Abstract
Context:
Numerous recovery strategies have been used in an attempt to minimize the symptoms of delayed-onset muscle soreness (DOMS). Whole-body vibration (WBV) has been suggested as a viable warm-up for athletes. However, scientific evidence to support the protective effects of WBV training (WBVT) on muscle damage is lacking.
Objective:
To investigate the acute effect of WBVT applied before eccentric exercise in the prevention of DOMS.
Design:
Randomized controlled trial.
Setting:
University laboratory.
Patients or Other Participants:
A total of 32 healthy, untrained volunteers were randomly assigned to either the WBVT (n = 15) or control (n = 17) group.
Intervention(s):
Volunteers performed 6 sets of 10 maximal isokinetic (60°/s) eccentric contractions of the dominant-limb knee extensors on a dynamometer. In the WBVT group, the training was applied using a vibratory platform (35 Hz, 5 mm peak to peak) with 100° of knee flexion for 60 seconds before eccentric exercise. No vibration was applied in the control group.
Main Outcome Measure(s):
Muscle soreness, thigh circumference, and pressure pain threshold were recorded at baseline and at 1, 2, 3, 4, 7, and 14 days postexercise. Maximal voluntary isometric and isokinetic knee extensor strength were assessed at baseline, immediately after exercise, and at 1, 2, 7, and 14 days postexercise. Serum creatine kinase was measured at baseline and at 1, 2, and 7 days postexercise.
Results:
The WBVT group showed a reduction in DOMS symptoms in the form of less maximal isometric and isokinetic voluntary strength loss, lower creatine kinase levels, and less pressure pain threshold and muscle soreness (P < .05) compared with the control group. However, no effect on thigh circumference was evident (P < .05).
Conclusions:
Administered before eccentric exercise, WBVT may reduce DOMS via muscle function improvement. Further investigation should be undertaken to ascertain the effectiveness of WBVT in attenuating DOMS in athletes.
Keywords: eccentric exercise, muscle strength, creatine kinase
Key Points.
After a bout of eccentric exercise, a whole-body vibration training session was associated with reduced symptoms of delayed-onset muscle soreness when compared with no vibration training.
Maximal isometric and isokinetic voluntary strength loss, plasma creatine kinase level, pressure point threshold, and muscle soreness were less than in the control group.
No effect on thigh circumference was seen.
Unaccustomed exercise, especially eccentric contractions, can cause muscle damage. This damage is characterized by decreased muscle force production,1,2 increased serum creatine kinase (CK) activity,3 ultrastructural disruption,1,2 inflammation,4 and increased proteolytic activity.5 The damage generally develops 24 to 48 hours after eccentric exercise and is usually described as delayed-onset muscle soreness (DOMS),1 which is evident as disruption of the normal banding patterns (alignment) of skeletal muscle and broadening or complete disruption of sarcomere Z lines.3,6 The disruption leads to release of CK, which in turn contributes to strength deficits.1,4 In eccentrically exercised muscle, edema, resulting from production of prostaglandin E2, has been observed at 24, 48, and 72 hours.7 Prostaglandin E2 also sensitizes the group IV afferent fibers of muscle connective tissue, which are responsible for dull, aching pain.7
Eccentric exercises are widely used as a basic component of strength-training programs. However, eccentric contractions may cause high-stress damage to recruited fibers8 (predominantly fast-twitch fibers2,6,9) when compared with concentric contractions.10,11 If this stress could be distributed over a greater number of active motor units, more fibers could be synchronously recruited during eccentric contractions and less damage would be expected.
During the last decade, a novel form of exercise based upon the application of sinusoidal vibrations to the body, called whole-body vibration (WBV), has been introduced for enhancing the force-generating capacity in humans.12 The WBV training (WBVT) aims to mechanically activate muscles by eliciting neuromuscular activity (muscle reflexes). Studies on the efficacy of WBVT are equivocal. Both positive (improved flexibility,13 power,14 muscle strength,15 and performance16) and negative or no results17,18 have been reported. In part, this ambiguity is caused by differences in experimental design, populations tested, and WBV devices used. Moreover, the exact mechanism that regulates how the body reacts to vibratory stimulus is currently unclear. The most-often mentioned mechanism is the elicited neuromuscular activation.12,19 Local tendon vibrations and local muscle vibrations induce activity of the muscle spindle Ia fibers, which are mediated by monosynaptic and polysynaptic pathways.20,21 A reflexive muscle contraction known as a tonic vibration reflex (TVR) arises in response to such vibratory stimulus.20 In WBVT, mechanical vibrations are usually transmitted to the body by oscillating platforms. Vibrations are then transferred from the platform to a specific muscle group. Stimulating the sensory receptors and afferent pathways with WBV may lead to a more efficient use of the stretch reflex. In addition, soft tissues act as wobbling masses vibrating in a damped manner in response to mechanical excitation.22 The muscle-tuning hypothesis suggests that the neuromuscular system works to dampen the soft tissue oscillation that occurs in response to vibrations; muscles alter their activity to dampen the vibrations, preventing any resonance phenomenon.22 This, in turn, can lead to a more efficient recruitment and synchronization of motor-unit populations.12
Regarding the role of WBV in improving muscle function, it would seem plausible to assume that WBVT may also be effective in preventing muscle damage. In fact, increased synchrony of motor-unit firing reduces myofibrillar stresses during a bout of eccentric exercise, based on the distribution of contractile stress over a larger number of active fibers. Therefore, less subsequent myofibrillar disruption would be expected. Accordingly, a recent study23 showed that applying local vibration of 50 Hz before downhill running was effective in attenuating DOMS.
