
Objective:
To examine the effects of isokinetic fatigue on muscle cocontraction ratios about the knee and ankle during running.
Design:
Cross-sectional study.
Setting:
Neuromechanics laboratory.
Patients or Other Participants:
Female middle-distance runners (age = 21.3 ± 1.93 years) with at least 5 years of training experience.
Intervention(s):
Participants ran on the treadmill at 3.61 m/s before and immediately after the fatigue protocol, which consisted of consecutive, concentric knee extension-flexion at 120°/s until they could no longer produce 30% of the maximum knee-extension moment achieved in the familiarization session for 3 consecutive repetitions.
Main Outcome Measure(s):
Electromyographic (EMG) amplitude of the vastus medialis (VM), biceps femoris (BF), gastrocnemius (GAS), and tibialis anterior (TA) was recorded using surface electrodes. Agonist∶antagonist EMG ratios for the knee (VM∶BF) and ankle (GAS∶TA) were calculated for the preactivation (PR), initial loading response (LR1), and late loading response (LR2) phases of running. Hip-, knee-, and ankle-joint angular displacements at initial foot contact were obtained from 3-dimensional kinematic tracings.
Results:
Fatigue did not alter the VM∶BF EMG ratio during the PR phase (P > .05), but it increased the ratio during the LR1 phase (P < .05). The GAS∶TA EMG ratio increased during the LR1 phase after fatigue (P < .05) but remained unchanged during the PR and LR2 phrases (P > .05).
Conclusions:
The increased agonist EMG activation, coupled with reduced antagonist EMG activation after impact, indicates that the acute decrease in muscle strength capacity of the knee extensors and flexors results in altered muscle-activation patterns about the knee and ankle before and after foot impact.
Keywords: biomechanics, muscle fatigue, joint stability
Key Points.
After an isokinetic fatigue protocol for the knee-extensor and knee-flexor muscles, participants contacted the ground with a greater knee-flexion angle.
An antagonist inhibition strategy about the knee and ankle was noted, as was a quadriceps-dominant strategy during the preactivation and initial contact phases of running.
Altering the agonist-antagonist muscle balance during the loading-response phase may affect joint stability.
Although running has beneficial effects for human health, runners experience frequent musculoskeletal injuries.1 Epidemiologic evidence indicates that the yearly injury-incidence rate varies from 37% to 56%.1 Running injuries result from a complex interaction of factors, including insufficient warm-up, running experience, characteristics of running practice and environment, fatigue, and muscular imbalances.1,2
In theory, the impulsive forces exerted when the foot contacts the ground may also contribute to musculoskeletal injuries.3 Compared with other movements, the ground reaction forces are relatively small,4 with magnitudes up to 2.32 × body weight (BW) and an impact load rate of 113 × BW/s.5 It has been suggested1,6–9 that when these impact loads are exerted continuously during repetitive running cycles, then injury risk might increase. For this reason, the biomechanics of fatigued running have been thoroughly investigated.10–18
The effects of fatigue on impact loading during running have been inconclusive, with some authors10 reporting that fatigue increases impact forces and others11,12 finding the opposite. However, exhaustive running is known to alter running cadence, step length, and lower extremity joint kinematics.2 In turn, joint kinematic adaptations include increased knee-flexion angle,13–16 altered subtalar-joint pronation,15 and decreased ankle dorsiflexion at impact.10,17,18 These joint kinematic changes result in a decrease in the portion of the body mass that is accelerated when the foot contacts the ground (ie, effective mass).3 The lower the effective mass, the greater the leg impact and attenuation.3 The kinematic changes in the fatigued runner are the result of muscle-performance impairments that contribute to the runner's inability to maintain the same technique for a long period of time. Such changes may increase injury potential, especially in less-experienced runners and runners with muscle weaknesses or imbalances.1,2
Rehabilitating or preventing running injuries requires appropriate exercise programs. If fatigue causes impairments in joint kinematics, then the aim of exercise programs would be to prepare the runner's musculoskeletal system to withstand fatigue and maintain consistent technique. This requires a deep understanding of the way the muscles work when the runner is fatigued. The associated joint kinematic changes in postfatigue running could be accompanied by 3 alterations in muscle-activation patterns.19
First, fatigue can affect muscle activation immediately before impact (preactivation). Normal running is characterized by very high preactivation (PR) of the biceps femoris (BF), which is followed by vastus medialis (VM) activation. Similarly, the tibialis anterior (TA) demonstrates high PR, whereas the gastrocnemius (GAS) is maximally activated just after contact.19–22 The high PR levels of several leg muscles during running suggest that PR is a preparatory requirement for enhancing muscle activation during the braking phase.20,23,24 Theoretically, reducing PR and force-generation capacity of the quadriceps and hamstrings muscles would impair the runner's ability to control the knee joint at impact.
Second, fatigue alters coactivation of the GAS and TA during running.19 When an imbalance between the muscles develops and the muscles that span the tensile surface of the bone become less active than those on the opposite side, the result is a decrease in the muscles' protective abilities19 and possible impairments in joint stability.25,26 Thus, it is important to investigate not only the individual muscles during running but also simultaneous activity of various muscles acting around a joint.
Third, evidence suggests that landing or hopping fatigue can redistribute the work produced around the lower limb joints6 or change muscle-coactivation patterns in several joints.27 To our knowledge, muscle-coactivation strategies in more than 1 joint during running under fatigue conditions have not been investigated.
