Abstract
Context:
Reduced hip-abductor strength and muscle activation may be associated with altered lower extremity mechanics, which are thought to increase the risk for anterior cruciate ligament injury. However, experimental evidence supporting this relationship is limited.
Objective:
To examine the changes in single-leg landing mechanics and gluteus medius recruitment that occur after a hip-abductor fatigue protocol.
Design:
Descriptive laboratory study.
Patients or Other Participants:
Twenty physically active women (age = 21.0 ± 1.3 years).
Intervention(s):
Participants were tested before (prefatigue) and after (postfatigue) a hip-abductor fatigue protocol consisting of repetitive side-lying hip abduction.
Main Outcome Measure(s):
Outcome measures included sagittal-plane and frontal-plane hip and knee kinematics at initial contact and at 60 milliseconds after initial contact during 5 single-leg landings from a height of 40 cm. Peak hip and knee sagittal-plane and frontal-plane joint moments during this time interval were also analyzed. Measures of gluteus medius activation, including latency, peak amplitude, and integrated signal, were recorded.
Results:
A small (<1°) increase in hip-abduction angle at initial contact and a small (<1°) decrease in knee-abduction (valgus) angle at 60 milliseconds after contact were observed in the postfatigue landing condition. No other kinematic changes were noted for the knee or hip at initial contact or at 60 milliseconds after initial contact. Peak external knee-adduction moment decreased 27% and peak hip adduction moment decreased 24% during the postfatigue landing condition. Gluteus medius activation was delayed after the protocol, but no difference in peak or integrated signal was seen during the landing trials.
Conclusions:
Changes observed during single-leg landings after hip-abductor fatigue were not generally considered unfavorable to the integrity of the anterior cruciate ligament. Further work may be justified to study the role of hip-abductor activation in protecting the knee during landing.
Keywords: anterior cruciate ligament, gluteus medius muscle, kinematics, kinetics, electromyography
Key Points.
Following a hip-abductor fatigue protocol, hip and knee kinematics at initial contact and at 60 milliseconds after landing did not change.
However, during the first 60 milliseconds of the postfatigue landing condition, external hip- and knee-adduction moments decreased by 24% and 27%, respectively. Gluteus medius onset latency was reduced after the fatigue protocol.
Lower extremity biomechanics were unaffected by hip-abductor fatigue in ways that are believed to increase the risk of anterior cruciate ligament injury.
Despite efforts in research and prevention, anterior cruciate ligament (ACL) injuries continue to occur 3 to 5 times more often in females compared with males.1–3 Many of these injuries are believed to occur during single-leg landings, with no direct blow to the knee.4–6 Although the causes of these injuries are not known, they are widely believed to include some combination of environmental, biomechanical, neuromuscular, structural, and hormonal factors.7–9
Authors10–12 studying biomechanical and neuromuscular risk factors for ACL injury have suggested that weakness of muscles that abduct the hip, such as the gluteus medius, may predispose an individual to greater hip adduction or internal-rotation excursion during weight-bearing activities such as jumping or landing. These aberrant hip movements are thought to lead to increased knee-abduction angles and moments for such individuals.10–12 In the presence of anterior tibiofemoral shear forces, such as those on the tibia from the quadriceps during jumping or landing, increased knee-abduction angles or moments may further increase ACL strain and risk for ACL injury.13,14
Experimental support for this intuitive link between hip-abductor weakness and hazardous knee-joint mechanics is mixed. For example, females with greater hip external-rotation strength experienced smaller peak external knee-abduction moments during a single-leg landing than females with smaller hip external-rotation strength values.15 An inverse relationship between hip-abductor eccentric peak torque and peak knee-abduction angle among females has also been reported.16 Finally, a computer model of lower extremity frontal-plane mechanics indicated increased frontal-plane hip stiffness as a consequence of either increased hip-abductor anticipatory muscle activation or increased hip-abductor strength serving to protect the ACL.17 However, contradictory findings have been reported by authors18,19 whose data revealed only a weak association between hip-abduction or external-rotation strength and knee-joint motions considered detrimental to the ACL during jumping or landing.
It is worth noting that previous studies of the link between hip-abductor weakness and altered lower extremity mechanics have been cross sectional in nature. As such, it was impossible to discern a cause-and-effect relationship between these phenomena. Experimental study designs that first change hip-abduction strength and then measure the effect of this change on lower extremity joint mechanics are necessary to examine this potential relationship. For example, investigators20 have recently experimentally reduced gluteus medius muscle function using intramuscular hypertonic saline injections to elicit a pain response that limits activation of the gluteus medius during walking. Muscle fatigue may be another way of experimentally inducing weakness of the hip abductors to study the effect on lower extremity mechanics during impact activities. Indeed, decreased ability of a muscle to produce force is a defining characteristic of muscle fatigue.
Presently, limited data describe relationships between hip-abductor fatigue and hip- or knee-joint mechanics during simulated athletic events such as single-leg landings. Further, those data that are available are limited to hip- and knee-joint kinematics.21 Additional analysis of the effect of hip-abductor weakness secondary to fatigue on joint kinetics during landing may be useful for the development of effective ACL injury-prevention and -treatment programs. Therefore, the primary purpose of our study was to analyze the effect of a hip-abductor fatigue protocol on hip- and knee-joint kinematics and kinetics during single-leg landings in recreational women athletes. We hypothesized that after hip-abductor fatigue, participants would demonstrate increased hip-adduction and knee-abduction angles and external hip-adduction and knee-abduction moments during single-leg landings.
A 2-dimensional computer model of the lower extremity indicated that delayed or reduced hip-abductor activation may also increase ACL injury risk during weight-bearing activities.17 Therefore, we also chose to evaluate potential changes in neuromuscular recruitment variables of the gluteus medius in response to prolonged exertion leading to muscle fatigue during single-leg landings. Such information may be useful to delineate whether observed changes in lower extremity mechanics were a consequence of altered neuromuscular recruitment, decreased muscular capacity to produce force, or both. We hypothesized that gluteus medius muscle activity would be diminished and delayed relative to contact with the floor during a single-leg landing after fatigue.
METHODS
Participants
In this study, we sought to identify a change in hip or knee frontal-plane kinematics greater than 3°. This change in joint kinematics represents a large effect size (0.7) relative to the variability of these measures established in a previous study22 using similar methods and instruments. A total of 16 participants was necessary to identify this difference between conditions with α = .05 and β = 0.2.23 Therefore, 20 physically active women from a university campus (age = 21.0 ± 1.3 years, height = 167.9 ± 5.9 cm, mass = 61.8 ± 8.4 kg) were recruited for this study. Being physically active was defined as participating in aerobic or athletic activity at least 3 times per week for at least 30 minutes each time. Participants were free of injury during the time of testing and had no history of lower extremity injury that required surgery. Seven of these participants were National Collegiate Athletic Association Division III track-and-field athletes, and 1 was left-leg dominant. The dominant leg was determined by asking which leg was preferred for kicking a ball. Participation was voluntary, and those who agreed to proceed with the study read and signed an informed consent form approved by the university's institutional review board (which also approved all procedures) before any testing began.
