Abstract
Reference:
Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463–472.
Clinical Questions:
In patients with low back pain (LBP) who do not have indications of a serious underlying condition, does routine, immediate lumbar imaging result in improved patient outcomes when compared with clinical care without immediate imaging?
Data Sources:
Studies were identified by searching MEDLINE (1966 through first week of August 2008) and the Cochrane Central Register of Controlled Trials (third quarter of 2008). The reference lists of identified studies were manually reviewed for additional citations. The search terms spine, low-back pain, diagnostic imaging, and randomized controlled trials were used in both databases. The complete search strategy was made available as an online supplement.
Study Selection:
The search criteria were applied to the articles obtained from the electronic searches and the subsequent manual searches with no language restrictions. This systematic review and meta-analysis included randomized, controlled trials that compared immediate, routine lumbar imaging (or routine provision of imaging findings) with usual clinical care without immediate lumbar imaging (or not routinely providing results of imaging) for LBP without indications of serious underlying conditions.
Data Extraction:
Data extraction and assessment of study quality were well described. The trials assessed one or more of the following outcomes: pain, function, mental health, quality of life, patient satisfaction, and overall patient-reported improvement. Two reviewers independently appraised citations considered potentially relevant, with disagreements between reviewers resolved by consensus. Two independent reviewers abstracted data from the trials and assessed quality with modified Cochrane Back Review Group criteria. The criterion for blinding of patients and providers was excluded because of lack of applicability to imaging studies. In addition, the criterion of co-intervention similarity was excluded because a potential effect of different imaging strategies is to alter subsequent treatment decisions. As a result of excluding these criteria, quality ratings were based on the remaining 8 criteria. The authors resolved disagreements about quality ratings through discussion and consensus. Trials that met 4 or more of the 8 criteria were classified as higher quality, whereas those that met 3 or fewer of the 8 criteria were classified as lower quality. In addition, the authors categorized duration of symptoms as acute (<4 weeks), subacute (4–12 weeks), or chronic (>12 weeks). The investigators also contacted the study authors for additional data if included outcomes were not published or if median (rather than mean) outcomes were reported. Statistical analysis was conducted on the primary outcomes of improvement in pain or function. Secondary outcomes of improvement in mental health, quality of life, patient satisfaction, and overall improvement were also analyzed. Outcomes were categorized as short term (≤3 months), long term (>6 months to ≤1 year), or extended (>1 year). For continuous outcomes, standardized mean differences (SMDs) of interventions for change between baseline and follow-up measurements were calculated. In studies reporting the same pain (visual analog scale [VAS] or Short Form-36 bodily pain score) or function (Roland-Morris Disability Questionnaire [RDQ]) outcomes, weighted mean differences (WMDs) were calculated. In all analyses, lower pain and function scores indicated better outcomes. For quality-of-life and mental health outcomes, higher scores indicated improved outcomes. All statistical analyses were performed with Stata 10.0. For outcomes in which SMDs were calculated, values of 0.2 to 0.5 were considered small, 0.5 to 0.8 were considered moderate, and values greater than 0.8 were considered large. For WMDs, mean improvements of 5 to 10 points on a 100-point scale (or equivalent) were considered small, 10-point to 20-point changes were considered moderate, and changes greater than 20 points were considered large. For the RDQ, mean improvements of 1 to 2 points were termed small, and improvements of 2 to 5 points were termed moderate.