Thus, we hypothesized that applying WBV before eccentric exercise may protect the muscle fibers against further damage. Consequently, our purpose was to address the potential effects of WBV in attenuating DOMS, muscle-strength loss, swelling, and CK activity.
METHODS
Participants
A total of 37 untrained university students volunteered for the study. Four participants did not fulfill the inclusion criteria, and 1 participant failed to finish the study. As a result, 22 women and 10 men were randomly allocated to the WBV-training (n = 15) and non–WBV-training (control; n = 17) groups. In the WBVT group, age, height, and mass were 21.46 ± 2.66 years, 162.81 ± 6.31 cm, and 65.13 ± 10.12 kg, respectively; in the control group, the values were 21.88 ± 1.93 years, 164.24 ± 10.85 cm, and 63.29 ± 14.11 kg, respectively. We excluded volunteers with a history of resistive or aerobic exercise training for at least 1 year before the study and anyone with a possible contraindication for WBV (eg, diabetes, epilepsy, metabolic or neuromuscular disease, osteoporosis, osteoarthrosis, prosthetics, menstrual irregularities, or orthopaedic injuries, similar to Roelants et al24). Participants were instructed not to take any medication or dietary supplements and not to perform any sports activities or unaccustomed exercise during the data-collection period. Each volunteer provided written informed consent before testing. The study was approved by the research ethics committee of the Tehran University of Medical Sciences.
Experimental Design
In 2 sessions separated by 2 to 3 days, participants were familiarized with the dynamometer by performing 2 trials of perceived maximal isometric and eccentric actions at 60°/s separated by 1 minute of rest. We chose this velocity based on results showing that muscular torque exerted during isokinetic testing decreased with increasing angular velocity of movement.25 Each participant performed a 5-minute warm-up on a stationary cycle ergometer. Both the WBVT and control groups performed 6 sets of 10 maximal voluntary eccentric contractions, separated by 3 minutes, to induce DOMS. Before performing the DOMS-inducing exercise, the WBVT group received vibration loading on a WBV platform in a half-squat position for 1 minute. Therefore, the independent variables were the 2 conditions: treatment (WBVT) and control.
Dependent variables consisted of maximal voluntary isometric and isokinetic knee extensor strength, thigh circumference, pressure pain threshold (PPT), plasma CK activity, and muscle soreness. Measurements were taken before and immediately after exercise and on days 1, 2, 3, 4, 7, and 14 postexercise. Changes in the measures over time were compared between the WBVT and control groups.
DOMS Inducement
Each participant began with a 5-minute warm-up on a stationary cycle ergometer at 50 W. Both experimental groups performed the exercise protocol to induce muscle damage. The exercise was performed on the dominant-limb knee extensors (based on kicking preference) against the lever arm of the isokinetic dynamometer (Biodex Medical Systems, Shirley, NY). Participants were secured to the dynamometer chair in a seated position using chest, waist, and thigh straps. Range of motion during exercise was 10° to 90° of physiologic knee flexion (0° = full extension). Six sets of 10 maximal voluntary eccentric contractions, separated by 3 minutes,26 were performed by the dominant limb, based on a pilot study that confirmed the production of muscle soreness. The concentric phase was set at 300°/s, and the eccentric phase was set at 60°/s. The movement began at a knee angle of 90°. At full knee extension, participants were instructed to resist maximally through the entire range of motion until full flexion had been reached. Then they were instructed to relax their muscles, and their limbs were returned to an extended position by the device at 300°/s after each eccentric contraction to ensure that only eccentric flexion was performed.