Data Collection
Maximum isometric strength of the hip abductors was tested using a Nicholas MMT handheld dynamometer (model 01160; Lafayette Instruments Company, Lafayette, IN) and stabilization strap.24 Within-testers reliability for this strength test has intraclass correlation coefficients between 0.93 and 0.97.25 Each participant was positioned such that she was lying on the side of the nondominant leg, with the dominant-leg knee straight and the dominant hip in 0° of flexion, abduction, and external rotation. To maintain consistent placement of the handheld dynamometer for all strength tests, a mark on the skin was placed 2.5 cm proximal to the lateral femoral epicondyle of the dominant leg. The handheld dynamometer was placed on this mark for all strength tests. Next, a stabilization strap was placed around the dynamometer, leg, and table with enough slack so that when the participant abducted her leg, the hip was in 0° of hip abduction.24 Participants performed three 5-second maximum voluntary isometric contraction (MVIC) trials, with 2 minutes' rest between trials. During each MVIC trial, the participant was instructed not to flex the knee and to keep the toes of the top leg pointing forward to help prevent alterations in muscle recruitment and compensation during testing.26 The largest isometric force obtained during one of the MVIC trials was used as the baseline hip-abduction strength measure.
Electromyographic (EMG) activity of the gluteus medius was recorded during all isometric strength tests, during all drop-landing trials, and during the first and last 30 seconds of the fatigue protocol. Before the electrodes were placed, the skin was abraded and cleaned with isopropyl alcohol. Next, the surface electrode was applied superior to the greater trochanter on a line to the most lateral aspect of the iliac crest, parallel to the direction of the gluteus medius muscle fibers.27 After visually inspecting the signal-to-noise ratio during resisted hip abduction, the single differential surface electrode (model DE-2.1; Delsys Inc, Boston, MA) with an interelectrode distance of 10 mm and a common mode rejection ratio of 92 dB was taped down to reduce movement and artifact.28 An elastic wrap was then applied over the surface electrode to limit movement artifact and reduce the likelihood that the electrode would move during testing. A ground electrode was placed over the clavicle. The surface electrode was sampled at 1200 Hz and was interfaced with an amplifier unit (model Bagnoli-8; Delsys Inc) with a gain setting of 1000. The EMG signals were processed through a 12-bit A/D board synchronized with the force platform and stored on a personal computer. The EMG signals were subsequently high-pass filtered using a bidirectional, fourth-order Butterworth filter with a cutoff frequency of 30 Hz, then full-wave rectified and low-pass filtered using a bidirectional fourth-order Butterworth filter with a cutoff frequency of 6 Hz and normalized to peak activity recorded during the MVIC trial.29
Processed EMG data were analyzed with a customized MATLAB program (The MathWorks, Inc, Natick, MA) to determine hip-abductor activity latency relative to initial contact with the force plate during single-leg landings, peak EMG amplitude, and integrated EMG (iEMG) in the prefatigue and postfatigue landing trials. Hip-abductor muscle activity onset was determined by examining a 500-millisecond window before contact with the force platform. A 10-N threshold was used to determine the onset of the vertical ground reaction force. The threshold voltage required for muscle onset was calculated from 5 SDs above the resting mean at baseline. Muscle activity onset before ground contact was determined by comparing data points with the threshold voltage. When the mean voltage of the 100-millisecond window exceeded this threshold voltage, that data point represented the onset of muscle activity.30 The area under the curve of the low-pass–filtered data then was calculated using trapezoidal integration to determine the iEMG for each landing. The reliability of our EMG variables was not calculated as part of this study. However, the reliability of these variables has been found to be good to excellent in previous studies31–33 of lower extremity muscle activation during dynamic movements (intraclass correlation coefficients = 0.81–0.93).
After completing the baseline strength test, volunteers were prepared for motion testing. Reflective markers (diameter = 14 mm) were placed on the participant's dominant leg and pelvis in a modified Helen Hayes configuration. The 3-dimensional coordinates of these markers were used to track motion of the pelvis, femur, shank, and foot, each modeled as a rigid body. Specifically, markers were placed on the right and left anterior-superior iliac spine, sacrum, thigh of the dominant leg approximately 15 cm above the superior pole of the patella (at the estimated line of the knee-joint center), lateral and medial femoral condyles, lateral tibia (halfway between the ankle and knee), tibial tuberosity, lateral and medial malleoli, posterior portion of the calcaneus (on the shoe), superior navicular region on the foot, and web space between metatarsals 1 and 2 (on the shoe) on the dominant leg.34 All participants wore the same type of running shoe (model 629; New Balance, Boston, MA) to reduce variability caused by different shoe-absorption properties.
All kinematic data were collected at 240 Hz using 8 Eagle digital cameras (Motion Analysis Corporation, Santa Rosa, CA) positioned around the performance area. Kinetic data from a force platform (model 4060 NC; Bertec Corporation, Columbus, OH) were sampled at 1200 Hz and synchronized with motion-capture data at the same frequency and cutoff.35 Marker trajectories were filtered at 15 Hz using a low-pass, fourth-order Butterworth recursive filter. Hip-, knee-, and ankle-joint centers were calculated using the coordinates from a static neutral standing trial with the participant facing the positive x-axis of the laboratory coordinate system. The hip-joint center was found relative to the anterior-superior iliac spine as a component of leg length and greater trochanter location.36 The knee-joint center was assumed to be at the midpoint of a line between the femoral condyles. The ankle-joint center was assumed to be at the midpoint of a line between the 2 ankle malleoli markers. Joint kinematics were calculated from the filtered 3-dimensional marker coordinate data using a Euler angle calculation with the assumption that flexion-extension was the first rotation, followed by abduction and internal-external rotation. Joint kinematic conventions were defined using the right-hand rule. Therefore, knee frontal-plane adduction (varus) and abduction (valgus) angular kinematics were assigned positive and negative values, respectively. Kinematic data were not normalized to the static neutral trial. That is, 0° corresponded with an erect posture at the hip and knee. At the ankle, 0° corresponded with the foot segment flat on the ground and the toes straight ahead at a right angle to the shank.
All joint moments referred to in this paper are external joint moments or moments applied from the impact forces to the structures within, transformed into the distal segment reference frame.37 Joint angles and moment values were calculated by Motion Monitor software (version 7.0; Innovative Sports Training Inc, Chicago, IL). Moments were calculated using the inverse dynamics approach38 and anthropometric data from Dempster.39 Knee-extensor moments and hip-flexor moments were assigned positive values. Knee-abduction and knee-adduction joint moments were assigned positive and negative values, respectively.