Main Results:
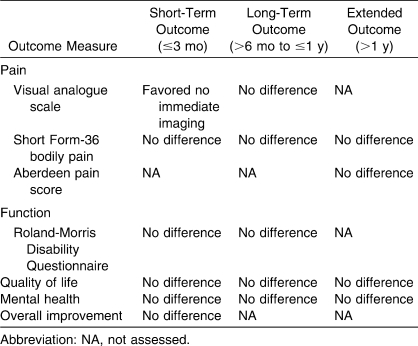
The total number of citations identified using the search criteria was 479 articles and abstracts. Of these, 466 were excluded because either they were not randomized trials or they did not use imaging strategies for LBP. At this step, 13 articles were retrieved for further analysis. This analysis resulted in 3 additional articles being excluded (1 was not a randomized trial and the other 2 compared 2 imaging techniques rather than immediate imaging versus no imaging). The final step resulted in the inclusion of 6 trials reported in 10 publications for the meta-analysis. In the studies meeting the inclusion criteria, 4 assessed lumbar radiography and 2 assessed magnetic resonance imaging (MRI) or computed tomography (CT) scans. In these 6 trials, 1804 patients were randomly assigned to the intervention group. The duration of patient follow-up ranged from 3 weeks to 2 years. In addition, 1 trial excluded patients with sciatica or other radiculopathy symptoms, whereas another did not report the proportion of patients with these symptoms. In the other 4 studies, the proportion of patients with sciatica or radiculopathy ranged from 24% to 44%. Of the included trials, 3 compared immediate lumbar radiography with usual clinical care without immediate radiography, and a fourth study compared immediate lumbar radiography and a brief educational intervention with lumbar radiography if no improvement was seen by 3 weeks. The final 2 studies assessed advanced imaging modalities. Specifically, one group compared immediate MRI or CT with usual clinical care without advanced imaging in patients with primarily chronic LBP (82% with LBP for >3 months) who were referred to a surgeon. In the other advanced imaging study, all patients with LBP for <3 weeks underwent MRI and were then randomized to routine notification of results or to notification of results only if clinically indicated. With respect to study quality, 5 trials met at least 4 of the 8 predetermined quality criteria, leading to a classification of higher quality. In addition, 5 trials were included in the primary meta-analysis on pain or function improvement at 1 or more follow-up periods. With regard to short-term and long-term improvements in pain, no differences were noted between routine, immediate lumbar imaging and usual clinical care without immediate imaging (Table 1). In studies using the VAS pain score, the WMD (0.62, 95% confidence interval [CI] = 0.03, 1.21) at short-term follow-up slightly favored no immediate imaging. No differences in outcome were seen in studies using the Short Form-36 bodily pain score. No improvements in function at short-term or long-term follow-up were noted between imaging strategies. Specifically, short-term function measured with the RDQ in 3 studies showed a WMD of 0.48 points (95% CI = −1.39, 2.35) between imaging strategies, whereas long-term function in 3 studies, also measured with the RDQ, showed a WMD of 0.33 points (95% CI = −0.65, 1.32). One included trial reported pain outcomes at extended (2-year) follow-up and found no differences between imaging strategies for pain (Short Form-36 bodily pain or Aberdeen pain score), with SMDs of −2.7 (95% CI = −6.17, 0.79) and −1.6 (−4.04, 0.84), respectively. The outcomes between immediate imaging and usual clinical care without immediate imaging did not differ for short-term follow-up in those studies reporting quality of life (SMD = −0.10, 95% CI = −0.53, 0.34), mental health (SMD = 0.12, 95% CI = −0.37, 0.62), or overall improvement (mean risk ratio = 0.83, 95% CI = 0.65, 1.06). In those studies reporting long-term follow-up periods, similar results can be seen for quality of life (SMD = −0.15, 95% CI = −0.33, 0.04) and mental health (SMD = 0.01, 95% CI = −0.32, 0.34). In the study reporting extended follow-up, immediate imaging was not better in terms of improving quality of life (SMD = 0.02, 95% CI = −0.02, 0.07) or mental health (SMD = −1.50, 95% CI = −4.09, 1.09) when compared with usual clinical care without immediate imaging. In the included studies, no cases of cancer, infection, cauda equina syndrome, or other serious diagnoses were reported in patients randomly assigned to either imaging strategy.
Conclusions:
Available evidence indicates that immediate, routine lumbar spine imaging in patients with LBP and without features indicating a serious underlying condition did not improve outcomes compared with usual clinical care without immediate imaging. Clinical care without immediate imaging seems to result in no increased odds of failure in identifying serious underlying conditions in patients without risk factors for these conditions. In addition to lacking clinical benefit, routine lumbar imaging is associated with radiation exposure (radiography and CT) and increased direct expenses for patients and may lead to unnecessary procedures. This evidence confirms that clinicians should refrain from routine, immediate lumbar imaging in primary care patients with nonspecific, acute or subacute LBP and no indications of underlying serious conditions. Specific consideration of patient expectations about the value of imaging was not addressed here; however, this aspect must be considered to avoid unnecessary imaging while also meeting patient expectations and increasing patient satisfaction.
