Abstract
Background
Rapeseed oil is the principal dietary source of monounsaturated and n-3 polyunsaturated fatty acids in the Northern Europe. However, the effect of rapeseed oil on the markers of subclinical atherosclerosis is not known. The purpose of this study was to compare the effects of dietary intake of cold-pressed turnip rapeseed oil (CPTRO) and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome.
Methods
Thirty-seven men with metabolic syndrome completed an open and balanced crossover study. Treatment periods lasted for 6 to 8 weeks and they were separated from each other with an eight-week washout period. Subjects maintained their normal dietary habits and physical activity without major variations. The daily fat adjunct consisted either of 37.5 grams of butter or 35 mL of VirginoR CPTRO. Participants were asked to spread butter on bread on the butter period and to drink CPTRO on the oil period. The fat adjunct was used as such without heating or frying.
Results
Compared to butter, administration of CPTRO was followed by a reduction of total cholesterol by 8% (p < 0.001) and LDL cholesterol by 11% (p < 0.001). The level of oxidized LDL was 16% lower after oil period (p = 0.024). Minimal differences in arterial elasticity were not statistically significant.
Conclusion
Cold-pressed turnip rapeseed oil had favourable effects on circulating LDL cholesterol and oxidized LDL, which may be important in the management of patients at high cardiovascular risk.
Trial registration
ClinicalTrial.gov NCT01119690
Background
Metabolic syndrome (MetS) is an international health problem. Risk factors included in the MetS are impaired glucose tolerance, elevated triglyceride levels, decreased HDL cholesterol concentration, elevated blood pressure and central obesity [1]. The risk of developing coronary heart disease is six to eight times greater and mortality in coronary heart disease two to three times greater among patients with MetS than among their healthy controls [2-5]. Furthermore, patients with MetS are at high risk to develop type 2 diabetes and myocardial infarction [4,5].
Atherosclerosis begins with an accumulation of lipoproteins, particularly low-density lipoprotein (LDL) into the intimae of arteries. In the arterial wall, LDL particles undergo oxidative modification which is suggested to play an important role in the atherosclerotic process [6]. Circulating oxidized LDL (oxLDL) seems to express the level of oxidative stress and associate with the risk factors of MetS [7]. Oxidized LDL has also been found to correlate with the extent of coronary heart disease and to be an independent predictor of an atherosclerotic plaque occurrence [8-10].
Oxidative modification of LDL damages the endothelium of the arterial wall [6]. Altered endothelial structure impairs the elasticity of the arteries already at an early stage of atherosclerosis [11]. Impaired elasticity of arteries may also result from factors affecting the elastic properties of the arterial wall, not only the endothelium. For example aging and hypertension may contribute to an overproduction of collagen and breaks in elastin fibers. Aortic stiffness has been reported to predict future coronary events and cardiovascular death in previous studies [12]. Thus, increased level of oxLDL and arterial stiffness might be used as early indicators of cardiovascular disease.
Despite of its' high total fat content, Mediterranean type diet rich in monounsaturated fatty acids (MUFA), decreases cardiovascular morbidity and mortality [13,14]. MUFA diminishes susceptibility of LDL to oxidation, which may contribute to this benefit [15-17]. The protective effect of n-3 polyunsaturated fatty acids (PUFA) from marine sources has been studied widely. However, the role of the plant derived n-3 PUFA, a principal dietary n-3 PUFA in the western diet, is less clear [18]. Rapeseed oil consists of high amounts of MUFA, a composition similar to that of olive oil. Rapeseed oil is also the main dietary source of plant-derived n-3 PUFA in the Northern Europe. However, the effect of rapeseed oil on the markers of subclinical atherosclerosis is not known.
Subjects with metabolic syndrome seem to have increased oxLDL levels and impaired arterial elasticity [19]. Manipulation of the fat quality in diet, to achieve alterations in plasma lipoproteins and arterial function, is an intriguing strategy in the prevention of atherosclerotic cardiovascular diseases. The aim of our study was to assess whether a dietary intake of cold-pressed turnip rapeseed oil (CPTRO) has beneficial effects on serum lipids, oxidized LDL and arterial elasticity compared to an intake of butter among men with metabolic syndrome.