Each participant performed 5 single-leg drop landings from the hang bar before the fatigue protocol (prefatigue) and 5 drop landings immediately after the fatigue protocol (postfatigue). All landing trials were performed from a height of 40 cm onto a force platform. The height of the hang bar was determined by having each participant hang with her arms and body completely extended and feet parallel to the ground. The height was adjusted such that the participant's feet were 40 cm from the ground. Participants were required to land on the dominant leg and were instructed to land as comfortably and normally as possible without falling over, stepping off the force platform, or touching the ground with either their hands or nondominant leg. For both conditions, participants were told that the landing trials were to be completed with as little delay between trials as possible. They were asked to practice the single-leg landing trials to ensure that they could complete 5 trials in fewer than 60 seconds during the prefatigue and postfatigue landing conditions. We chose to investigate single-leg landing trials instead of double-leg landings because most ACL injuries are thought to occur during single-leg landings and asymmetries often occur between legs in double-leg landings with regard to force and impulse.5,40
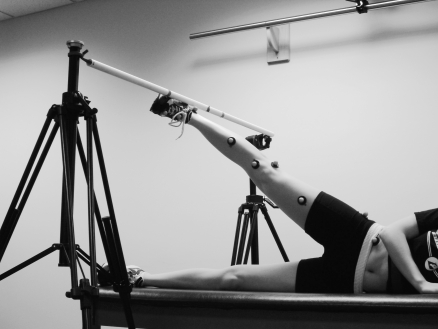
Our intent was to examine the effects of isolated hip-abductor fatigue on lower extremity landing mechanics in recreationally active women. Therefore, the fatigue protocol for the gluteus medius required participants to repeatedly abduct the hip to 30° while in a side-lying position, rather than in a functional weight-bearing activity that might have involved several muscle groups. A plastic bar was positioned over the participant's feet and set to a height that corresponded to 30° of hip abduction with the knee fully extended as measured using a standard goniometer (Figure 1). The bar gave the participant a fixed target for achieving 30° of hip abduction for every repetition and also offered tactile feedback relative to goal achievement. Each participant was then instructed to raise and lower the leg to this reference angle at a pace of 60 beats per minute as provided by a digital metronome (model 96204X; Mel Bay Publications, Pacific, MO) until she reported a Borg perceived exertion scale rating of 19 or greater (on a 6–20 scale) and she failed to touch the bar on 2 consecutive repetitions at the proper tempo. Volunteers were coached by the investigator not to flex or externally rotate the hip during the protocol. Hip-abduction strength was tested again when the 2 fatigue criteria were met, followed immediately by 5 drop landing trials (postfatigue condition). All 5 drop landings had to be completed within 60 seconds of the end of the fatigue protocol.
Figure 1.
Participant positioning for the hip abductor fatigue protocol.
We made 3 efforts to quantify fatigue and validate the exertion protocol used in this study. First, ratings of perceived exertion were recorded every 10 seconds during the exertion protocol. These ratings have been found to increase during activities intended to induce local muscle fatigue.41 Second, average EMG (aEMG) amplitude and mean power frequency (MPF) in the first and last 30 seconds of the hip-abduction fatigue protocol were calculated to examine how the musculature responded to this isolated fatigue protocol. The filtered EMG data over this time were divided into six 5-second sections. The aEMG amplitude was determined by summing these EMG values for each section and dividing by the number of samples. The MPF for each section was determined based on the power density spectrum of these data, obtained using a Hamming window function followed by a fast Fourier transform. The aEMG and MPF values for all 6 sections were then calculated and used for analysis. Previous authors42–44 reported increased aEMG amplitude and decreased MPF (spectral compression) associated with both isotonic and dynamic muscular contractions in a fatigued state. Third, fatigue has been defined as a reduction in the force-generating capacity of the neuromuscular system that occurs during sustained activity.45 Therefore, peak hip-abductor strength immediately after the exertion protocol was compared with hip-abductor strength values before the protocol.
Injuries to the ACL have been reported46 as likely to occur within the first 60 milliseconds of ground contact. Thus, EMG, kinematic, and kinetic data from the first 60 milliseconds of landing performance were analyzed. Specific EMG variables included hip-abductor muscle-activation latency relative to initial contact and peak EMG and iEMG amplitude during the first 60 milliseconds of landing. Kinematic variables of interest included frontal-plane pelvis orientation and hip and knee frontal-plane and sagittal-plane joint angles at initial contact and at 60 milliseconds after initial contact. Joint kinetic variable of interest included maximum knee-extension moment, knee-abduction moment, hip-flexion moment, and hip-adduction moment during the first 60 milliseconds after initial contact with the force plate during each landing.
A paired-samples t test was performed for each dependent variable of interest using SPSS for Windows (version 16.0; SPSS Inc, Chicago, IL). The independent variable was fatigue state (prefatigue, postfatigue). All postfatigue landing trials were completed within 1 minute (mean, 47.2 ± 8.5 seconds) of completion of the fatigue protocol. Previous analysis47 of the recovery time of the quadriceps muscle after fatigue from repetitive exertion revealed that muscle weakness was reduced for up to 1 minute after exertion ceased. Although the recovery time for the gluteus medius may be greater or smaller than the recovery time for the quadriceps, we calculated the average of the dependent variables from the 5 prefatigue and postfatigue landing trials in our statistical analysis. The alpha level was set to .05 for all statistical tests.48
RESULTS
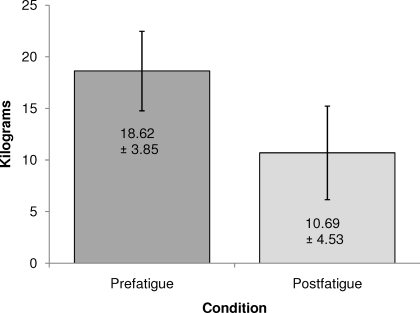
Average time to fatigue was 179.4 ± 43.2 seconds. Average time to complete all 5 postfatigue landing trials was 47.2 ± 8.5 seconds. Peak isometric hip-abduction strength decreased by 43% at the end of the fatigue protocol (effect size = 1.89, P < .05; Figure 2).
Figure 2.
Average isometric hip-strength prefatigue and postfatigue.
Electromyography
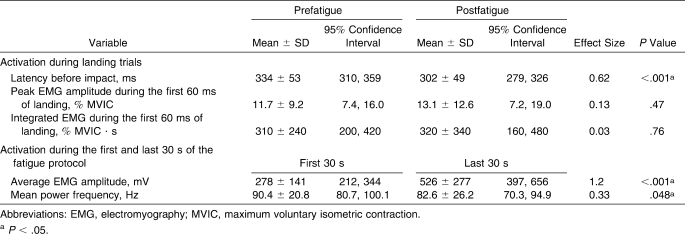
Participants demonstrated an 89% increase in hip-abductor aEMG amplitude (P < .05) and 8.6% decrease in MPF (P < .05) over the course of the fatigue protocol (Table 1). After the fatigue protocol, hip-abductor latency decreased by 9.5% (32 milliseconds) during single-leg landings (P < .05). No differences were found in normalized peak EMG amplitude or iEMG for the hip abductors between the prefatigue and postfatigue landing trials.
Table 1.