WBV Training
Volunteers in the WBV-training group were exposed to a vibration treatment using a vibration platform (Pro 5; Power Plate North America, Inc, Irvine, CA) before performing the exercises. They were asked to stand in half-squat position on the vibration platform with the knee bent at 100° for 60 seconds. Participants wore no shoes but wore similar cotton socks to avoid the dampening effect of the sole of the shoes. The half-squat position was used to improve upon the performance enhancement noted by Cardinale and Lim,27 who observed that standing on a vibrating platform in half-squat position elicited greater electromyographic responses in the vastus lateralis muscle than standing in the same position in the absence of vibration. Additionally, regarding the duration of WBV application, Bazett-Jones et al28 found that a single 45-second bout of WBV at 2.80g (40 Hz, 2 to 4 mm) was an effective stimulus in eliciting improved countermovement jump performance in untrained women, lasting for approximately 5 minutes after exposure. The amplitude allowed by the vibration platform (peak to peak) was 5 mm. The frequency used in the experiment was 35 Hz; in professional women volleyball players, Cardinale and Lim27 reported that a WBV frequency of 30 Hz (10 mm) elicited greater electromyographic root-mean-square values than 40 Hz and 50 Hz. Investigators29 have proposed that in highly trained athletes, the effects of vibration training may be diminished due to less adaptive potential compared with untrained volunteers. Given the positive results found by Cardinale and Lim27 in the trained population, we assumed that the physically inactive participants in our study might benefit from the settings used in their research.
Measurements
We measured maximal voluntary isometric and isokinetic knee extensor strength, thigh circumference, PPT, plasma CK activity, and muscle soreness for the exercised limb. All measurements except CK activity were taken twice during the familiarization session. Measurements were taken before and immediately after exercise and 1, 2, 3, 4, 7, and 14 days postexercise. Plasma CK activity was measured at the same time points as those described previously with the exception of immediately and 4 and 14 days postexercise.30 Muscle strength was measured at the same time points except for 3 and 4 days postexercise because Paddon-Jones et al30 found that acute bouts of slow-velocity eccentric exercise resulted in a shorter period of muscle soreness.
Muscular Strength
Unilateral limb isokinetic testing was performed on the isokinetic dynamometer to record isometric and isokinetic eccentric torque of the knee extensors.25 The dynamometer was calibrated before the sessions in accordance with the manufacturer's instructions. The volunteers were seated in the 5° reclined position and firmly strapped at the shoulders, hips, and thighs at a knee-joint angle of 90° below full extension. The knee of the tested leg was aligned with the axis of the dynamometer. The tibial pad was secured to the shank 3 cm superior to the lateral malleolus. Appropriate gravity correction was performed before each testing session. For the maximal voluntary isometric contractions (MVICs), participants were asked to sustain maximal effort for 6 seconds at fixed knee-joint angles of 15°, 30°, 45°, and 60° (where 0° was referred to as full extension) so that we could examine a possible shift in optimal angle. Visual feedback from the dynamometer and verbal encouragement from the investigator encouraged maximal effort throughout the protocol. The highest peak torque value of 2 measurements for each angle was used for subsequent analysis. The rest between MVICs was 1 minute, and a 3-minute recovery period was allowed between tests at different joint angles.
After the MVIC measurements, we assessed eccentric maximal voluntary torque of the knee extensors isokinetically at 60°/s velocity with the same positioning as in the isometric assessment; the 80° range of motion was identified as extension (10°) to flexion (90°). The highest peak torque of 5 trials was accepted.
Pressure Pain Threshold
We measured PPT at 5, 10, and 15 cm above the patella using a 20-mL syringe with a spring inside that was scaled from 0 to 10. The rounded tip of the syringe was placed at the above-mentioned points in a vertical position, and the piston was pressed down while the participant was in the long-sitting position with a relaxed quadriceps muscle. He or she was asked to announce any unpleasant sensation (ie, pain), and then we recorded the number on the syringe as the PPT.23 The mean value of the 3 sites was calculated and used for statistical analysis. This device was evaluated in a pilot study in our laboratory, and the intertester and intratester reliabilities were calculated as 0.95 and 0.89, respectively.
Plasma CK Activity
A blood sample of approximately 5 mL was drawn from the antecubital vein at each measurement time point. Serum was separated and frozen at −20°C before analysis. Serum CK activity was assessed on a Technicon RA 1000 (Technicon Ltd, Basingstoke, United Kingdom) random access analyzer using a Technicon test kit.
Muscle Soreness
We measured subjective pain via a 10-mm visual analog scale, with the far-left endpoint representing no pain and the far-right endpoint representing very sore muscles. Lying prone, volunteers actively flexed the knee and maintained the position for 10 seconds.31 They then placed a mark on the scale representing the soreness experienced in the knee-extensor region. In the same manner, they rated the soreness experienced during full extension of the knee.
Thigh Circumference
We measured thigh circumferences at 5, 10, and 15 cm above the patella by a tape measure while the participant was in the long-sitting position with a relaxed quadriceps muscle. The marks were maintained using a permanent ink marker during the experimental period. Two measurements were taken from each marked site and averaged, and the mean value of the 3 measurement sites was used for statistical analysis.