Previous protocols1,6–9,19 on fatigue effects on running biomechanics are based on running until exhaustion. Exhaustive running protocols fail to distinguish which muscles are responsible for specific kinematic or kinetic changes observed after fatigue.10 For example, changes in knee-joint kinematics after fatiguing running might be a protective response to better absorb impact forces and to reduce the risk of injury.13 However, kinematic changes may also result from fatigue of the muscles that surround the hip, knee, or ankle. Yet because general running fatigue protocols involve more than 1 muscle, it is difficult to distinguish the role of 1 muscle relative to another. Thus, guiding rehabilitation or injury-prevention programs is challenging because clinicians lack a clear picture of how knee-muscle function contributes to injuries in a fatigued runner.
Selective muscle-fatigue protocols may prove helpful in this area by providing the opportunity to relate kinematic changes from fatigue with specific muscle behaviors.10,28 For example, examining changes in running kinematics after fatiguing only the knee muscles would offer specific information on how reduced force capacity of these muscles affects joint kinematics, not only of the knee but of other joints as well. To our knowledge, such information about running after fatigue is missing. Therefore, the purpose of our study was to examine the effects of a localized muscle-fatigue protocol on hip-, knee-, and ankle-joint kinematics and muscle-coactivation ratios about the knee and the ankle before and immediately after foot contact in running. We had 2 main hypotheses: (1) that localized muscle fatigue of the knee muscles would increase the VM∶BF coactivation ratio before and after foot impact, and (2) that fatigue would also alter the GAS∶TA muscle-activation ratio.
METHODS
Participants
Thirteen women (age = 21.3 ± 1.93 years, height = 168.1 ± 6.13 cm, body mass = 70.1 ± 5.21 kg) who were middle-distance runners with a minimum of 5 years' training experience (at least 3 training sessions per week) volunteered after signing informed consent. Their weekly mileage during the experiment period ranged from approximately 25 to 40 mi (40 to 64 km) per week with official records in an 800-m event of less than 2 minutes, 15 seconds. The participants had no history of lower extremity injury or pain resulting in inability to run for more than 1 month for at least 1 year before testing. From the medical records, it appeared that 3 athletes had experienced mild hamstrings strains 2 to 3 years before the study, and 1 athlete had experienced an ankle sprain. None of the athletes had a medical record of patellofemoral pain. The study was approved by the University Ethics Committee.
Design
A single-group pretest-posttest design was applied. Participants visited the laboratory on 2 occasions 1 week apart. The aim of the first visit was to familiarize them with the muscle-strength testing and treadmill running. During the second visit, each volunteer performed a fatigue protocol while we recorded treadmill-running kinematics and muscle-electromyographic (EMG) activity before and after fatigue.
EMG Measurements
A BTS TELEMG remote system (BTS Bioengineering, Milan, Italy) including shielded electrode lead assemblies (bipolar silver/silver chloride electrodes, center-to-center interelectrode distance = 2 cm) interfaced to a portable amplifier/transmitter (model 920 DD; BTS Bioengineering; common mode rejection ratio >110 dB at 50/60 Hz, bandwidth = 10–500 Hz, gain = 1000) was used for EMG data collection. The system uses a portable telemetry unit that was secured around the waist of the participant. Bipolar surface electrodes were placed on the VM, BF, GAS, and TA. The EMG electrode locations were prepared by shaving the skin at each electrode site and cleaning it with alcohol wipes. Skin resistance, always checked using a simple DC ohmmeter, was less than 5 kΩ. The electrode locations were identified during a maximal voluntary isometric contraction (MVIC) from the seated (VM, GAS, TA) and prone (BF) positions based on previous recommendations.29 For the VM, electrodes were located approximately 5 cm medial to a point that was 25% of the distance from the superior aspect of the patella to the anterior-superior iliac spine. The BF electrodes were placed 2.5 cm medial to the midpoint of a line from the ischial tuberosity to the midpopliteal crease. For the GAS, electrodes were directly placed over the lateral head, at approximately 25% of the distance from the fibular head to the middle of the calcaneal tendon. For the TA, electrodes were placed over the ventral side of the lower leg, just lateral to the tibia at one-third of the distance from the tip of the fibula to the tip of the lateral malleolus. The ground electrode was positioned on the bony surface on the lateral femoral epicondyle. Electrode positions and the EMG signal were checked continuously for consistency. Attention was paid to stabilizing the cables during testing, as their movement can induce artifact while also pulling the electrodes. The position of the electrodes was not altered throughout the protocol.
The EMG data were transmitted to the main control unit of the BTS system and converted into digital form at a rate of 1000 Hz. The EMG system was interfaced to the data-acquisition board of the Vicon system (model 612; Oxford Metrics Ltd, Oxford, United Kingdom), allowing synchronization of the EMG and kinematic signals. Data collection was initialized and controlled by the Vicon software (version 3.1). Because of differences in initial sampling rates, the EMG signals were resampled offline after data collection to match the kinematic data. For each participant, the EMG signal was filtered using a digital high-pass filter at 10.0 Hz with zero-phase shift. It was then rectified and down sampled at 120 Hz to match the kinematic data. After rectification, the EMG amplitude signals were normalized as a percentage of EMG values recorded during the MVIC.