Hip-Abductor EMG Measures Before and After Fatigue
Initial-Contact Joint Kinematics
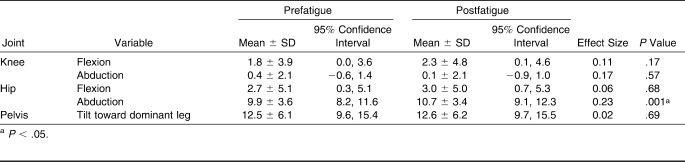
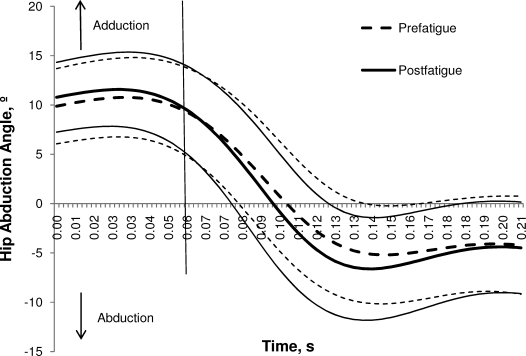
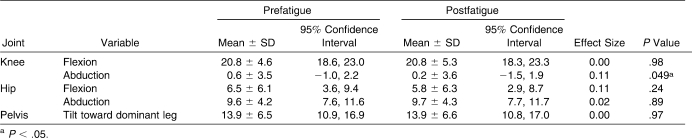
Hip- and knee-joint kinematics at initial contact were similar between the prefatigue and postfatigue conditions (Table 2). No differences between conditions were found for knee-flexion angle, knee-adduction angle, hip-flexion angle, or frontal-plane pelvic tilt. A small (0.8°) increase in hip-abduction angle was observed after the fatigue protocol (P < .05; Figure 3).
Table 2.
Joint Kinematics at Initial Contact and Before and After Fatigue, °
Figure 3.
Average time-normalized (±1 SD) hip-abduction angle during the landing trials before and after fatigue. The vertical line represents 60 milliseconds.
Joint Kinematics at 60 Milliseconds After Landing
Knee-flexion angles were similar during the prefatigue and postfatigue landing conditions (P > .05; Table 3). A very small (0.4°) yet significant reduction in knee abduction was noted after the fatigue protocol (P < .05). Participants displayed no differences in hip sagittal-plane, hip frontal-plane, or pelvis frontal-plane kinematics at 60 milliseconds after the single-leg landing.
Table 3.
Joint Kinematics at 60 ms Following Initial Contact During a Single-Leg Landing Before and After Fatigue, °
Peak External Joint Moments During the First 60 Milliseconds of Landing
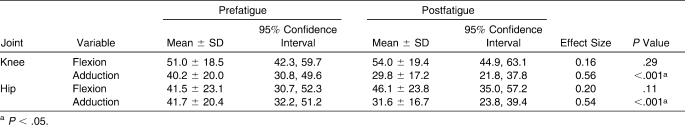
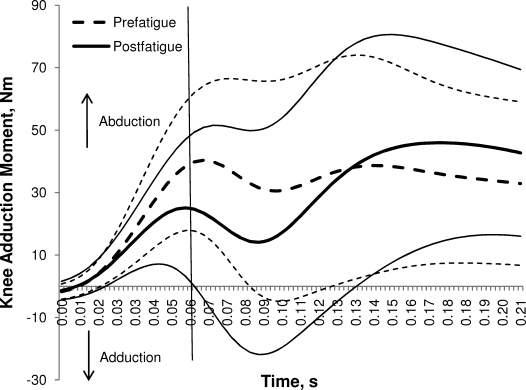
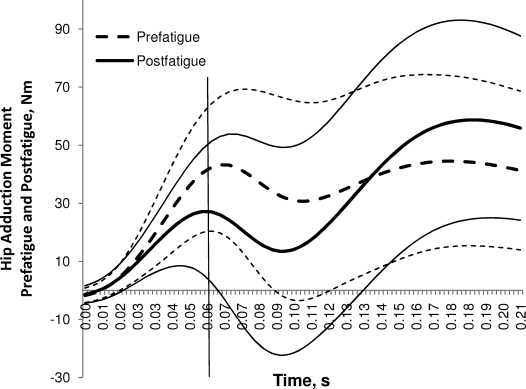
Participants demonstrated 27% (10 Nm) smaller peak knee-adduction moments (P < .05) and 24% (10 Nm) smaller peak hip-adduction moments (P < .05) in the postfatigue condition (Table 4; Figures 4 and 5). No differences were found with respect to peak sagittal-plane knee-flexion or hip-flexion moment between conditions (P > .05).
Table 4.
Peak External Joint Moments in the First 60 ms Following Initial Contact During Single-Leg Landings Before and After Fatigue, Nm
Figure 4.
Average time-normalized (±1 SD) knee-adduction moment for the first 60 milliseconds of landing prefatigue and postfatigue. The vertical line represents 60 milliseconds.
Figure 5.
Average (±1 SD) hip-adduction moment for the first 60 milliseconds prefatigue and postfatigue. The vertical line represents 60 milliseconds.
Vertical Ground Reaction Force
Peak vertical ground reaction force was 2% smaller after the fatigue protocol (Table 5). However, this difference was not statistically significant (P > .05).
Table 5.
Peak Vertical Ground Reaction Force Before and After Fatigue, N
DISCUSSION
Hip-muscle weakness has been implicated as a risk factor for ACL injuries in women during high-risk activities such as landing.8,10,12 The purpose of our study was to induce weakness of the hip abductors through prolonged exertion and then test for changes in lower extremity mechanics and hip-abductor activation thought to be relevant to ACL injuries during single-leg landings. The fatigue protocol coincided with a 43% reduction in peak hip-abduction isometric strength immediately before the single-leg landings. The effect size for the change in strength after the protocol suggests that a large, clinically significant change in hip-abductor strength existed between the prefatigue and postfatigue conditions and speaks to the validity of the isolated fatigue protocol for the purpose of this study.
Electromyography
Hip-abductor EMG data were recorded during the first and last 30 seconds of the fatigue protocol to provide further objective evidence of skeletal muscle fatigue resulting from the protocol. Authors of previous EMG studies of skeletal muscle response to prolonged exertion have examined the quadriceps muscles. Most of these researchers49,50 reported increased EMG activity and decreased MPF associated with a reduction in force-generating capacity from fatigue. However, the 89% increase in aEMG amplitude we recorded at the end of the protocol was larger than expected, and the 8.6% decrease in MPF was less than in previous reports. These findings may have occurred because most gluteus medius muscle fibers are type I, whereas most quadriceps muscle fibers are type II.51 Type I fibers may be more prone to increases in EMG amplitude and more resistant to reductions in MPF associated with fatigue than type II fibers.49,50,52,53 Thus, greater increases in aEMG and smaller decreases in MPF relative to prolonged exertion exercise may be expected for measurements recorded from the gluteus medius compared with the quadriceps muscle group. Future study of the EMG response gluteus medius muscle fatigue appears warranted to substantiate this hypothesis. However, we believe that these EMG changes in conjunction with the 43% reduction in peak hip-abductor force and Borg rating of perceived exertion values of 19/20 during the final 30 seconds of the fatigue protocol provide sufficient evidence of hip-abductor fatigue before the postfatigue landing condition. It is interesting to note that gluteus medius iEMG and peak EMG values did not change from prefatigue to postfatigue, which suggests that the number of motor units recruited during landing may not have changed between conditions. In this case, decreased capacity of the gluteus medius to create force may be attributed to diminished effectiveness of the biochemical cross-bridge cycle within the muscle fiber, rather than a reduction in the number of motor units recruited during landing between the prefatigue and postfatigue landing conditions.