Reliability of the Measurements
We obtained 2 measurements for all variables except CK and calculated the means. Measurement reliability and precision were quantified through the calculation of the intraclass correlation coefficient (ICC) (2,1) with the 95% confidence interval, using the data taken from the 5 volunteers during 2 pre-exercise sessions. The ICC (2,1) values for isometric and isokinetic strength, thigh circumference, PPT, and muscle soreness were 0.90, 0.92, 0.85, 0.95, and 0.89, respectively.
Statistical Analysis
We used SPSS (version 15; SPSS Inc, Chicago, IL) to conduct the analysis. Normal distribution of data was determined by the 1-sample Kolmogorov-Smirnov test, and parametric tests were used to analyze the data. With an independent-samples t test, we compared the baseline measurements between the groups at the beginning and end of training. Changes in variables over time were compared between the WBVT and control conditions using a 2-factor analysis of variance (treatment × time). When the analysis of variance showed a difference between conditions, we applied a Bonferroni post hoc test to identify the significance. Statistical significance was set at P < .05 for all analyses. Data are presented as mean ± SEM unless otherwise stated.
RESULTS
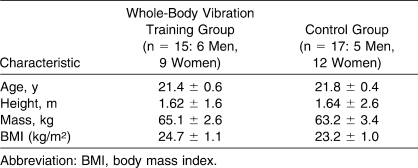
No differences were noted between the WBVT and CT groups for the demographic variables (Table 1). Baseline values for the all dependent variables showed no differences between the groups (Tables 2 through 4).
Table 1.
Participants' Characteristics, Mean ± SEM
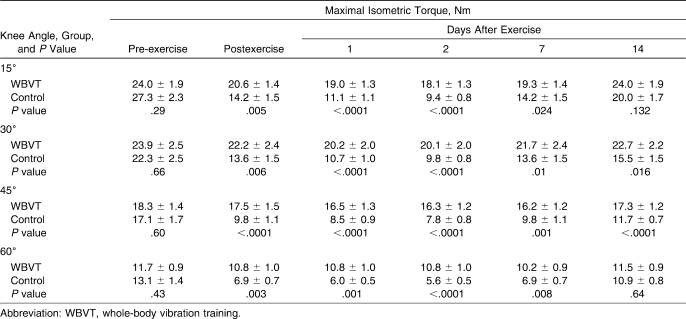
Table 2.
Changes in Maximal Isometric Torque Before, Immediately After, and Days 1, 2, 7, and 14 After Exercise for the Whole-Body Vibration Training (n = 15) and Control Groups (n = 17), Mean ± SEM
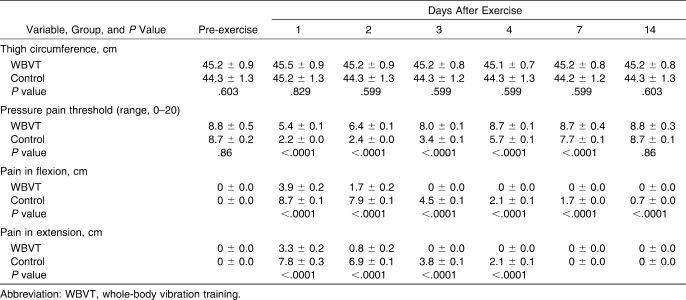
Table 4.
Changes in Thigh Circumference, Pressure Pain Threshold, Muscle Soreness (Flexion and Extension) Before and Days 1 to 14 After Exercise for the Whole-Body Vibration Training (n = 15) and Control Groups (n = 17), Mean ± SEM
Muscular Strength
Maximal isometric torque was larger at the 15° knee angle (27.3 ± 2.3 Nm) than at other angles before exercise and throughout the measurements; however, the magnitude of decrease in torque postexercise was similar among the 4 angles. A decrease was seen in maximal isometric torque for the control group at all angles in all sessions (Table 2). In the control group, the torque decreased immediately postexercise by 42.70% below baseline, compared with a 7.50% decrease in the WBVT group. This decremental trend was shown in the control group at 24 hours (by 51.75%) and 48 hours (by 55.44%), whereas decreases of 12.21% and 13.45% were demonstrated at 24 and 48 hours, respectively, in the WBVT group. By day 14, a mean percentage decrease of 22.22% was still evident in the control group, compared with a 2.28% decrease in the WBVT group (P < .0001).