Kinematics
Sagittal-plane kinematic data were collected with a passive, 6-camera, 3-D Vicon motion-analysis system sampling at 120 Hz. The cameras were calibrated to a volume of 2.0 m3 and calibration errors were all less than 3 mm. We chose to fatigue muscles that we perceived as primarily controlling the sagittal-plane movement of the knee. For this reason, only running kinematics in the sagittal plane were examined.
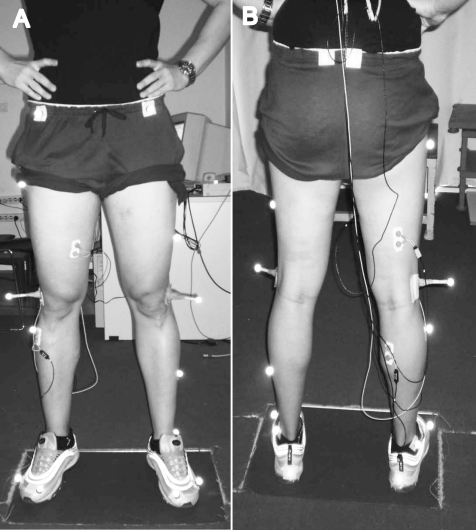
Retroreflective markers were placed on the head of the first and fifth metatarsals, heel, lateral malleolus, midshank, lateral epicondyle, midthigh, posterior- and anterior-superior iliac spines, sacrum, and right and left shoulders (Figure). Before each running trial (prefatigue and postfatigue), a standing trial was recorded to establish initial joint-angle positions. Two standing trials were used to account for the effects of any marker movement during the fatiguing exercise. Marker position was automatically tracked using the Plug-in-Gait module of the Workstation software (Oxford Metrics Ltd). Three-dimensional marker-position coordinates were computed using the direct linear-transformation method. The resulting displacement-time data for each marker were filtered using a low-pass, fourth-order, Butterworth dual-pass filter. Optimal cutoff frequencies were chosen by comparing the residuals of the difference between filtered and unfiltered signals at several cutoff frequencies. The filter cutoffs ranged between 4 and 7 Hz for all markers.
Figure.
Marker and electrode placement. A, anterior view. B, posterior view.
Embedded right-hand Cartesian coordinate systems were defined for the thigh, shank, and foot of each limb to describe the position and orientation of each segment. With these embedded coordinate systems, the orientation angles between segments were determined using Euler angle equations. The angles between the trunk and thigh (hip flexion-extension), thigh relative to the tibia (knee flexion-extension), and tibia relative to the foot about the mediolateral axis (ankle plantar flexion-dorsiflexion) of the right lower extremity were calculated and used for analysis. For reference, hip extension and knee extension during the standing trial were set equal to 0°, whereas the ankle in neutral position was equal to 0° (angles greater than 0° indicated dorsiflexion and angles less than 0° indicated plantar flexion).
Procedures
Familiarization Session
On day 1, the participants came to the laboratory for familiarization with the isokinetic-testing and treadmill-running protocol. A Cybex Norm isokinetic dynamometer (Lumex Corporation, Ronkonkoma, NY) was used for the strength and fatigue tests. The tests were performed in a seated position (hip-flexion angle of 85°) and the trunk, waist, and thigh of the right lower extremity were stabilized with Velcro straps (Velcro USA Inc, Manchester, NH). Each participant performed 3 concentric flexion-extension maneuvers at 120°/s to determine maximum extension and flexion torque. Motion ranged from 0° (full extension) to 90° of knee flexion. The volunteers were instructed to exert maximal effort through the whole range of motion. For all measurements, the rotation axis of the dynamometer was approximately aligned with the rotation axis of the joint tested. Maximum values were then used to set the target levels of fatigue tests performed during the main testing session. The participants were also familiarized with treadmill running over a range of speeds until they reported feeling comfortable running at 3.61 m/s. This treadmill speed was selected after pilot testing in which these athletes showed signs of inconsistent technique in repeated sessions when running at speeds greater than 3.61 m/s.
Main Testing Session
On day 2, the main experimental protocol was performed. This included treadmill running before and immediately after the fatigue protocol. The session started with our obtaining reference EMG activity during the MVIC. Participants performed MVICs of the knee extensors and flexors and ankle plantar flexors and dorsiflexors consisting of three 5-second maximal isometric efforts of the knee extensors at 65° of knee flexion and the knee flexors at 35° of knee flexion and ankle plantar-flexion and dorsiflexion MVICs performed from the seated position with the foot securely fastened to the footplate at 0° of ankle plantar flexion (neutral position).30,31 These tests were performed to normalize the surface EMG signals.30 The average EMG signal for a period of 2 seconds, during which the recorded maximum torque was relatively consistent (±5%), was used for further analysis.
After MVIC testing, the running task was performed. Participants warmed up by running on a treadmill (Runrace 1200 HC; Technogym S.P.A., Forli, Italy) at 3.61 m/s until they reported feeling comfortable. They then ran for 10 seconds at 3.61 m/s. During this 10-second period, sagittal-plane kinematics from the right lower limb of 5 nonconsecutive strides were collected.
Immediately after the treadmill run, participants performed the fatigue protocol. This consisted of consecutive, concentric knee–extension-flexion efforts at 120°/s until they could no longer produce 30% of the maximum knee-extension moment achieved in the prior session for 3 consecutive repetitions. This force limit has been applied previously,32 and we selected it to ensure appropriate localized muscle fatigue. The volunteers received standardized verbal instructions to maintain maximal effort and full range of motion throughout the test.