Hip-abductor muscle activity occurred 32 milliseconds later during the postfatigue landing condition. Prolonged exertion is believed to cause changes in both peripheral and central elements of neuromuscular control, referred to as peripheral fatigue and central fatigue, respectively. Peripheral fatigue refers to a decrease in the ability of a muscle to produce force because of changes that occur at or distal to the neuromuscular junction, whereas central fatigue refers to an exercise-induced reduction in voluntary activation of a muscle because of changes that occur in a motor unit proximal to the neuromuscular junction. A recent study54 provides evidence that central fatigue alone may be sufficient to produce measureable changes in lower extremity mechanics during single-leg landings. The reduction in gluteus medius activation latency we observed may also be the result of central fatigue effects, such as delayed anticipatory activation from the motor cortex or decreased α motor neuron conduction velocity from reduced excitability of the motor neuron membrane after repetitive activations.55
A decrease in activation latency of the gluteus medius during the postfatigue condition is equivalent to a reduction in anticipatory activation. It has been argued17 that a decrease in the anticipatory activation or strength of the hip abductors would decrease the stiffness of the hip joint in the frontal plane. Joint torque is the product of muscle force and muscle moment arm. Therefore, decreased peak strength would directly affect the capacity of a muscle to produce joint torque. Depending on the magnitude of the delay between neural stimulation of a muscle and the development of muscle tension (electromechanical delay), decreased anticipatory activation may also have an effect on joint torque. Participants in this study demonstrated both decreased peak isometric strength and decreased anticipatory activation of the hip abductors after the fatigue protocol. Theoretically, then, each of these changes may contribute to the decreased external hip-adductor moments recorded during the first 60 milliseconds after impact from the single-leg landing. To our knowledge, electromechanical delay of the gluteus medius has not been reported. Thus, the clinical significance of the decreased gluteus medius activation latency observed in this study with respect to decreased hip-adduction moment during the postfatigue landing condition is unclear. However, the data from this study are consistent with those of previous authors indicating that decreased activation latency or muscle strength may decrease external hip-adduction moment.
As noted earlier, decreased gluteus medius activation latency may be a consequence of some change in central control associated with participation in the isolated fatigue protocol. Some54 have argued that targeted training of central control mechanisms may more effectively minimize the likelihood of altered lower extremity mechanics linked with ACL injury risk than interventions focused on strengthening alone. Indeed, adding visual feedback to a general lower extremity strengthening program has recently been found to elicit greater changes in frontal-plane hip kinematics during jumping than a strengthening program alone.56,57 Additionally, Mizner et al58 reported decreases in peak vertical ground reaction force, peak knee-abduction angle, and peak external knee-abduction moment after verbal instruction before bilateral jump landings. The mechanism for these greater changes is not entirely clear, but the feedback provided in these studies may have led to earlier anticipatory muscle activation, increased hip- and knee-joint stiffness, and smaller hip- and knee-joint frontal-plane kinematic excursion.59
Kinematic and Kinetic Data
Weakness of the hip abductors has been suspected of contributing to many lower extremity injuries, including ACL tears, as a consequence of altered lower extremity mechanics.10,11,60,61 Authors18,22,62,63 of several cross-sectional studies have linked hip-abductor weakness with increased knee-abduction and hip-adduction motion during dynamic weight-bearing activities in female athletes. However, our main finding was that fatigue effects, including a 43% decrease in peak hip-abduction strength, did not lead to meaningful changes in hip or knee kinematics or kinetics during the first 60 milliseconds of a single-leg landing, which are traditionally considered detrimental to the ACL.
Participants demonstrated 0.8° and 0.1° greater hip abduction at initial contact and 60 milliseconds after impact, respectively, during the postfatigue condition. Therefore, average hip-adduction excursion was 0.7° greater during the first 60 milliseconds of landing after the fatigue protocol. Although the greater hip abduction at initial contact was statistically significant, the clinical relevance of such a small change is questionable. Willson et al19 also reported no meaningful change in hip-adduction kinematics during single-leg jumps, despite a 21% decrease in peak hip-abduction strength. Conversely, potentially meaningful effects of hip-abductor exertion were reported by Jacobs et al,18 who found a 2.5° increase in hip-adduction motion during single-leg landings after a 30-second submaximal hip-abductor exercise protocol. However, these authors also reported a low correlation between hip-abduction strength and hip-adduction motion (r = −0.40), which suggests that hip-abduction peak strength accounts for only a small proportion of the change in hip-adduction motion. The seemingly small effect decreased hip-abductor strength had on lower extremity kinematics may result from the gluteus medius not being highly active during single-leg landings in our participants. Activity during the first 60 milliseconds of the prefatigue landing condition was only 12% of maximum voluntary contraction. Therefore, the demands of this task may have been insufficient to elicit large changes in frontal-plane kinematics. Tasks that involve greater acceleration or deceleration in the frontal plane may require greater gluteus medius activation and a more significant change in kinematics after fatigue.
Women in the current study also did not demonstrate a meaningful increase in knee-abduction angles at initial contact or at 60 milliseconds following the single-leg landing after the hip-abductor fatigue protocol. This result supports the findings of previous related studies. For example, recreationally active college students (males and females) demonstrated less than 1° greater knee abduction at initial contact and an even smaller change in knee-abduction excursion during a double-leg landing immediately after a hip-abductor fatigue protocol. 21 Similarly, male and female volunteers had no change in frontal-plane knee motion after a submaximal hip-abductor exercise protocol.18 Taken together, these studies suggest that both hip- and knee-joint frontal-plane kinematics are largely unaffected by the neuromuscular effects of hip-abductor fatigue during the activities tested to date. Activities that place greater demands on the hip abductors may yield larger kinematic effects than those shown in this investigation. Additionally, participants in this study were not selected based on their tendency to demonstrate altered hip or knee frontal-plane kinematics. However, consistent evidence supports the possibility that extraordinary emphasis placed on increasing hip-abductor strength or endurance may not have a meaningful effect on frontal-plane hip or knee kinematics.
In contrast with the kinematic results, the external hip- and knee-joint frontal-plane moments were markedly reduced during the postfatigue landing condition. Kinetic analysis of single-leg landing mechanics revealed a 27% decrease in knee-adduction moment after the hip-abductor fatigue protocol. External knee-abduction moments experienced during bilateral drop landings are among the best known predictors of ACL injury risk.14,64 However, external knee-adduction moments, rather than internal abduction moments, were observed in this study. This is not unprecedented: external knee-adduction moments have been previously reported in females during single-leg activities.15,65 Although a prospective study14 suggested that external knee-abduction moments best predict ACL injury risk, cadaveric data13 indicated that ACL loads increase when either a 10-Nm external knee-abduction or -adduction moment is applied to the knee in the presence of an anterior tibial force. In the context of our findings, these cadaveric data suggest that kinetic changes at the knee as a consequence of an isolated hip-abductor fatigue protocol may actually reduce ACL loads during single-leg landings.