Methods
Subjects
The present study is a part of Hämeenlinna Metabolic Syndrome research program HMS. It is a regional entity investigating atherosclerotic risk factors in men with MetS, who were referred from private and public consultations in primary and secondary health care. The inclusion criteria were male sex, age 30 to 65 years and a fulfilment of the criteria of MetS according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel [1]. NCEP defines MetS as the presence of at least three of the following five criteria: 1) waist circumference > 102 cm, 2) serum triglycerides ≥ 1.7 mmol/L, 3) HDL cholesterol < 1.03 mmol/L, 4) blood pressure ≥ 130/85 mmHg and 5) fasting plasma glucose ≥ 6.1 mmol/L or diabetes.
43 study subjects, already recruited into HMS, participated in this dietary intervention study. Subjects were individually interviewed on their medical history, dietary habits, smoking and alcohol consumption. Physical examination consisted of height, weight, waist circumference and blood pressure measurements. Body mass index (BMI) was calculated as weight (kg)/height² (m²). Blood pressure was measured four times after at least ten minutes of rest in a semi-sitting position. The individual blood pressure was taken as a mean of these values. The weight and blood pressure measurements were repeated at the end of both study periods. Research personnel performing the laboratory and arterial elasticity measurements were blinded about the diet periods.
The Ethics Committee of the Kanta-Häme Hospital District approved the study protocol and the study followed the ethical principles outlined in the Declaration of Helsinki. Each study subject gave a written informed consent.
Design and Diets
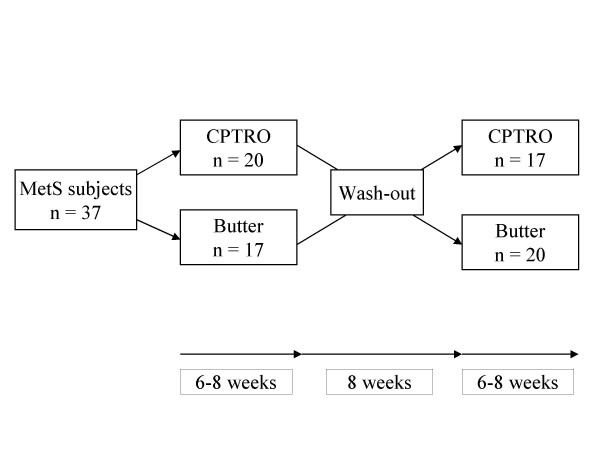
Subjects' usual diet was supplemented with CPTRO and butter in an open, balanced and randomized crossover design. The study consisted of two study periods (oil and butter) lasting from 6 to 8 weeks, and they were separated from each other by an 8-week washout period (Figure 1). To avoid the effect of seasonal variation on the results, the first study period occurred from October to December and the second period from January to March.
Figure 1.
Design of the open, randomized cross-over study. Six of 43 subjects withdrew during the study because they were unable to comply with the dietary regimens.
At the beginning of each fat supplementation period the participants were given both verbal and written detailed instructions to be followed. Butter with a maximal water content of 16% and cold-pressed turnip rapeseed oil (0.92 g/ml) were provided free of charge. The daily fat adjunct consisted either of 37.5 grams of butter (Valio Ltd, Finland) on the saturated fatty acid (SFA) period and 35 mL of VirginoR CPTRO (Kankaisten Öljykasvit Ltd) on the oil period, which comprised approximately 11-12% of daily energy intake. We recommended participants to use the fat adjunct as such, without heating or frying. To keep the diets as stable as possible, participants were asked to spread the butter supplement on bread during the butter period and to drink the oil supplement as well as to eat their bread without spread during the oil period. Besides these instructions subjects were asked to maintain their normal dietary habits and physical activity without major variations. Heating or frying of other fat included in the subjects' every-day diet was not forbidden. To ensure that subjects' everyday diet and physical activity remained similar during both periods, they were asked to fill in a structured questionnaire on their diet as well as on the amount, intensity and type of physical exercise during the study.
Analysis of Fatty Acids and Phenolic Compounds
The fatty acid compositions of cold-pressed turnip rapeseed oil and butter were analysed at MTT Agrifood Research Finland (Jokioinen, Finland). Fatty acids were analysed as their methyl esters. The analysis was carried out using a Hewlett Packard 5890 capillary gas chromatograph equipped with a DB-23 column coated with a cyanopropyl-stationary phase (60 m * 0.25 mm id, film thickness 0.25 μm) and a mass selective detector. A mixture of standard fatty acids (C4:0 - C24:1) purchased from Nu-Check-Prep Inc. (Minnesota, USA) was used as the standard to identify the peaks.