Electrode movement is a common issue for most studies using dynamic movements such as ours. There is no way to extract the signal coming from accidental movement of the electrodes relative to the muscle because it is impossible to monitor changes in skin movement relative to the muscle. Artificial EMG signal tracings can be introduced when cable movement pulls the electrode. We took 3 steps to avoid this error: (1) visually inspecting that the electrodes remained in the same position throughout the test; (2) monitoring the EMG signal during the movement and, more importantly, when the participant moved from one activity (eg, isokinetic dynamometer) to the next (eg, running); and (3) filtering the raw signal for frequencies below 10 Hz to account for standardized cable movements. When electrodes are pulled off or starting to pull off, the pattern of raw EMG signal changes: the signal deviates significantly from the baseline and shows a consistent characteristic signal form. Finally, if we were unable to avoid these errors, then we dropped all the data for the participant.
When the isokinetic fatigue test ended, the participant immediately got off the dynamometer and then performed the running task as quickly as possible to reduce the effects of recovery from muscular fatigue. The average time period between terminating the fatigue protocol and beginning the posttest running trials was 1.35 minutes.
Data Analysis
The means of 5 right steps for all kinematic variables before and after fatiguing exercise were examined. Ground contact was determined from the kinematic (vertical linear position and velocity) tracings of the toe marker with respect to the treadmill during consecutive cycles.33 Examination of data from consecutive running cycles showed that upon initial impact, a visible oscillation was evident in the vertical position on the time graph of the toe marker. This oscillation was better identified by examining the first derivative of the vertical position (ie, the vertical velocity of the marker), which showed a sudden increase immediately after contact. This point was considered to represent initial impact. The reliability (intraclass correlation) coefficient of initial contact across the 5 recorded trials was 0.92. Kinematic variables included joint-flexion–angle measurements at the time of initial ground contact as well as maximum joint excursion during the loading-response phase.
For each trial, the normalized EMG was averaged for each of the following phases: the PR phase, defined as the period of 100 milliseconds before initial ground contact; the initial loading response (LR1) phase, defined as the 50-millisecond interval immediately after ground contact; and the main loading response (LR2) phase, defined as the period between 50 and 200 milliseconds after ground contact. We selected these intervals based on previous studies of running biomechanics.13,24 Subsequently, the mean value from the 5 running trials for each participant was calculated.
The normalized VM∶BF and GAS∶TA EMG ratios were computed and average values calculated for each phase. We used this specific coactivation index to compare our results with recent findings on fatigue effects on movement biomechanics.27,32 Agonist∶antagonist EMG ratios of 1.0 indicate equal activation of the 2 antagonistic muscles, whereas coactivation ratios greater than 1.0 indicate increased agonist (VM or GAS) activation compared with the antagonist (BF or TA) muscles and vice versa.27 Ratios less than 1 indicate high activity of the antagonist relative to the agonist muscles.
Statistical Analysis
Separate, repeated-measures 2-way analyses of variance for each of the EMG variables (VM, BF, GAS, and TA EMG value; VM∶BF EMG ratio; GAS∶TA EMG ratio) were used to determine whether fatigue (pretest, posttest) had an effect on the variables tested across 3 movement phases (PR, LR1, LR2). One-way analysis-of-variance designs were also used to examine fatigue effects on hip, knee, and ankle angles at contact. For each comparison, effect sizes were calculated to better show the statistical power of post hoc results. Effect sizes higher than 0.50 indicate a moderate difference between means; values higher than 0.80 are indicative of a large difference.34 Finally, for each testing condition, the 95% confidence intervals were also estimated. A significance level of α < .05 was set a priori for all analyses.
RESULTS
During the isokinetic fatigue protocol, participants performed an average number of 44.4 ± 1.5 repetitions. The fatigue protocol ended when the knee-extension moment reached 30% of maximum. The corresponding decline of the knee-flexor moment during the protocol was 46.8% ± 4.7%.
Knee-Muscle Activation
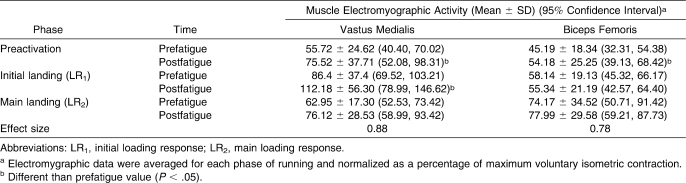
The average VM EMG activation ranged from 55.72% ± 24.62% MVIC (prefatigue) to 112.18% ± 56.30% MVC (postfatigue), whereas the BF EMG activation ranged from 45.19% ± 18.34% MVIC (prefatigue) to 77.99% ± 29.58% MVIC (postfatigue; Table 1).
Table 1.
Vastus Medialis and Biceps Femoris Muscle Electromyographic Activity Before and After the Isokinetic Fatigue Protocol
A significant interaction (time × phase) effect on VM EMG activation was noted (F2,24 = 4.47, P = .03). Post hoc Tukey tests showed that the VM activation increased during the PR and LR1 phases (Table 1) but not during the LR2 phase. We also found an interaction effect on BF EMG activation (F2,24 = 3.81, P = .04). Post hoc comparisons indicated that the BF increased after fatigue only during the PR phase (P < .05).