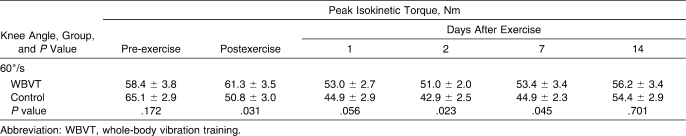
No time effect was found in maximal isokinetic torque at 60°/s between baseline and any other sessions (immediately after or 1, 2, 7, or 14 days postexercise) in the WBVT group over time (P = .999, P = .167, P = .211, P = .183, and P = .999, respectively). Maximal isokinetic torque at 60°/s changed over time within the control group (P < .001), decreasing by 42.53% below baseline immediately postexercise and by 49.02% and 52.88% at 24 and 48 hours, respectively. Between-groups comparisons of maximal isokinetic torque showed a decrease in the control group compared with the WBVT group (Table 3).
Table 3.
Changes in Peak Isokinetic Torque Before, Immediately After, and Days 1, 2, 7, and 14 After Exercise for the Whole-Body Vibration Training (n = 15) and Control Groups (n = 17), Mean ± SEM
Pressure Pain Threshold
A between-groups comparison of the mean change at 3 measured PPT sites showed a greater decrease in the control group than in the WBVT group (Table 4). Both groups showed similar trends with decrements of 38.11% and 74.56% at 24 hours and 25.58% and 71.62% at 48 hours for the WBVT and control groups, respectively. However, the decreasing trend was steeper in the control group. After this time point, PPT in the WBVT group recovered to near pre-exercise level (P = .072), whereas it had not recovered by day 14 postexercise in the control group (P < .0001).
Plasma CK Activity
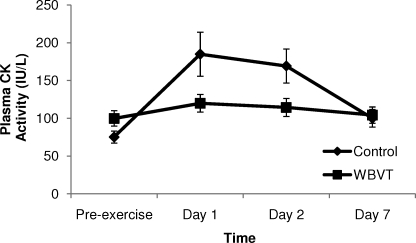
No difference in plasma CK activity between groups was evident before exercise (P = .065). At 24 hours postexercise, the CK peak value for the control group was 46% higher than that for the WBVT group (Figure). In addition, the CK-level percentage increments at 24 and 48 hours were less in the WBVT group than in the control group (P = .003). At day 7, postexercise CK level was 24.82% above baseline in the control group and 4.46% above baseline in the WBVT group (Figure).
Figure.
Changes in plasma creatine kinase activity before (pre-exercise) and 1 to 7 days postexercise for the whole-body vibration training and control groups. Abbreviations: CK, creatine kinase; WBVT, whole-body vibration training.
Muscle Soreness
Before exercise, no participant reported any soreness during assessments. The DOMS developed after exercise in both groups in the flexion and extension conditions of the knee joint (Table 4). At 24 hours, the mean pain increment equated to 36.02% and 82.25% in the WBVT and control groups, respectively. The control group reported greater perception of 36.44% and 46.03% postexercise DOMS than the WBVT group by 24 and 48 hours, respectively (P < .0001).
Thigh Circumference
Baseline thigh circumference was not different between the groups (P = .603). Between-groups comparison of limb girth showed no difference in the control group compared with the WBVT group (P = .59) (Table 4).
DISCUSSION
We investigated the possible effects of a pre-eccentric exercise WBVT intervention on DOMS and other indicators of eccentric-exercise–induced muscle damage. The WBVT had an alleviative effect on the responses to DOMS-inducing exercise in terms of changes in maximal isometric and isokinetic force, PPT, plasma CK activity, and muscle soreness.
The magnitude of strength loss was different between the groups. Also, the magnitude of maximal isometric torque loss among different knee angles and the eccentric peak torque loss was lower in the WBVT group than in the control group. These results may reflect vibration's enhancement of the level of motor-unit activity, slow-twitch fiber recruitment, and motor-unit synchronization in accordance with Bakhtiary and colleagues'23 results. They showed that applying local vibration to the lower limb for 1 minute before downhill running was effective in attenuating DOMS compared with untreated volunteers.23
Several proposed mechanisms may explain how the body reacts to vibratory stimulus. One theory19,29 suggests that acute changes to motor output due to WBV are most likely attributed to neural factors, such as enhanced muscle-spindle sensitivity and gamma activation, leading to increased motor-unit recruitment and neuromuscular facilitation. With the muscle-spindle threshold reduced, increased activity from Ia afferent fibers would increase muscle activation through facilitation of homonymous α motoneurons.29 Accordingly, less muscle damage in the WBVT group compared with the control group in our study could be attributed to the heightened sensitivity of the muscle spindles. Enhanced spindle sensitivity could increase the level of preactivation and muscle stiffness and lower firing thresholds. A reduced threshold would also bring other spindles into play.17 Increased synchrony of motor-unit firing could lead to a more efficient distribution of contractile stress over a larger number of active fibers. In line with these postulations, Cardinale and Lim27 reported that the greatest electromyographic activity in the vastus lateralis muscle occurred when the frequency of the vibrating platform was at 30 Hz with the knee bent to 100°.