To analyze the amount of phenolic compounds, sample of CPTRO was dissolved into hexane and extracted with 80% methanol according to a modified method of Vuorela et al [20]. Phenolic compounds were identified and quantified by high performance liquid chromatography with diode array detection using analytical conditions similar to those used for the determination of alkylresorcinols [21]. Vinylsyringol (2,6-dimethoxy-4-vinylpheol) was quantified as syringic acid.
The fatty acid compositions of the dietary fat supplements i.e. VirginoR cold-pressed turnip rapeseed oil and butter are shown in Table 1. Butter consisted mainly of saturated fatty acids (61.7%). Oleic acid was the main unsaturated fatty acid found in butter. CPTRO consisted mainly of unsaturated fatty acids (95.6%), over half of which were monounsaturated ones. The major fatty acids in oil were oleic acid, linoleic acid and alfa-linolenic acid. CPTRO contained also a small amount of phenolic compounds. Total amount of sinapine and sinapic acid was 0.94 μmol/100 g and of vinylsyringol 0.89 μmol/100 g. Also some smaller peaks of unknown phenolic compounds were seen in the high performance liquid chromatograms.
Table 1.
Distribution of fatty acids in cold-pressed VirginoR turnip rapeseed oil (CPTRO) and butter used in the study
| Fatty acids | Distribution % | |
|---|---|---|
| CPTRO | Butter | |
| 10:0 capric acid | 0 | 1.6 |
| 12:0 lauric acid | 0 | 2.7 |
| 14:0 myristic acid | 0 | 11.0 |
| 14:1 (ω-5) myristoleic acid | 0 | 0.9 |
| 15:0 pentadecanoic acid | 0 | 0.9 |
| 16:0 palmitic acid | 2.9 | 30.0 |
| 16:1 (ω-7) palmitoleicacid | 0.1 | 1.5 |
| 18:0 stearic acid | 1.3 | 14.6 |
| 18:1 (ω-7) vaccenic acid | 2.9 | 0.9 |
| 18:1 (ω-9) oleic acid | 56.7 | 30.4 |
| 18:2 (ω-6) linoleic acid | 21.9 | 2.0 |
| 18:3 (ω-3) alpha-linolenic acid | 13.1 | 0.4 |
| 20:1 (ω-9) eicosenoic acid | 0.9 | 0 |
| Others (8:0, 17:0, 18:2, 20:0) | 0.3 | 1.4 |
| Unidentified fatty acids | 0 | 1.8 |
| Saturated fatty acids | 4.5 | 61.7 |
| Monounsaturated fatty acids | 60.6 | 33.7 |
| Polyunsaturated fatty acids | 35.0 | 2.9 |
The following fatty acids analyzed did not exist in CPTRO nor in butter: 16:0(i), 16:1, 17:1, 18:3 (ω-6), 18:4 (ω-3), 18:4 (ω-3), 19:1, 20:2 (ω-6), 20:3, 20:3 (ω-3), 20:4 (ω-6), 20:5 (ω-3), 22:0, 22:1 (ω-9), 22:2, 22:3, 22:4 (ω-6), 22:5 (ω-3), 22:6 (ω-3), 24:0, and 24:1 (ω-9).
Blood Sampling and Biochemical Analysis
At the end of both dietary periods, blood samples were collected into 10 mL EDTA tubes, 5 mL lithium-heparin gel tubes and 2 mL natrium-fluoride tubes after a 12-hour overnight fast and at least ten minutes of rest. Levels of HDL, LDL and total cholesterol and triglycerides as well as glucose and HbA1C were analyzed by Cobas Integra procedure (Roche). HbA1C was assessed in % and conversed to mmol/mol [22]. The laboratory practices strict internal quality control with daily and monthly control samples performed by a national external quality assurance program (Labquality Oy).