Ankle-Muscle Activation
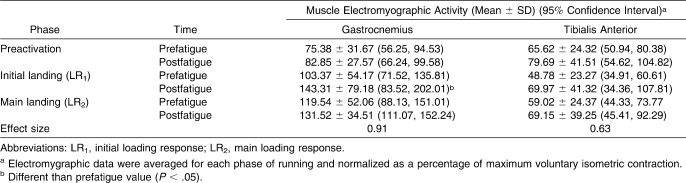
The GAS EMG activation ranged from 75.38% ± 31.67% MVIC (prefatigue) to 143.31% ± 79.18% MVIC (postfatigue), whereas the TA activation ranged from 48.78% ± 23.27% MVIC (prefatigue) to 79.69% ± 41.51% MVIC (postfatigue; Table 2).
Table 2.
Gastrocnemius and Tibialis Anterior Muscle Electromyographic Activity Before and After the Isokinetic Fatigue Protocol
An interaction effect (F2,24 = 3.91, P = .03.) was seen on the GAS EMG activation. Post hoc comparisons showed an increase in GAS EMG activation during the LR1 phase (P < .05) but not during the PR or LR2 phase (P > .05). No effects of fatigue on TA muscle activation were demonstrated (F2,24 = 0.82, P > .05).
Muscle Coactivation Ratios
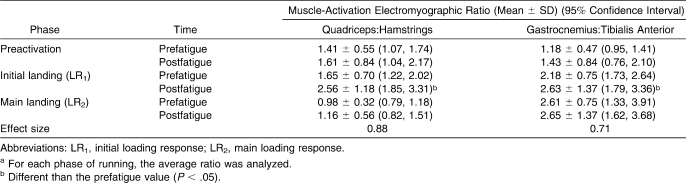
An interaction effect was seen on the VM∶BF (F2,24 = 5.16, P = .01) and GAS∶TA EMG ratios (F2,24 = 3.65, P = .04). Post hoc comparisons showed nonsignificant (P > .05) effects of fatigue on the VM∶BF and GAS∶TA EMG ratios for the PR and LR2 phases (Table 3). During the LR1 phase, an increase (P < .05) in the VM∶BF ratio was noted postfatigue. The GAS∶TA EMG ratio also increased (P < .05) postfatigue.
Table 3.
Quadriceps:Hamstrings and Gastrocnemius:Tibialis Anterior Muscle-Activation Electromyographic Ratios Before and After the Isometric Fatigue Protocola
Joint Angles
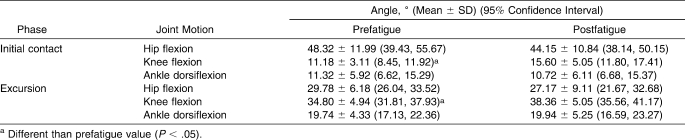
Knee-flexion angle increased at initial contact (F1,12 = 11.339, P = .01), as did knee-joint excursion (F1,12 = 8.67, P = .01) postfatigue, with high effect sizes for each of these variables (Table 4). Nonsignificant changes in hip and ankle angles were observed (P > .05).
Table 4.
Hip, Knee, and Ankle Angles at Initial Foot Contact and Maximum Joint Excursion During the Loading Response Phase Before and After the Isometric Fatigue Protocol
DISCUSSION
Our objective was to examine the effects of an isokinetic fatigue protocol of the knee musculature on muscle-coactivation ratio about the knee and the ankle before and immediately after the foot contact of running. Muscle-coactivation ratios were altered after fatigue during the initial response phase. To our knowledge, the effects of localized muscle fatigue on muscle- coactivation ratios in running have not been previously investigated. Because the effects of fatigue differed among running phases, it is important to examine and discuss the results separately for each phase.
Preactivation
We hypothesized that knee-muscle fatigue would alter muscle-coactivation levels before the foot contacted the ground. However, this hypothesis was not confirmed: no differences in the estimated ratios postfatigue were found (Table 3). Each ratio remained unaffected by the fatigue protocol for different reasons. For the ankle, the similar PR levels of both muscles suggest that any changes in muscle preactivity levels observed during long-distance running do not appear to be influenced by reduced force capacity of the knee muscles. For the knee, the activation ratio was unchanged because fatigue increased both VM and BF activation (Table 1). This can be attributed to the role of muscle PR for joint stability, as previous authors24,35 have suggested that PR aims to enhance the stiffness of the musculotendinous system to tolerate and absorb high-impact loads at the beginning of ground contact. Increased knee muscle PR after the protocol may be seen as a compensatory response to the reduced force-generation capacity of the fatigued muscles. Despite the absence of change in the coactivation ratio, the higher activation of both agonist and antagonist knee muscles postfatigue would indicate a stiffer joint before impact, which may increase joint stability.20,27
Contact and Initial Response
During foot contact, the quadriceps muscle group acts eccentrically to maintain hip and knee movement while the hamstrings coactivate concentrically.24,36 In this phase, both muscles display high activation levels and, therefore, both contribute to joint stability by cocontracting. The increased knee-flexion angle (Table 4) coupled with the higher VM∶BF coactivation ratio during the LR1 phase (Table 3) indicates reduced knee-joint stiffness, because the amount of simultaneous muscle activation changed in favor of the quadriceps muscle.