In addition, less torque loss in the WBVT group compared with the control group might be explained by the “muscle-tuning” hypothesis.22 Vibrations produce fast and short changes in the length of the muscle-tendon complex. Sensory receptors, probably muscle spindles, initiate reflexive muscle contractions in an attempt to dampen the vibratory waves,22 referred to as the TVR.20,21 Dampening of the vibrations depends on the individual's viscoelastic properties (ie, stiffness), vibration frequency, and muscle length.32 It has been suggested33 that WBV also leads to a rapid increase in intramuscular temperature. Because the increase in temperature leaves the muscle less stiff, the muscle may require greater neuromuscular activation to dampen the WBV stimulus33 and, therefore, less damage would result.
Another theory19 suggests that WBV can produce changes in gravitational conditions during the intervention. An increase in gravitational load increases the cross-sectional area and force-generating capability of the muscles.19 Several studies have indicated that TVR is composed of motor-unit activity synchronized and unsynchronized with the vibration cycle. Martin and Park34 showed that at low vibration frequencies (≤100 Hz), harmonic synchronization is predominant and TVR magnitude increases with vibration frequency. A TVR increase in the frequency range of 40 to 80 Hz results primarily from an increase in motoneuron depolarization with the firing frequency of Ia afferents, which leads to a recruitment of motor units of increasing threshold.34 Conversely, Hopkins et al17,18 reported no alteration in stretch reflex magnitude or peroneus longus activation after ankle-inversion perturbation. These differences in the vibration effect may be caused by differences in the types of WBV devices used and differences in study design or footwear conditions; athletic shoes may have a dampening effect on the amplitude and frequency of the actual vibration when the stimulus is absorbed by the sole of the shoe. Marin et al35 found that athletic shoes may alter the response to WBV.
Additionally, it is equally well established that repeated bouts of the same or similar eccentrically based exercise result in markedly fewer symptoms of damage than the initial bout. The effect of WBV in attenuating DOMS might be similar to the “repeated-bout effect” proposed by Nosaka and Clarkson,36 who suggested that a neural adaptation can lead to a better workload distribution among fibers. Furthermore, some researchers10,11 suggested that reduced electromyographic activity observed during a maximum eccentric contraction is due to incomplete activation of the motoneurons that innervate the muscle. Based on our results, we might hypothesize that WBV can more efficiently recruit motor units thorough incremental motor-unit activation, reflexive recruitment of previously inactive motor units, synchronization among active motor units, or a shift to slow-twitch fiber activation (or a combination of these).
We found less serum CK activity, higher PPT, and less muscle soreness in the WBVT group compared with the control group. Some authors1,2,6 defined plasma CK activity as a sign of eccentric muscle damage. Release of this cytoplasmic protein can be due to either temporary muscle fiber damage accompanied by membrane leakage or death of the muscle fiber.37 Due to preferential recruitment of fast-twitch motor units in eccentric contractions, fast-twitch fibers seem to be more susceptible to disruption.6,9 Thus, a change in motor-unit activation might reduce high fiber stresses and consequently limit myofibrillar disruption. Hence, it would seem reasonable that vibration could increase recruitment of slow-twitch motor units, distribute the contractile stress over a larger number of active fibers, and consequently leave the individual with a less damaged muscle. Furthermore, we could deduce that the lower level of muscle damage in WBVT group might lead to less production of pain substrates.
We found no differences between groups for thigh circumference. Previous authors7,38,39 attributed the pain of DOMS to edema and swelling within the exercised muscle fibers. However, Smith40 and Armstrong41 argued that monocytes, which convert to macrophages, accumulate after injury and produce substances which, in turn, sensitize the type III and IV nerve endings within 24 to 48 hours. In addition, Buroker and Schwane42 and Gulick et al43 found that girth measurements of eccentrically exercised limbs did not increase at any postexercise assessment time, in accordance with our findings.
Our study was limited to a recreationally active college-aged population. Whether these results can be attributed to athleticism warrants investigation. Also, our findings are limited by the lack of other measures (eg, electromyography) that would have allowed further explanation of these results. Studies should be performed to find an ideal combination of various vibration settings to achieve more efficient results.
CONCLUSIONS
We investigated the possible effects of WBVT on muscle damage. Based on these results, a single WBVT session, consisting of one 60-second bout at 35 Hz transmitted through a vibration platform with the participant in the half-squat position, seemed to be effective in attenuating DOMS, PPT, and plasma CK activity and controlling strength loss after a bout of eccentric exercise.