Determination of Oxidized LDL
Plasma levels of oxidized LDL were determined as duplicates after both diet intervention periods. Determinations were made according to a validated monoclonal antibody 4E6-based capture ELISA (Mercodia AB, Uppsala, Sweden) [7,9]. The monoclonal antibody mAb-4E6 is the same as in the assays previously described by Holvoet et al [8,10]. Samples were diluted with sample buffer in two steps to gain a final dilution 1/6561. 25 μl of each calibrator, control and diluted sample were pipetted into wells containing mouse monoclonal anti-oxidized LDL. 100 μl assay buffer was added into each well. Samples were washed 6 times with automatic washer before 100 μl peroxidase conjugated mouse monoclonal anti-apoB was added. After a 60-minute incubation at room temperature, samples were washed again and the bound conjugate was detected by reaction with 200 μl 3,3', 5,5'-tetramethylbenzidine. Reaction was stopped by 50 μl 0.5 M H2SO4 and the colorimetric endpoint was read spectrophotometrically at 450 nm. Same experienced researcher at Kanta-Häme Central Hospital performed all determinations of oxidized LDL.
Determination of Arterial Elasticity
An experienced nurse measured the elasticity of large and small arteries after at least 10 minutes of rest in a semi-sitting position. The recording was carried out in a temperature-controlled room after an overnight fast. Subjects were asked to refrain from eating, smoking, drinking caffeinated drinks and taking medication for 12 hours and drinking alcohol for two days prior to measurement. Radial artery pulse wave was recorded non-invasively with an arterial tonometry (HDI/PulseWave™CR-2000) that uses a modified Windkessel pulse-contour method [11]. The capacitive elasticity of large arteries (C1) and the reflective elasticity of small arteries (C2) were automatically assessed as a mean of five most similar pulse waves appearing during thirty seconds of measurement. Four measurements were performed to gain mean large and small arterial elasticity for every subject. Studies were performed at the end of both diet periods.
Statistical Analysis
Statistical analysis was carried out with the SPSS software (version 17.0, SPSS 2009). Basic results are reported as mean ± SD. Paired samples' T-test was used to compare the outcome measurements after diets in case of normality. Wilcoxon's test was used in case of non-normality. These results are reported as mean ± SEM. Statistical significance was accepted at p value < 0.05.
Results
Six men withdrew because they were unable to comply with the dietary regimens. Results include thirty-seven subjects who completed both study periods. Baseline characteristics of these 37 men are shown in Table 2.
Table 2.
Basic clinical and laboratory characteristics of 37 men with metabolic syndrome accomplishing all clinical phases of the study
| Mean (SD) | Range | |
|---|---|---|
| Height, cm | 177.1 (5.7) | 163.0 - 191.0 |
| Weight, kg | 97.4 (16.3) | 68.0 - 142.0 |
| Waist circumference, cm | 111.1 (13.1) | 92.0 - 150.0 |
| BMI, kg/m2 | 31.0 (5.0) | 23.9 - 46.4 |
| SBP, mmHg | 145.5 (12.3) | 122.0 - 172.0 |
| DBP, mmHg | 90.8 (5.6) | 80.0 - 105.0 |
| Fasting glucose, mmol/L | 6.62 (1.19) | 3.3 - 9.5 |
| HbA1C | ||
| (%) | 6.42 (0.66) | 5.3 - 8.7 |
| (mmol/mol) | 46.7 (7.2) | 34.4 - 71.6 |
| Total cholesterol, mmol/L | 5.15 (1.49) | 2.6 - 8.4 |
| HDL-C, mmol/L | 1.15 (0.27) | 0.5 - 1.7 |
| LDL-C, mmol/L | 3.33 (1.25) | 1.1 - 6.0 |
| Triglycerides, mmol/L | 2.19 (1.13) | 0.9 - 6.3 |
| ALAT, U/L | 53.0 (27.4) | 25.0 - 163.0 |
| GT, U/L | 72.4 (62.9) | 15.0 - 275.0 |
| Afos, U/L | 67.6 (19.7) | 30.0 - 115.0 |
| Fibrinogen, g/L | 3.49 (0.96) | 2.3 - 5.5 |
There were six current smokers, twenty-one ex-smokers and nine non-smokers. Twenty-one men had earlier been diagnosed with hypertension, nineteen with type 2 diabetes and six with coronary heart disease. Pharmacological therapy consisted of aspirin or other antiplatelet agent in fourteen, beta-blockers in sixteen, and ACE inhibitors or angiotensin1 receptor inhibitors in fourteen subjects, but only four were on glucose-lowering drugs. Fifteen men were on statin medication, two of these on combination therapy of statin and fibrate.