The increase in quadriceps activation after fatigue has often been called a quadriceps-dominant strategy,27,32,37 and it might indicate that either that participants placed greater reliance on the quadriceps muscles postfatigue or that fatigue resulted in increased VM motor-unit recruitment to produce the same amount of force. Greater quadriceps muscle activation with lengthening24,38 may be required to prevent the knee from collapsing into flexion. This possibility is further supported by the observation that the quadriceps muscle has a higher torque-generation capacity at high knee-flexion angles.39 Consequently, the shift to higher knee-flexion angles postfatigue may reduce ground reaction forces and absorb the shock at impact,13 but it may also bring the quadriceps into a better position lengthwise for torque production. Consequently, the increased VM∶BF coactivation ratio at impact may indicate the neuromuscular system's attempt to maintain quadriceps-force exertion and, through that, to control the knee and hip during initial foot impact.
Authors19,27,32 have suggested that a strategy to compensate for local muscle-fatigue effects is the decline in the antagonist activation patterns of the knee and the ankle. Our results do not confirm these findings because neither BF (Table 1) nor TA (Table 2) activation were altered postfatigue. These differences can be mainly attributed to differences in the movement type and fatigue protocol examined. Specifically, drop landings are associated with greater ground reaction forces and different joint kinematics than running. Further, general running fatigue protocols such as the one used by Mizrahi et al19 caused impairments in the knee musculature and may have affected the hip and ankle muscles. Such impairments may lead to additional adjustments, thus resulting in different muscle-activation levels compared with the local muscle-fatigue protocol applied in the present study.
In our study, the GAS∶TA ratio increased postfatigue (Table 3). Previous investigators19,27 have reported similar results after multijoint fatigue protocols. This finding suggests that localized muscle fatigue may also result in changes in the activation patterns of muscles that are not fatigued. In particular, this enforces a previous observation27 that fatigue caused a shift toward greater reliance on nonfatigued musculature. The GAS is a biarticular muscle that flexes the knee and plantar flexes the ankle. Given that the knee-flexor muscles (hamstrings) were fatigued (although to a lesser degree than the knee extensors), the increase in GAS activation may compensate for the reduced hamstrings force-generation capacity around the knee while it simultaneously continues to produce force around the ankle. Such a pattern may represent a compensatory strategy to maintain leg stiffness during impact movements.40
Main Loading-Response Phase
The nonsignificant fatigue effects on muscle-coactivation ratios during the LR2 phase (Table 3) indicate that most adjustments due to muscle fatigue take place before or immediately after impact. Perhaps fatigue causes alterations in muscle synergies only when joint stability is threatened. Preparatory movements and muscle-activation patterns occur during the prelanding and initial-response phases; once stability is preserved, then alterations in muscle-coactivation patterns are unlikely.
Limitations
One of the limitations of using EMG signals to examine dynamic movements is the risk of signal errors from cable and electrode movement artifacts. To reduce the likelihood of these errors, we focused on stabilizing the cables during the protocol and continuously monitoring the EMG electrode position and resulting signal. In addition, we did not quantify the reliability of EMG measurements; instead, we averaged 5 running cycles before and after fatigue. Although this method does not ensure a high level of reliability, it takes into consideration the intertrial variability frequently observed during EMG testing. Another limitation of this study is that EMG changes postfatigue may be influenced by simultaneous changes in muscle temperature.41 Although corrective techniques have been proposed41 to overcome this problem, they are invasive and their applicability for correcting EMG amplitude data has yet to be confirmed.
A further limitation is the existence of large confidence intervals of the measured EMG variables (Tables 1–4). The considerable variability in individual EMG signals also affected the variability in the estimated coactivation ratios. The variability originates mainly from 2 factors: (1) the EMG signal displays considerable variability, especially when measured under dynamic conditions; and (2) a certain variability exists among participants and sessions in running performance. These factors might have had an effect on our results, given that significant differences between pairs of means were not detected because of the presence of variability. We used a standardized EMG analysis protocol to analyze and treat the EMG signal. Future authors could examine the effects of advanced filtering or smoothing techniques to reduce EMG signal variability.
CLINICAL RELEVANCE
Running is a multiarticular movement that is associated with various types of injuries. Most of these injuries may result from training or technique errors, especially when the runner is fatigued.42 Injury-prevention and injury-rehabilitation programs aim to improve muscle function and coordination in runners. These goals could be better accomplished by understanding the role of specific muscles for running performance under fatigue conditions. Our findings showed that fatiguing the knee-extensor and, to a lesser extent, the knee-flexor muscles increases muscle activation around the knee before running impact. Knee-flexion angle increases at impact, and a general shift occurs toward greater quadriceps and GAS activation after impact. Because most of these adaptations are also observed after general running fatigue protocols,19 fatigue of the knee muscles may play a major role for the observed alterations in lower limb joint kinematics in fatiguing running. From a clinical point of view, these results suggest that quadriceps-muscle strengthening and endurance training may help runners cope with the effects of fatigue. Such training, however, should be performed so that muscular imbalances around the knee are avoided. This factor is more important when the athlete has a history of previous knee injury, which may cause muscle atrophy (in the early stages of rehabilitation) as well as muscle imbalances. The value of improving the knee-musculature endurance capacity is enforced by the observation that fatigue of the knee muscles can affect other joint muscles as well, such as the ankle. Yet these results should be considered within the global framework of changes in the kinematics and muscle-activation patterns in the lower body as a whole during fatiguing running.