Keywords: spine, assessment, outcomes
COMMENTARY
Low back pain (LBP) accounts for about 2% of all physician office visits; only visits for routine examinations, hypertension, and diabetes occur with greater frequency.1 In addition, the rate of LBP in athletes has been observed to vary from 1% to >30% and is influenced by sport, sex, training frequency and intensity, and technique.2 Therefore, athletic trainers, physicians, and other practitioners will encounter athletes among their patients presenting with LBP. Some athletes may also demonstrate a desire for imaging and an expectation that immediate imaging will be a component of the evaluation of their LBP, particularly because injured elite athletes often undergo immediate imaging.
The results of this systematic review continue to support the clinical practice guidelines published by the Agency for Health Care Policy and Research in 1994.3 These guidelines advocated against immediate imaging in patients with acute LBP, and this systematic review does not challenge that approach. In contrast, since the publication of these guidelines, imaging utilization rates have continued to increase.3 For example, lumbar magnetic resonance imaging (MRI) increased by 307% in the Medicare population from 1994 to 2005.3 Although no specific data on the utilization rate of imaging in the non-Medicare adult population are available, we can postulate that the data from Martin et al1 would support increased imaging in this group (which likely includes adult athletes). However, the evidence on the usefulness of imaging and, in particular, immediate imaging in managing LBP supports an approach toward less immediate imaging.4,5 Deyo et al4 recognized that clinicians are challenged in taking this approach given patient demands and the fear of lawsuits that our current system engenders. In addition, Rhodes et al6 presented evidence demonstrating the powerful nature of imaging with regard to patients' beliefs about their back pain and its legitimacy when an abnormality is found. Yet as Deyo et al4 and Chou et al5 have noted, positive findings on imaging are common in asymptomatic individuals, which indicates that a number of positive results in patients with LBP may have preceded their current episode.
Modic et al7 studied patients with acute onset (<3 weeks) of LBP or radiculopathy to assess the effect of MRI findings on patient prognosis and outcome. One group of patients (along with their treating physicians) was not blinded to the MRI results, whereas the other group was blinded. At the 6-week follow-up, no differences in function (Roland-Morris Disability Questionnaire) or patient satisfaction were observed. Further, patient knowledge of the MRI findings did not alter outcome; however, knowledge of the findings was associated with a lesser sense of well-being. With regard to the effects on therapeutic decision making, MRI results did not provide any additive value over clinical assessment in these patients. Based on these observations, Modic et al7 concluded that MRI does not seem to have a measurable value with respect to planning conservative care, and it may be deleterious in terms of unnecessary therapy for or concern by patients. In a subsequent report by this same research group, Ash et al8 observed no differences in patient outcomes between a group that was blinded to MRI results and a group that was not blinded to MRI results, with one exception. The blinded group scored higher than the unblinded group (improved) on the Short Form-36 general health subscale at the 6-week follow-up. At the 1-year follow-up, the blinded group also scored higher on this subscale, but the difference was not statistically significant. These findings led Ash et al8 to conclude that patient knowledge of MRI findings did not alter the primary patient outcomes in their patients. However, blinded patients did show a better sense of well-being at the short-term follow-up point. Ash et al8 suggested that the knowledge of MRI results may have a negative psychological effect by labeling patients based on test results rather than clinical symptoms. Chou et al5 also recognized that the imaging strategies (radiography, computed tomography [CT], and MRI) used can be harmful to patients. For example, radiography and CT expose patients to significant doses of radiation, and any imaging technique runs the risk of labeling a patient with a pathoanatomic diagnosis unrelated to the actual cause of his or her symptoms.4,7 Once labeled, a patient may then be on a path toward invasive or surgical interventions with no clear improvement in outcome when compared with usual clinical care.4,5,7
As significant as these findings are in favor of no immediate imaging in patients with nonspecific LBP, certain limitations remain. According to Chou et al,5 a number of authors did not clearly describe their randomization methods, did not use blinded outcome evaluators, and failed to report intention-to-treat analyses, making it difficult to examine the study's methodologic strength. Also noted as a limitation was the lack of standardization in reporting outcomes among trials. Further, assessing the applicability of these studies was impeded by the use of different exclusion criteria for patients with risk factors for serious underlying conditions. Many of these criteria correspond with the signs and symptoms, often referred to as “red flags,” that warrant further investigation, which can include immediate imaging (Table 2). Awareness and understanding of these red flags is essential for the athletic trainer when conducting the examination and initiating an appropriate physician referral when warranted.