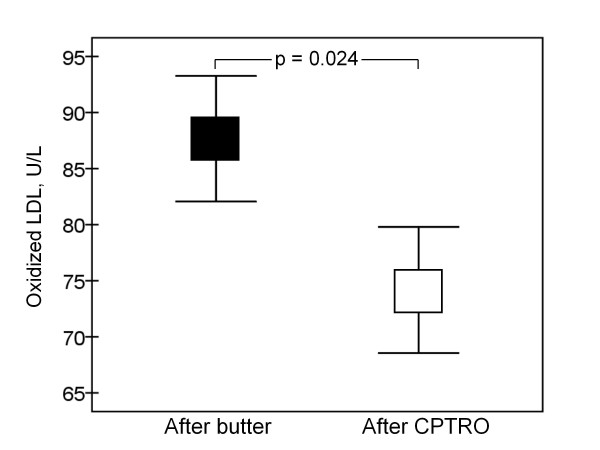
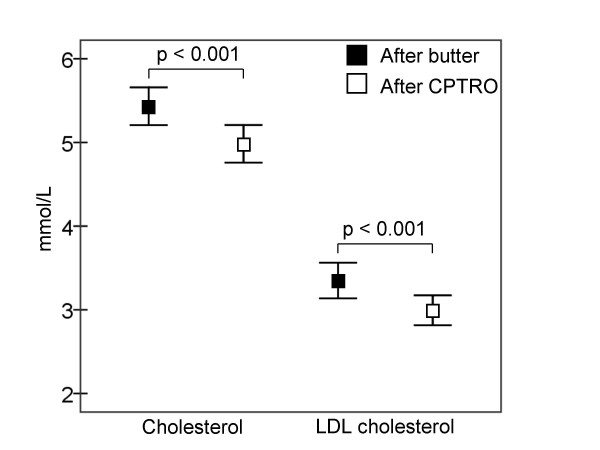
Physical activity, medication and every-day diet of participants, evaluated by a structured questionnaire, remained stable during the study. As presented in Figure 2, the level of oxidized LDL was significantly lower (16%) after the cold-pressed turnip rapeseed oil period compared to the butter period (p = 0.024). Total and LDL cholesterol were also significantly lower after the CPTRO period compared to the levels after the butter period, 8% (p < 0.001) and 11% (p < 0.001), respectively (Figure 3). No significant differences in HDL cholesterol, triglycerides, glucose or HbA1C were found between the periods. The concentration of HDL cholesterol was 1.15 ± 0.04 mmol/L after both diet periods. Triglycerides were 2.72 ± 0.30 mmol/L after the butter and 2.56 ± 0.38 mmol/L after the CPTRO periods (NS).
Figure 2.
Plasma concentration of oxidized LDL at the end of fat supplementation periods. OxLDL was 87.7 ± 5.6 U/L after the butter and 74.2 ± 5.6 U/L after the CPTRO (cold-pressed turnip rapeseed oil) period (p = 0.024).
Figure 3.
Concentrations of total and LDL cholesterol. Total cholesterol was 5.43 ± 0.23 mmol/L at the end of the butter and 4.98 ± 0.23 mmol/L at the end of the CPTRO (cold-pressed turnip rapeseed oil) period (p < 0.001). LDL cholesterol was 3.35 ± 0.21 mmol/L and 3.00 ± 0.18 mmol/L, respectively (p < 0.001).
There were no significant differences in the mean large or small arterial elasticity between the diet periods (Table 3). Systolic blood pressure was 138.5 ± 2.2 mmHg after the butter and 138.5 ± 2.6 mmHg after the CPTRO period. Diastolic blood pressures were 81.8 ± 1.4 mmHg and 81.3 ± 1.6 mmHg, respectively. Weight remained stable through the study, 97.1 ± 2.8 after the butter and 97.1 ± 2.7 after the oil period. Total blood count showed no significant differences between rapeseed oil and butter consumption except in the mean corpuscular haemoglobin (31.1 ± 0.2 pg vs. 30.8 ± 0.2 pg, p = 0.01) and in the platelet count (221.6 ± 9.0 E9/L vs. 235.4 ± 9.5 E9/L, p = 0.003).