CONCLUSIONS
After an isokinetic fatigue protocol of the knee extensors and flexors, individuals contacted the ground with a greater knee-flexion angle. This change was accompanied by an antagonist-inhibition strategy around the knee and ankle and a quadriceps-dominant strategy during the PR and initial-contact phases. An altered balance between the agonist and antagonist muscles during the loading response of running alters joint stability and may have negative implications for joint injuries in runners.
Acknowledgments
We thank Christina Liasou, MSc, and Vicki Kouvelioti, MSc, for assisting with data collection.
REFERENCES
- 1.van Mechelen W. Running injuries: a review of the epidemiological literature. Sports Med. 1992;14(5):320–335. doi: 10.2165/00007256-199214050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gerlach K. E., White S. C., Burton H. W., Dorn J. M., Leddy J. J., Horvath P. J. Kinetic changes with fatigue and relationship to injury in female runners. Med Sci Sports Exerc. 2005;37(4):657–663. doi: 10.1249/01.mss.0000158994.29358.71. [DOI] [PubMed] [Google Scholar]
- 3.Derrick T. R., Dereu D., McLean S. P. Impacts and kinematic adjustments during an exhaustive run. Med Sci Sports Exerc. 2002;34(6):998–1002. doi: 10.1097/00005768-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Miller D. I. Ground reaction forces in distance running. In: Cavanagh P. R., editor. Biomechanics of Distance Running. Champaign, IL: Human Kinetics; 1990. pp. 202–224. [Google Scholar]
- 5.Munro C. F., Miller D. I., Fuglevand A. J. Ground reaction forces in running: a re-examination. J Biomech. 1987;20(2):147–155. doi: 10.1016/0021-9290(87)90306-x. [DOI] [PubMed] [Google Scholar]
- 6.Madigan M. L., Pidcoe P. E. Changes in landing biomechanics during a fatiguing landing activity. J Electromyogr Kinesiol. 2003;13(5):491–498. doi: 10.1016/s1050-6411(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 7.Nigg B. M., Cole G. K., Bruggemann G. P. Impact forces during heel-toe running. J Appl Biomech. 1995;11(4):407–432. [Google Scholar]
- 8.Nyland J. A., Shapiro R., Caborn D. N., Nitz A. J., Malone T. R. The effect of quadriceps femoris, hamstring, and placebo eccentric fatigue on knee and ankle dynamics during crossover cutting. J Orthop Sports Phys Ther. 1997;25(3):171–184. doi: 10.2519/jospt.1997.25.3.171. [DOI] [PubMed] [Google Scholar]
- 9.Burr D. B. Bone, exercise, and stress fractures. Exerc Sport Sci Rev. 1997;25:171–194. [PubMed] [Google Scholar]
- 10.Christina K. A., White S. C., Gilchrist L. A. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum Mov Sci. 2001;20(3):257–276. doi: 10.1016/s0167-9457(01)00048-3. [DOI] [PubMed] [Google Scholar]
- 11.Bruggemann G. P. Influence of fatigue on lower extremity function. Paper presented at: XIV Symposium on Biomechanics in Sports; June 25–29, 1996; Funchal, Portugal.
- 12.Nicol C., Komi P. V., Marconnet P. Fatigue effects of marathon running on neuromuscular performance, II: changes in force, integrated electromyographic activity and endurance capacity. Scand J Med Sci Sports. 1991;1:18–24. [Google Scholar]
- 13.Derrick T. R. The effects of knee contact angle on impact forces and accelerations. Med Sci Sports Exerc. 2004;36(5):832–837. doi: 10.1249/01.mss.0000126779.65353.cb. [DOI] [PubMed] [Google Scholar]
- 14.Bates B., Osternig L., Mason B., James S. Lower extremity function during the support phase of running. In: Asmussen E., Jorgensen K., editors. Biomechanics. Vol I-B. Baltimore, MD: University Park Press; 1978. pp. 30–39. [Google Scholar]
- 15.Kim W., Voloshin A. S., Johnson S. H. Modeling of heel strike transients during running. Hum Mov Sci. 1994;13(2):221–244. [Google Scholar]
- 16.Stergiou N., Bates B. T., James S. L. Asynchrony between subtalar and knee joint function during running. Med Sci Sports Exerc. 1999;31(11):1645–1655. doi: 10.1097/00005768-199911000-00023. [DOI] [PubMed] [Google Scholar]
- 17.van Gheluwe B., Madsen C. Frontal rearfoot kinematics in running prior to volitional exhaustion. J Appl Biomech. 1997;13(1):66–75. [Google Scholar]
- 18.Dutto D., Levy M., Lee K. K., Sidthalaw S., Smith G. A. Effect of of fatigue and gender on running mechanics [abstract] Med Sci Sports Exerc. 1997;29(5):S82. [Google Scholar]
- 19.Mizrahi J., Verbitsky O., Isakov E., Daily D. Effect of fatigue on leg kinematics and impact acceleration in long distance running. Hum Mov Sci. 2000;19(2):139–151. [Google Scholar]
- 20.Gazendam M. G., Hof A. L. Averaged EMG profiles in jogging and running at different speeds. Gait Posture. 2007;25(4):604–614. doi: 10.1016/j.gaitpost.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery W. H., 3rd, Pink M., Perry J. Electromyographic analysis of hip and knee musculature during running. Am J Sports Med. 1994;22(2):272–278. doi: 10.1177/036354659402200220. [DOI] [PubMed] [Google Scholar]