Our kinetic analysis also revealed a 24% decrease in external hip-adduction moment after the hip-abductor fatigue protocol. Thus, the internal moment required by the hip muscles is reduced after fatigue. These kinetic findings support the recent work of Henriksen et al,20 who reported decreased external knee- and hip-adduction moments during walking as a consequence of impaired gluteus medius muscle function from intramuscular injections. Peak vertical ground reaction force was unchanged from the prefatigue to the postfatigue condition. Therefore, it is unlikely that these findings are due to decreased vertical ground reaction force between conditions. Kinematic compensations may have occurred in response to hip-abductor fatigue that explain the change in hip- and knee-adductor moments during the postfatigue landing condition. Increased lateral trunk sway has been found to decrease hip- and knee-joint adduction external moments during walking.66 Hence, it is possible that participants compensated for hip-abductor weakness with increased lateral trunk flexion toward the dominant leg before or shortly after contact during landing, thereby reducing the internal moment required by the hip musculature. The slightly greater hip-abduction angle at initial contact may support this explanation. Unfortunately, we did not place markers on the trunk or upper extremities to test this hypothesis.
The methods used to generate hip-abductor fatigue vary significantly in the recent literature. Some authors employed a weight-bearing protocol, presumably under the premise that fatigue elicited in this manner may generalize better to functional activities.21 Other researchers used non–weight-bearing protocols, presumably for increased control in generating a fatigued state specific to muscles that abduct the hip. The non–weight-bearing hip-abductor fatigue protocol we used has also been used by previous investigators.67 However, in contrast to the previous study, we applied no additional resistance to the participant's leg during the fatigue protocol for 2 reasons. First, through pilot studies, we found that even modest external resistance dramatically decreased the time volunteers could participate in the fatigue protocol to under 1 minute. Many athletes are active in events that last much longer than 1 minute without rest, and we felt that our results might not generalize well to such athletes if the fatigue protocol was too short. Second, low-intensity, long-lasting fatigue protocols have resulted in greater and more persistent reductions in quadriceps muscle force-generating capacity.47 Therefore, this low-intensity, long-lasting protocol may result in a greater reduction in gluteus medius force-generating capacity than if external resistance had been applied to the leg during the protocol, resulting in a shorter exercise bout.
Our study had several limitations. First, because of movement of the electrode over the underlying muscles as well as contamination of the EMG signal from nearby muscles, we were restricted in our ability to discern which muscles were affected by the hip-abductor fatigue protocol. Similarly, although human performance studies using surface-based motion analysis are considered traditional and acceptable techniques for estimating joint kinematics, a marker placed on the skin and the motion artifact associated with the data collection may not accurately reflect underlying bone translations and rotations, particularly in the transverse plane.68 These errors are further propagated by the estimation of joint forces and moments derived from the inverse dynamic approach.
Fatigue and the subsequent recovery from fatigue are complex phenomena apparently mediated by both central and peripheral mechanisms. Peripheral muscle fatigue recovery is thought to vary with how much lactate the individual produces, as well as phosphorylcreatine resynthesis efficiency.69 For most people, phosphorylcreatine resynthesis is believed to require roughly 2 minutes; significant strength deficits in lower extremity skeletal muscles such as the quadriceps have been found despite 1 minute of rest.47,69 Perhaps as a result of afferent signals from fatigued muscles, central fatigue continues as long as peripheral fatigue exists.70 Participants completed the single-leg landing postfatigue motion tests in less than 1 minute after the fatigue protocol ended. Despite this timeline, however, our data could indicate signs of recovery from completion of the fatigue protocol to completion of the postfatigue landing trials. Although aEMG increased 89% during the fatigue protocol, iEMG and peak EMG data were not different between the prefatigue and postfatigue drop-landing conditions, which may indicate that the effects of the fatigue protocol did not persist throughout the postfatigue landing condition.
For the purpose of this study, fatigue of the hip abductors was intended to simulate decreased hip-abductor strength. Fatigue has been defined as any reduction in the maximal capacity to generate force or power output.45 Indeed, the maximum isometric force produced by the hip abductors after the fatigue protocol decreased by 43%. Yet this decrease in force is likely accompanied by central fatigue, as well as muscle neurologic and physiologic effects that would not exist among individuals with decreased hip-abduction strength before exertion. Therefore, the generalizability of these findings to all people with decreased hip strength can be questioned.
Lastly, as with all studies, these results should be viewed with respect to the characteristics of the participants. Our volunteers were highly active women with no current injuries or history of lower extremity surgery, including ACL reconstruction. Seven of our participants were National Collegiate Athletic Association athletes. Thus, these were healthy women who managed to participate in recreational or competitive sports, presumably for many years, and yet avoid injury to their ACLs. Perhaps they demonstrated unique lower extremity biomechanics or possessed neuromuscular recruitment strategies that inherently limited their risk of ACL injury. As such, these findings may not generalize well to less active or gifted athletes.
CONCLUSIONS
We examined single-leg landing mechanics and gluteus medius EMG response to a hip-abduction fatigue protocol in women. Our results revealed no meaningful changes in hip or knee kinematics at initial contact or at 60 milliseconds after landing. However, external hip-adduction moment decreased 24% and knee-adduction moment decreased 27% during the first 60 milliseconds of the postfatigue landing condition. Further, gluteus medius onset latency was reduced after the fatigue protocol, potentially indicating reduced anticipatory muscle activation because of fatigue of centrally mediated neuromuscular control mechanisms. Based on these findings, lower extremity mechanics did not appear to change as a result of hip-abductor fatigue in ways that are consistent with biomechanics believed to increase the risk of ACL injury. Training programs intended to reduce the incidence of ACL injury may not benefit from extraordinary emphasis on hip-abductor strength or endurance.
Acknowledgments
We gratefully acknowledge funding from the University of Wisconsin Graduate Student Research, Service, and Educational Leadership (RSEL) grant program.