Table 3.
Large (C1) and small (C2) arterial elasticity at the end of butter and oil periods.
| Butter period | Oil period | p value | |
|---|---|---|---|
| C1, mL/mmHg × 10 | 15.5 ± 0.46 | 15.9 ± 0.71 | NS |
| C2, mL/mmHg × 100 | 6.26 ± 0.48 | 6.34 ± 0.44 | NS |
Data are presented as mean ± SEM
Discussion
In this study among men with MetS dietary cold-pressed turnip rapeseed oil was associated with favourable changes in lipid parameters compared to the use of butter. The lower level of total cholesterol after CPTRO consumption was due to the lower level of LDL cholesterol. However, the concentration of circulating oxLDL was proportionally even lower than that of LDL cholesterol after the CPTRO period.
To our knowledge, this is the first report on the beneficial effect of rapeseed oil on circulating oxLDL levels. The significant reductions in total and LDL cholesterol levels are in accordance with earlier rapeseed oil studies [23,24]. In agreement with most of the previous studies [23,25,26] the use of rapeseed oil did not have significant effect on HDL cholesterol levels, although a slight increase in HDL cholesterol by rapeseed oil has also been reported [24].
LDL particles enriched with MUFA seem to be resistant against oxidation [15-17]. Since MUFA are the main fatty acids in rapeseed oil, this protective effect may contribute to the decreased level of oxLDL after CPTRO enriched diet in the present study. Previously, a diet with high content of MUFA has been shown to decrease oxLDL levels among healthy non-obese men as well as among subjects at high cardiovascular risk [27,28]. Although effects of turnip rapeseed oil among metabolic syndrome patients have not been studied earlier, high MUFA-rich diet has been reported to improve oxidative stress parameters in patients with MetS [29]. Also in a study by Perona et al [30], MUFA-rich virgin olive oil protected LDL against oxidation among type 2 diabetics.
The origin of circulating oxLDL is unknown. Oxidative modification of LDL can generate minimally or fully oxidized LDL [31]. It is generally thought that oxidation of LDL occurs mainly in the arterial wall and only minimally oxidized LDL exists in the circulation [6,31]. However, increased circulating oxLDL, determined by a method using the same monoclonal antibody as in the present study, is known to associate both with subclinical and clinical atherosclerosis as well as to predict future cardiovascular events [8,9,32]. Thus, dietary manipulations that protect LDL against oxidation may delay development of atherosclerotic lesions in patients with MetS.
Increased glucose levels, often present among subjects with MetS, may enhance the oxidation of lipoprotein(a) [33]. Previously, a low-carbohydrate (LC) diet had more favourable effects on MetS features compared to a low-fat diet [34]. A low-carbohydrate diet has also been reported to prevent the accumulation of oxLDL in the arterial wall [35]. Whether a LC-diet supplemented with CPTRO would cause even greater reductions in oxLDL and prevent future cardiovascular events among MetS subjects would be interesting to study.
Most dietary approaches to prevent hyperlipidaemia and atherosclerosis have focused on the alteration of fat composition and quantity in diet. In the present study, CPTRO had a cholesterol-lowering effect as a supplement to the habitual diet. Turnip rapeseed oil is the main Finnish source of unsaturated fatty acids, mainly monounsaturated ones. By improved cultivation, MUFA in rapeseed oil consist of an important amount of oleic acid. Mediterranean type diet, also rich in MUFA, has been shown to decrease total cardiovascular mortality [13]. Compared to olive oil, common in the Mediterranean region, VirginoR rapeseed oil contains less MUFA and SFA but a considerable amount of n-3 PUFA, namely alfa-linolenic acid (see Table 1).
Low dose of n-3 PUFA supplement reduced early mortality after myocardial infarction in the GISSI-Prevenzione trial [36]. On the other hand LDL susceptibility to oxidation has been assumed to associate with an increased amount of PUFA in the small and dense LDL particles [37]. In addition, PUFA-rich diet increased susceptibility of LDL to oxidation when compared to MUFA-rich diet among subjects with impaired glucose intolerance [38]. However, moderate amounts of highly unsaturated n-3 fatty acids in a diet supplemented with rapeseed oil did not increase LDL oxidizability when provided in the context of a diet rich in MUFA [15].