Acknowledgments
This study was supported by a grant from the Postgraduate Studies and Research Program, Tehran University of Medical Sciences. We acknowledge the generous assistance of the staff and students of the Rehabilitation Faculty, Tehran University of Medical Sciences.
REFERENCES
- 1.Armstrong R. B., Warren G. L., Warren J. L. Mechanisms of exercise induced muscle fiber injury. Sports Med. 1991;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Proske U., Morgan D. L. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(pt 2):333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stauber W. T., Clarkson P. M., Fritz V. K., Evans W. J. Extracellular matrix disruption after eccentric muscle action. J Appl Physiol. 1990;69(3):868–874. doi: 10.1152/jappl.1990.69.3.868. [DOI] [PubMed] [Google Scholar]
- 4.Pyne D. B. Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport. 1994;26(3–4):49–58. [PubMed] [Google Scholar]
- 5.Belcastro A. N. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. J Appl Physiol. 1993;74(3):1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- 6.Fridén J., Sjøstrøm M., Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4(3):170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 7.Fridén J., Sfakianos P., Hargens A. Muscle soreness and intramuscular fluid pressure: comparison between eccentric and concentric load. J Appl Physiol. 1986;61(6):2175–2179. doi: 10.1152/jappl.1986.61.6.2175. [DOI] [PubMed] [Google Scholar]
- 8.Moritani T., Murasmatsu S., Muro M. Activity of motor units during concentric and eccentric contractions. Am J Phys Med. 1988;66(6):338–350. [PubMed] [Google Scholar]
- 9.Lieber R. L., Woodburn T. M., Fridén J. Muscle damage induced by eccentric contractions of 25% strain. J Appl Physiol. 1991;70(6):2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- 10.Enoka R. M. Eccentric contractions require unique activation strategies by the nervous system. J Appl Physiol. 1996;81(6):2339–2346. doi: 10.1152/jappl.1996.81.6.2339. [DOI] [PubMed] [Google Scholar]
- 11.Nardone A., Romano C., Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989;409(1):451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosco C., Cardinale M., Tsarpela O., Locatelli E. New trends in training science: the use of vibrations for enhancing performance. New Stud Athlet. 1999;14(4):55–62. [Google Scholar]
- 13.Fagnani F., Giombini A., Di Cesare A., Pigozzi F., Di Salvo V. The effects of a whole-body vibration program on muscle performance and flexibility in female athletes. Am J Phys Med Rehabil. 2006;85(12):956–962. doi: 10.1097/01.phm.0000247652.94486.92. [DOI] [PubMed] [Google Scholar]
- 14.Bosco C., Cardinale M., Tsarpela O. Influence of vibration on mechanical power and electromyogram activity in human arm flexor muscles. Eur J Appl Physiol Occup Physiol. 1999;79:306–311. doi: 10.1007/s004210050512. [DOI] [PubMed] [Google Scholar]
- 15.de Ruiter C. J., van der Linden R. M., van der Zijden M. J. A., Hollander A. P., de Haan A. Short-term effects of whole-body vibration on maximal voluntary isometric knee extensor force and rate of force rise. Eur J Appl Physiol. 2003;88(4–5):472–475. doi: 10.1007/s00421-002-0723-0. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva M. E., Nunez V. M., Vaamonde D., et al. Effect of different frequencies of whole body vibration on muscular performance. Biol Sport. 2006;23(3):267–281. [Google Scholar]
- 17.Hopkins J. T., Fredericks D., Guyon P. W., et al. Whole body vibration does not potentiate the stretch reflex. Int J Sports Med. 2009;30(2):124–129. doi: 10.1055/s-2008-1038885. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins T., Pak J. O., Robertshaw A. E., Feland J. B., Hunter I., Gage M. Whole body vibration and dynamic restraint. Int J Sports Med. 2008;29(5):424–428. doi: 10.1055/s-2007-965362. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale M., Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hagbarth K., Eklung G. Motor effects of vibratory stimuli. In: Granit R., editor. Muscular Afferents and Motor Control: Proceedings of First Symposium. Stockholm, Sweden: Almqvist & Wiksell; 1985. pp. 177–186. [Google Scholar]
- 21.Seidel H. Myoelectric reactions to ultra-low frequency and low-frequency whole body vibration. Eur J App Physiol Occup Physiol. 1988;57(5):558–562. doi: 10.1007/BF00418462. [DOI] [PubMed] [Google Scholar]
- 22.Nigg B. M. Impact forces in running. Curr Opin Orthop. 1997;8(6):43–47. [Google Scholar]