REFERENCES
- 1.Agel J., Arendt E. A., Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld T. N., Schmitt D. J., Hendy M. P., Mangine R. E., Noyes F. R. Incidence of injury in indoor soccer. Am J Sports Med. 1994;22(3):364–371. doi: 10.1177/036354659402200312. [DOI] [PubMed] [Google Scholar]
- 3.Malone T. R., Hardaker W. T., Garrett W. E., Feagin J. A., Bassett F. H. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J South Orthop Assoc. 1993;2(1):36–39. [Google Scholar]
- 4.Mercer T. H., Gleeson N. P., Wren K. Influence of prolonged intermittent high-intensity exercise on knee flexor strength in male and female soccer players. Eur J Appl Physiol. 2003;89(5):506–508. doi: 10.1007/s00421-003-0830-6. [DOI] [PubMed] [Google Scholar]
- 5.Olsen O. E., Myklebust G., Engebresten L., Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 6.Yu B., Kirkendall D. T., Garrett W. E., Jr Anterior cruciate ligament injuries in female athletes: anatomy, physiology, and motor control. Sports Med Arthrosc Rev. 2002;10(1):58–68. [Google Scholar]
- 7.Arendt E. A., Agel J., Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Davis I., Ireland M. L., Hanaki S. ACL injuries: the gender bias. J Orthop Sports Phys Ther. 2007;37(2):A2–A7. [PubMed] [Google Scholar]
- 9.Griffin L. Y., Albohm M. J., Arendt E. A., et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 10.Ireland M. L. The female ACL: why is it more prone to injury? Orthop Clin North Am. 2002;33(4):637–651. doi: 10.1016/s0030-5898(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 11.Powers C. M. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 12.Willson J. D., Dougherty C. P., Ireland M. L., Davis I. M. Core stability and its relationship to lower extremity function and injury. J Am Acad Orthop Surg. 2005;13(5):316–325. doi: 10.5435/00124635-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Markolf K. L., Burchfield D. M., Shapiro M. M., Shepard M. F., Finerman G. A., Slauterbeck J. L. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 14.Hewett T. E., Myer G. D., Ford K. R., et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence R. K., 3rd, Kernozek T. W., Miller E. J., Torry M. R., Reuteman P. Influences of hip external rotation strength on knee mechanics during single leg drop landings in females. Clin Biomech (Bristol, Avon) 2008;23(6):806–813. doi: 10.1016/j.clinbiomech.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs C. A., Mattacola C. G. Sex differences in eccentric hip-abductor strength and knee-joint kinematics when landing from a jump. J Sport Rehabil. 2005;14(4):346–355. [Google Scholar]
- 17.Chaudhari A. M., Andriacchi T. P. The mechanical consequences of dynamic frontal plane limb alignment for non-contact ACL injury. J Biomech. 2006;39(2):330–338. doi: 10.1016/j.jbiomech.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs C. A., Uhl T. L., Mattacola C. G., Shapiro R., Rayens W. S. Hip abductor function and lower extremity landing kinematics: sex differences. J Athl Train. 2007;42(1):76–83. [PMC free article] [PubMed] [Google Scholar]
- 19.Willson J. D., Davis I. S. Lower extremity strength and mechanics during jumping in women with patellofemoral pain. J Sport Rehabil. 2009;18(1):76–90. doi: 10.1123/jsr.18.1.76. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen M., Aaboe J., Simonsen E. B., Alkjaer T., Bliddal H. Experimentally reduced hip abductor function during walking: implications for knee joint loads. J Biomech. 2009;42(9):1236–1240. doi: 10.1016/j.jbiomech.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Carcia C., Eggen J., Shultz S. J. Hip-abductor fatigue, frontal-plane landing angle, and excursion during a drop jump. J Sport Rehabil. 2005;14(4):321–331. [Google Scholar]
- 22.Heinert B. L., Kernozek T. W., Greany J. F., Fater D. C. Hip abductor weakness and lower extremity kinematics during running. J Sport Rehabil. 2008;17(3):243–256. doi: 10.1123/jsr.17.3.243. [DOI] [PubMed] [Google Scholar]
- 23.Dupont W. D., Plummer W. D. Power and sample size calculation. Department of Biostatistics, Vanderbilt University. http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize. Accessed July 21, 2010.
- 24.Ireland M. L., Willson J. D., Ballantyne B. T., Davis I. M. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671–676. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- 25.Willson J. D., Binder-Macleod S., Davis I. S. Lower extremity jumping mechanics of female athletes with and without patellofemoral pain before and after exertion. Am J Sports Med. 2008;36(8):1587–1596. doi: 10.1177/0363546508315592. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs C., Uhl T. L., Seeley M., Sterling W., Goodrich L. Strength and fatigability of the dominant and nondominant hip abductors. J Athl Train. 2005;40(3):203–206. [PMC free article] [PubMed] [Google Scholar]
- 27.Cram J. R., Kasman G. S. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen; 1998. pp. 368–373. [Google Scholar]
- 28.Zazulak B. T., Ponce P. L., Straub S. J., Medvecky M. J., Avedisian L., Hewett T. E. Gender comparison of hip muscle activity during single-leg landing. J Orthop Sports Phys Ther. 2005;35(5):292–299. doi: 10.2519/jospt.2005.35.5.292. [DOI] [PubMed] [Google Scholar]
- 29.Besier T. F., Lloyd D. G., Ackland T. R. Muscle activation strategies at the knee during running and cutting maneuvers. Med Sci Sports Exerc. 2003;35(1):119–127. doi: 10.1097/00005768-200301000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Brindle T. J., Mattacola C., McCrory J. Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):244–251. doi: 10.1007/s00167-003-0353-z. [DOI] [PubMed] [Google Scholar]
- 31.Cowan S. M., Bennell K. L., Hodges P. W., Crossley K. M., McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82(2):183–189. doi: 10.1053/apmr.2001.19022. [DOI] [PubMed] [Google Scholar]
- 32.Bolgla L. A., Uhl T. L. Reliability of electromyographic normalization methods for evaluating the hip musculature. J Electromyogr Kinesiol. 2007;17(1):102–111. doi: 10.1016/j.jelekin.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Moliga J. M., Myers J. B., Redfern M. S., Lephart S. M. Reliability and precision of EMG in leg, torso, and arm muscles during running. J Electromyogr Kinesiol. 2010;20(1):e1–e9. doi: 10.1016/j.jelekin.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Kernozek T. W., Torry M. R., Van Hoof H., Cowley H., Tanner S. Gender differences in frontal and sagittal plane biomechanics during drop landings. Med Sci Sports Exerc. 2005;37(6):1003–1012. [PubMed] [Google Scholar]
- 35.Bisseling R. W., Hof A. L. Handling of impact forces in inverse dynamics. J Biomech. 2006;39(13):2438–2444. doi: 10.1016/j.jbiomech.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Bush T. R., Gutowski P. E. An approach for hip joint center calculation for use in seated postures. J Biomech. 2003;36(11):1739–1743. doi: 10.1016/s0021-9290(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 37.Mow V. C., Huiskes R. Basic Orthopaedic Biomechanics and Mechano-Biology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 97–101. [Google Scholar]
- 38.Kadaba M. P., Ramakrishnan H. K., Wootten M. E. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- 39.Dempster W. WADC technical report: space requirements of the seated operator. Med Sci Sports Exerc. 1959;35:1754–1750. [Google Scholar]