In addition to MUFA, phenolic compounds of olive oil have been shown to reduce oxidative damage [39]. Rapeseed oil, supplemented in the present study, contained only slight amounts of phenolic compounds. Therefore, the antioxidative effect of rapeseed oil is presumably mediated mainly by the high contents of MUFA and n-3-PUFA.
In our study, arterial elasticity seemed to be minimally better after the CPTRO than after the butter period, but the difference was not statistically significant. One explanation might be that the intervention period was too short to affect the function of the arterial wall. However, McVeigh et al [40] have found improved arterial compliance already after a 6-week fish oil administration in patients with non-insulin-dependent diabetes. Arterial stiffness is not merely affected by endothelial dysfunction. For example, untreated long-lasting hypertension and aging may cause increase in the amount of collagen and decrease in the amount of elastin fibers in the arterial wall [12]. Alterations in the elastic properties of the arterial wall contribute to the impairment of elasticity.
Since endothelial dysfunction is a key mechanism in atherosclerosis, a measurement of mere endothelial function might be more sensitive in a short-term intervention study [11]. Previously, a walnut diet rich in antioxidants and alfa-linolenic acid for four weeks showed an improvement in endothelial function of hypercholesterolaemic subjects [41]. Even one meal supplemented with walnuts rich in n-3-PUFA, has been reported to improve flow-mediated dilation of arteries as a sign of improved endothelial function [42]. The same effect was however not seen after a meal supplemented with MUFA-rich olive oil [42]. Differences in fat type and study groups or methods used to evaluate arterial function might explain the seeming discrepancy of the results between the abovementioned studies and the present one. It would be interesting to study the effects of rapeseed oil among subjects with evidently decreased arterial elasticity.
The platelet count was lower after the CPTRO period in our study. It has been revealed earlier that fish oils decrease fibrinogen level and platelet count [43]. Vegetable n-3 PUFA, like alfa-linolenic acid, is an important constituent of the VirginoR CPTRO. During fat metabolism it can be converted to longer chain fatty acids found also in fish oils. Hence, the significant decrease in platelet count after CPTRO consumption may be attributed to the n-3 PUFA.
Strength of this study, unlike of many other dietary studies, was the balanced crossover design used to diminish the variability between the subjects. We did not measure the fat and energy intake by dietary records, which was a limitation of the study. However, we ensured the stability of both habitual diet and physical activity during the study periods by analyzing the detailed questionnaire that subjects filled in at the end of the intervention periods. Previously a similar food frequency questionnaire has been reported to be a suitable tool for ranking energy and nutrient intake [44]. In addition, there was no difference in weight between the two dietary periods which is in agreement with previous studies on n-3-PUFA and MUFA rich diets [45,46]. Hence, the difference in energy balance did not explain our findings.
A healthy cohort would have been an interesting addition to the study. However, we have previously reported increased oxLDL levels as well as impaired large arterial elasticity among MetS subjects compared to their healthy, age-matched counterparts [19]. Therefore, in the present study we wanted to focus on MetS subjects because of their increased risk for atherosclerotic cardiovascular diseases.
Vegetable oils are often consumed in cooking and frying. However, repeated heating may destroy the beneficial compounds of the oils and generate unhealthy effects instead [47,48]. Therefore, study subjects were asked to drink CPTRO as such, without heating or frying. Heating or frying of fat included in the subjects' every-day diet was not forbidden. We cannot determine the optimal daily dose of CPTRO based on the results of this study, in which a constant dose of CPTRO and a comparable amount of butter were used. Previously whipped cream with a similar dose of SFA as used in the present study did not cause a significant increase in postprandial oxidative stress [49]. Thus, at least the dose of CPTRO, used in the present study, seems to be beneficial as a supplement to a diet. In practise, it may be used as such for example in salad and dip sauces. It is worth to notice that the study included only men with MetS. Hence, the results cannot necessarily be generalized to the whole population including women and men without MetS.
Conclusion
A short-time modification of the diet with cold-pressed turnip rapeseed oil compared to butter decreased the concentration of circulating LDL cholesterol and oxidized LDL. In men with metabolic syndrome, modification of the diet with cold-pressed turnip rapeseed oil may be beneficial in treating hyperlipidaemia and diminishing oxidative stress and thereby delaying the progression of atherosclerosis.