- 23.Bakhtiary A. H., Safavi-Farokhi Z., Aminian-Far A. Influence of vibration on delayed onset of muscle soreness following eccentric exercise. Br J Sports Med. 2007;41(3):145–148. doi: 10.1136/bjsm.2006.031278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelants M., Verschueren S., Delecluse C., Levin O., Stijnen V. Whole-body-vibration–induced increase in leg muscle activity during different squat exercises. J Strength Cond Res. 2006;20(1):124–129. doi: 10.1519/R-16674.1. [DOI] [PubMed] [Google Scholar]
- 25.Baltzopoulos V., Brodie D. A. Isokinetic dynamometery: applications and limitations. Sports Med. 1989;8(2):101–116. doi: 10.2165/00007256-198908020-00003. [DOI] [PubMed] [Google Scholar]
- 26.Ariki P., Davies G. J. Rest interval between isokinetic velocity spectrum rehabilitation sets. Phys Ther. 1985;65(5):733–734. [Google Scholar]
- 27.Cardinale M., Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibration of different frequencies. J Strength Cond Res. 2003;17(3):621–624. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Bazett-Jones D. M., Finch H. W., Dugan E. L. Comparing the effects of various whole-body vibration accelerations on counter-movement jump performance. J Sports Sci Med. 2008;7(1):144–150. [PMC free article] [PubMed] [Google Scholar]
- 29.Rehn B., Lidström J., Skoglund J., Lindström B. Effects on leg muscular performance from whole-body vibration exercise: a systematic review. Scand J Med Sci Sports. 2007;17(1):2–11. doi: 10.1111/j.1600-0838.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 30.Paddon-Jones D., Keech A., Lonergan A., Abernethy P. Differential expression of muscle damage in humans following acute fast and slow velocity eccentric exercise. J Sci Med Sport. 2005;8(3):255–263. doi: 10.1016/s1440-2440(05)80036-2. [DOI] [PubMed] [Google Scholar]
- 31.Minder P. M., Noble J. G., Alves-Guerreiro J., et al. Interferential therapy: lack of effect upon experimentally induced delayed onset muscle soreness. Clin Physiol Funct Imaging. 2002;22(5):339–347. doi: 10.1046/j.1475-097x.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- 32.Jordan M. J., Norris S. R., Smith D. J., Herzog W. Vibration training: an overview of the area, training consequences, and future considerations. J Strength Cond Res. 2005;19(2):459–466. doi: 10.1519/13293.1. [DOI] [PubMed] [Google Scholar]
- 33.Cochrane D. J., Stannard S. R., Sargeant A. J., Rittweger J. The rate of muscle temperature increase during acute whole-body vibration exercise. Eur J Appl Physiol. 2008;103(4):441–448. doi: 10.1007/s00421-008-0736-4. [DOI] [PubMed] [Google Scholar]
- 34.Martin B. J., Park H. S. Analysis of the tonic vibration reflex: influence of vibration variables on motor unit synchronization and fatigue. Eur J Appl Physiol Occup Physiol. 1997;75(6):504–511. doi: 10.1007/s004210050196. [DOI] [PubMed] [Google Scholar]
- 35.Marin P. J., Bunker D., Rhea M. R., Ayllon F. N. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: implications for prescription of vibratory stimulation. J Strength Cond Res. 2009;23(8):2311–2316. doi: 10.1519/JSC.0b013e3181b8d637. [DOI] [PubMed] [Google Scholar]
- 36.Nosaka K., Clarkson P. M. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27(9):1263–1269. [PubMed] [Google Scholar]
- 37.McNeil P. L., Khakee R. Disruptions of muscle fiber plasma membranes: role in exercise-induced damage. Am J Pathol. 1992;140(5):1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 38.Fridén J., Sjøstrøm M., Ekblom B. A. A morphological study of delayed muscle soreness. Experientia. 1981;37(5):506–507. doi: 10.1007/BF01986165. [DOI] [PubMed] [Google Scholar]
- 39.Hasson S., Barnes W., Hunter M., Williams J. Therapeutic effects of high speed voluntary muscle contractions on muscle soreness and muscle performance. J Orthop Sports Phys Ther. 1989;10(12):499–507. doi: 10.2519/jospt.1989.10.12.499. [DOI] [PubMed] [Google Scholar]
- 40.Smith L. L. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23(5):542–551. [PubMed] [Google Scholar]
- 41.Armstrong R. B. Mechanism of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16(6):529–538. [PubMed] [Google Scholar]
- 42.Buroker K. C., Schwane J. A. Does post-exercise static stretching alleviate delayed muscle soreness? Physician Sportsmed. 1989;17(6):65–83. doi: 10.1080/00913847.1989.11709806. [DOI] [PubMed] [Google Scholar]
- 43.Gulick D. T., Kimura I. F., Sitler M., Paolone A., Kelly J. D. Various treatment techniques on signs and symptoms of delayed onset muscle soreness. J Athl Train. 1996;31(2):145–152. [PMC free article] [PubMed] [Google Scholar]