- 40.McNair P. J., Marshall R. N., Matheson J. A. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103(901):537–539. [PubMed] [Google Scholar]
- 41.Pincivero D. M., Coelho A. J., Campy R. M. Gender differences in perceived exertion during fatiguing knee extensions. Med Sci Sports Exerc. 2004;36(1):109–117. doi: 10.1249/01.MSS.0000106183.23941.54. [DOI] [PubMed] [Google Scholar]
- 42.Potvin J. R. Effects of muscle kinematics on surface EMG amplitude and frequency during fatiguing dynamic contractions. J Appl Physiol. 1997;82(1):144–151. doi: 10.1152/jappl.1997.82.1.144. [DOI] [PubMed] [Google Scholar]
- 43.Tesch P. A., Dudley G. A., Duvoisin M. R., Hather B. M., Harris R. T. Force and EMG signal patterns during repeated bouts of concentric or eccentric muscle actions. Acta Physiol. Scand. 1990;138(3):263–271. doi: 10.1111/j.1748-1716.1990.tb08846.x. [DOI] [PubMed] [Google Scholar]
- 44.Christensen H., Sogaard K., Jensen B. R., Finsen L., Sjogaard G. Intramuscular and surface EMG power spectrum from dynamic and static contractions. J Electromyogr Kinesiol. 1995;5(1):27–36. doi: 10.1016/s1050-6411(99)80003-0. [DOI] [PubMed] [Google Scholar]
- 45.Bigland-Ritchie B., Johansson R., Lippold O. C. J., Woods J. J. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50(1):313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- 46.Kernozek T. W., Ragan R. J. Estimation of anterior cruciate ligament tension from inverse dynamics data and electromyography in females during drop landing. Clin Biomech (Bristol, Avon) 2008;23(10):1279–1286. doi: 10.1016/j.clinbiomech.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Ullrich B., Brüggemann G. P. Force-generating capacities and fatigability of the quadriceps femoris in relation to different exercise modes. J Strength Cond Res. 2008;22(5):1544–1555. doi: 10.1519/JSC.0b013e318173c4ec. [DOI] [PubMed] [Google Scholar]
- 48.Pollard P., Richardson J. T. On the probability of making Type I errors. Psychol Bull. 1987;102(1):159–163. [Google Scholar]
- 49.Ebersole K. T., O'Connor K. M., Wier A. P. Mechanomyographic and electromyographic responses to repeated concentric muscle actions of the quadriceps femoris. J Electromyogr Kinesiol. 2006;16(2):149–157. doi: 10.1016/j.jelekin.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Perry-Rana S. R., Housh T. J., Johnson G. O., Bull A. J., Berning J. M., Cramer J. T. MMG and EMG responses during fatiguing isokinetic muscle contractions at different velocities. Muscle Nerve. 2002;26(3):367–373. doi: 10.1002/mus.10214. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T., Kawahara H., Miyashita H., Watarai K., Takagi M., Tachibana S. Cross reactive identification of types 1 and 2C fibers in human skeletal muscles with monoclonal anti-neurofilament (200 kd) antibody. Histochemistry. 1987;87(1):39–45. doi: 10.1007/BF00518722. [DOI] [PubMed] [Google Scholar]
- 52.Komi P. V., Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42(1):41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- 53.Moritani T., Nagata A., Muro M. Electromyographic manifestations of muscular fatigue. Med Sci Sports Exerc. 1982;14(3):198–202. [PubMed] [Google Scholar]
- 54.McLean S. G., Samorezov J. E. Fatigue-induced ACL injury risk stems from a degradation in central control. Med Sci Sports Exerc. 2009;41(8):1661–1672. doi: 10.1249/MSS.0b013e31819ca07b. [DOI] [PubMed] [Google Scholar]
- 55.Butler J. E., Taylor J. L., Gandevia S. C. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23(32):10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herman D. C., Weinhold P. S., Guskiewicz K. M., Garrett W. E., Yu B., Padua D. A. The effects of strength training on the lower extremity biomechanics of female recreational athletes during a stop-jump task. Am J Sports Med. 2008;36(4):733–740. doi: 10.1177/0363546507311602. [DOI] [PubMed] [Google Scholar]
- 57.Herman D. C., Onate J. A., Weinhold P. S., et al. The effects of feedback with and without strength training on lower extremity biomechanics. Am J Sports Med. 2009;37(7):1301–1308. doi: 10.1177/0363546509332253. [DOI] [PubMed] [Google Scholar]
- 58.Mizner R. L., Kawaguchi J. K., Chmielewski T. L. Muscle strength in the lower extremity does not predict postinstruction improvements in the landing patterns of female athletes. J Orthop Sports Phys Ther. 2008;38(6):353–361. doi: 10.2519/jospt.2008.2726. [DOI] [PubMed] [Google Scholar]
- 59.Reichenbach A., Thielscher A., Peer A., Bülthoff H. H., Bresciani J. P. Seeing the hand while reaching speeds up on-line responses to a sudden change in target position. J Physiol. 2009;587(pt 19):4605–4616. doi: 10.1113/jphysiol.2009.176362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fredericson M., Cookingham C. L., Chaudhari A. M., Dowdell B. C., Oestreicher N., Sahrmann S. A. Hip abductor weakness in distance runners with iliotibial band syndrome. Clin J Sport Med. 2000;10(3):169–175. doi: 10.1097/00042752-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Nadler S. F., Malanga G. A., DePrince M., Stitik T. P., Feinberg J. H. The relationship between lower extremity injury, low back pain, and hip muscle strength in male and female collegiate athletes. Clin J Sport Med. 2000;10(2):89–97. doi: 10.1097/00042752-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Willson J. D., Davis I. S. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech (Bristol, Avon) 2008;23(2):203–211. doi: 10.1016/j.clinbiomech.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 63.Dierks T. A., Manal K. T., Hamill J., Davis I. S. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38(8):448–456. doi: 10.2519/jospt.2008.2490. [DOI] [PubMed] [Google Scholar]
- 64.McLean S. G., Su A., van den Borgert A. J. Development and validation of a 3-D model to predict knee joint loading during dynamic movement. J Biomech Eng. 2003;125(6):864–874. doi: 10.1115/1.1634282. [DOI] [PubMed] [Google Scholar]
- 65.Stefanyshyn D. J., Stergiou P., Lun V. M., Meeuwisse W. H., Worobets J. T. Knee angular impulse as a predictor of patellofemoral pain in runners. Am J Sports Med. 2006;34(6):1844–1851. doi: 10.1177/0363546506288753. [DOI] [PubMed] [Google Scholar]
- 66.Mündermann A., Asay J. L., Mündermann L., Andriacchi T. P. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J Biomech. 2008;41(1):165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Vuillerme N., Sporbert C., Pinsault N. Postural adaptation to unilateral hip muscle fatigue during human bipedal standing. Gait Posture. 2009;30(1):122–125. doi: 10.1016/j.gaitpost.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Benoit D. L., Ramsey D. K., Lamontagne M., Xu L., Wretenberg P., Renstrom P. Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. Gait Posture. 2006;24(2):152–164. doi: 10.1016/j.gaitpost.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Harris R. C., Edwards R. H., Hultman E., Nordesjo L. O., Nylind B., Sahlin K. The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Arch. 1976;367(2):137–142. doi: 10.1007/BF00585149. [DOI] [PubMed] [Google Scholar]
- 70.Taylor J. L., Todd G., Gandevia S. C. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33(4):400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]