- 22.Swanson S. C., Caldwell G. E. An integrated biomechanical analysis of high speed incline and level treadmill running. Med Sci Sports Exerc. 2000;32(6):1146–1155. doi: 10.1097/00005768-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Komi P. V., Kaneko M., Aura O. EMG activity of the leg extensor muscles with special reference to mechanical efficiency in concentric and eccentric exercise. Int J Sport Med. 1987;8(suppl 1):22–29. doi: 10.1055/s-2008-1025700. [DOI] [PubMed] [Google Scholar]
- 24.Kyrolainen H., Avela J., Komi P. V. Changes in muscle activity with increasing running speed. J Sport Sci. 2005;23(10):1101–1109. doi: 10.1080/02640410400021575. [DOI] [PubMed] [Google Scholar]
- 25.Hortobagyi T., Westerkamp L., Beam S., et al. Altered hamstring-quadriceps muscle balance in patients with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2005;20(1):97–104. doi: 10.1016/j.clinbiomech.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kellis E. Quantification of quadriceps and hamstring antagonist activity. Sport Med. 1998;26(1):37–62. doi: 10.2165/00007256-199825010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Padua D. A., Arnold B. L., Perrin D. H., Gansneder B. M., Carcia C. R., Granata K. P. Fatigue, vertical leg stiffness, and stiffness control strategies in males and females. J Athl Train. 2006;41(3):294–304. [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn J. M., Holmes J. D., Andrews D. M. The effect of localized leg muscle fatigue on tibial impact acceleration. Clin Biomech (Bristol, Avon) 2004;19(7):726–732. doi: 10.1016/j.clinbiomech.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Hermens H. J., Freriks B., Merletti R., Hagg G., Stegeman D., Blok J. SENIAM 8: European Recommendations for Surface Electromyography. Enschede, The Netherlands: Roessingh Research and Development; 1999. [Google Scholar]
- 30.Kellis E., Baltzopoulos V. The effects of normalization method on antagonistic activity during concentric and eccentric isokinetic knee extension and flexion. J Electromyogr Kinesiol. 1996;6(4):235–245. doi: 10.1016/s1050-6411(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 31.Patikas D., Michailidis C., Bassa H., et al. Electromyographic changes of agonist and antagonist calf muscles during maximum isometric induced fatigue. Int J Sport Med. 2002;23(4):285–289. doi: 10.1055/s-2002-29079. [DOI] [PubMed] [Google Scholar]
- 32.Kellis E., Kouvelioti V. Agonist versus antagonist muscle fatigue effects on thigh muscle activity and vertical ground reaction during drop landing. J Electromyogr Kinesiol. 2009;19(1):55–64. doi: 10.1016/j.jelekin.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Heiderscheit B. C., Hoerth D. M., Chumanov E. S., Swanson S. C., Thelen B. J., Thelen D. G. Identifying the time of occurrence of a hamstring strain injury during treadmill running: a case study. Clin Biomech (Bristol, Avon) 2005;20(10):1072–1078. doi: 10.1016/j.clinbiomech.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Thomas J. R., Nelson J. K. Research Methods in Physical Activity. Champaign, IL: Human Kinetics; 1990. pp. 343–363. [Google Scholar]
- 35.Gollhofer A., Kyrolainen H. Neuromuscular control of the human leg extensor muscles in jump exercises under various stretch-load conditions. Int J Sports Med. 1991;12(1):34–40. doi: 10.1055/s-2007-1024652. [DOI] [PubMed] [Google Scholar]
- 36.Abe D., Muraki S., Yanagawa K., Fukuoka Y., Niihata S. Changes in EMG characteristics and metabolic energy cost during 90-min prolonged running. Gait Posture. 2007;26(4):607–610. doi: 10.1016/j.gaitpost.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Mizrahi J., Verbitsky O., Isakov E. Fatigue-induced changes in decline running. Clin Biomech (Bristol, Avon) 2001;16(3):207–212. doi: 10.1016/s0268-0033(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 38.Kyrolainen H., Komi P. V., Belli A. Mechanical efficiency in athletes during running. Scand J Med Sci Sports. 1995;5(4):200–208. doi: 10.1111/j.1600-0838.1995.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 39.Kellis E., Baltzopoulos V. Agonist and antagonist EMG-angle relationship during isokinetic eccentric and concentric exercise. Isokinet Exerc Sci. 1996;6(2):79–87. [Google Scholar]
- 40.Bonnard M., Sirin A. V., Oddsson L., Thorstensson A. Different strategies to compensate for the effects of fatigue revealed by neuromuscular adaptation processes in humans. Neurosci Lett. 1994;166(1):101–105. doi: 10.1016/0304-3940(94)90850-8. [DOI] [PubMed] [Google Scholar]
- 41.Madigan M. L., Pidcoe P. E. A muscle temperature compensation technique for EMG fatigue measures. Med Sci Sports Exerc. 2002;34(5):780–784. doi: 10.1097/00005768-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 42.James S. L., Jones D. C. Biomechanical aspects of distance running injuries. In: Cavanagh P. R., editor. Biomechanics of Distance Running. Champaign, IL: Human Kinetics; 1990. pp. 249–270. [Google Scholar]