It is also unclear if the studies were comparing advanced imaging (MRI or CT) with no imaging or if they were comparing the incremental value of advanced imaging in patients who had previously undergone lumbar radiography. Addressing these issues would be helpful in terms of allowing us to determine with greater precision the value and timing of imaging in the management of nonspecific LBP.1,5,6 An additional limitation of the review of Chou et al5 is that the trials included in the meta-analysis did not specifically address a young athletic population, which may affect the generalizability of the findings to this population. For example, Bono2 cited data indicating a higher rate of spondylolysis in adolescents than in adults. Further, he stated that significant examination findings in these patients included LBP exacerbated by repeated extension. Therefore, these observations (adolescent age group, LBP exacerbated by repeated extension) may be potential red flags for the athletic trainer to consider when examining an adolescent patient. In contrast, current evidence indicates that the guidelines suggested by Chou et al5 should not be applied to the young athletic population.
According to Bono,2 most LBP in athletes is nonspecific in nature upon presentation to the practitioner; therefore, implementing a sound strategy for the use of immediate imaging is warranted. Athletic trainers can have an important role in this strategy because identifying risk factors for serious underlying conditions (eg, red flags) during the initial patient examination is important (Table 2). Furthermore, athletic trainers are positioned to help patients understand the current evidence about the limited value of immediate imaging in cases involving nonspecific LBP. In view of this current evidence, individuals presenting with LBP in the absence of red flags can achieve positive treatment outcomes with the usual clinical care and without undergoing immediate imaging. In addition, reducing the rate of immediate imaging would diminish patient exposure to potentially harmful doses of radiation and unneeded invasive procedures, which may be particularly important in younger patients. As primary health care providers, athletic trainers can facilitate an evidence-based approach for the use of immediate imaging in patients with nonspecific LBP without jeopardizing positive patient outcomes.
Table 1.
Summary of Main Results for Immediate Imaging Versus Usual Clinical Care Without Immediate Imaging
Table 2.
“Red Flags” in Patients With Nonspecific Low Back Pain
REFERENCES
- 1.Martin B. I., Deyo R. A., Mirza S. K., et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Bono C. M. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86(2):382–396. doi: 10.2106/00004623-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Bigos S. J., Bowyer R., Braen R., et al. Clinical Practice Guideline 14: acute low back pain in adults. US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research (AHCPR) http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsarchive&part=A25870. Accessed November 2, 2009.
- 4.Deyo R. A., Mirza S. K., Turner J. A., Martin B. I. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62–68. doi: 10.3122/jabfm.2009.01.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R., Fu R., Carrino J. A., et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463–472. doi: 10.1016/S0140-6736(09)60172-0. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes L. A., McPhillips-Tangum C. A., Markham C., Klenk R. The power of the visible: the meaning of diagnostics tests in chronic back pain. Soc Sci Med. 1999;48(9):1189–1203. doi: 10.1016/s0277-9536(98)00418-3. [DOI] [PubMed] [Google Scholar]
- 7.Modic M. T., Obuchowski N. A., Ross J. S., et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237(2):597–604. doi: 10.1148/radiol.2372041509. [DOI] [PubMed] [Google Scholar]
- 8.Ash L. M., Modic M. T., Obuchowski N. A., Ross J. S., Brant-Zawadzki M. N., Grooff P. N. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098–1103. doi: 10.3174/ajnr.A0999. [DOI] [PMC free article] [PubMed] [Google Scholar]