List of Abbreviations
BMI: body mass index; C1: large arterial elasticity; C2: small arterial elasticity; CPTRO: cold-pressed turnip rapeseed oil; HbA1C: glycosylated haemoglobin; HDL: high density lipoprotein; HMS: Hämeenlinna Metabolic Syndrome Research Program; LC: low-carbohydrate; LDL: low density lipoprotein; MetS: metabolic syndrome; MUFA: mono-unsaturated fatty acids; NCEP: National Cholesterol Education Program; oxLDL: oxidized low density lipoprotein; PUFA: poly-unsaturated fatty acids; SFA: saturated fatty acids
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AP designed the study with the help of KO. AP, HPM and MW participated in the acquisition of data, analysis and drafting of the manuscript. PK, HA, SH, and JMP made a substantial contribution in the acquisition of data and helped in drafting the manuscript, KO participated in the acquisition of data and gave the final approval of the version to be published. All authors read and approved the final manuscript.
Contributor Information
Ari Palomäki, Email: ari.palomaki@khshp.fi.
Hanna Pohjantähti-Maaroos, Email: hanna.pohjantahti-maaroos@kuh.fi.
Marja Wallenius, Email: marja.wallenius@armas.fi.
Päivi Kankkunen, Email: paivi.kankkunen@ttl.fi.
Heikki Aro, Email: heikki.aro@tekes.fi.
Sari Husgafvel, Email: sari.husgafvel@khshp.fi.
Juha-Matti Pihlava, Email: juha-matti.pihlava@mtt.fi.
Kalevi Oksanen, Email: kalevi.oksanen@khshp.fi.
Acknowledgements and Funding
This study was supported by grants from the Ministry of Health and Social Welfare in Finland through the Medical Research Fund of Kanta-Häme Central Hospital, the Häme Regional Fund under the auspices of the Finnish Cultural Foundation, Kankaisten Öljykasvit Ltd, and Hilkka and Väinö Kiltti Foundation. None of them had a role in data collection, analysis of the results, or preparation of the manuscript.
We sincerely acknowledge Mrs Leena Puura for determining the fatty acid profiles, Mrs Tuula Kurtélius for analyzing phenolic compounds of CPTRO, and RN Paula Lahtinen for measuring arterial elasticity. We acknowledge sincerely the professional aid of MD Ruth Laitinen. We appreciate the professional technical aid of Mrs Kirsti Räsänen. The authors gratefully acknowledge the cooperation of the study subjects.
References
- Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- Vanhala MJ, Kumpusalo EA, Pitkajarvi TK, Takala JK. Metabolic syndrome in a middle-aged Finnish population. J Cardiovasc Risk. 1997;4:291–295. doi: 10.1097/00043798-199708000-00010. [DOI] [PubMed] [Google Scholar]
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Sattar N, Gaw Allan, Scherbakova O, Ford I, O´Reilly D, Haffner S, Isles C, Macfarlane P, Packard C, Cobbe S, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland coronary prevention study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir V, Fagerberg B, Hulthe J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year-old men. J Intern Med. 2002;252:440–447. doi: 10.1046/j.1365-2796.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (Air Study) Arterioscler Thromb Vasc Biol. 2002;22:1162–1167. doi: 10.1161/01.ATV.0000021150.63480.CD. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Mertens A, Verhamme P, Bogaerts K, Beyens G, Verhaeghe R, Collen D, Muls E, Van de Werf F. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21:844–848. doi: 10.1161/hq1201.100221. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- De Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- Kratz M, Cullen P, Kannenberg F, Kassner A, Fobker M, Abuja PM, Assmann G, Wahrburg U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur J Clin Nutr. 2002;56:72–81. doi: 10.1038/sj.ejcn.1601288. [DOI] [PubMed] [Google Scholar]
- Cicero A, Nascetti S, López-Sabater M, Elosua R, Salonen J, Nyyssönen K, Poulsen H, Zunft H-J, Kiesewetter H, de la Torre K, Covas M, Kaikkonen J, Mursu J, Koenbick C, Bäumler H, Gaddi A. EUROLIVE study group. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: the EUROLIVE study. J Am Coll Nutr. 2008;2:314–320. doi: 10.1080/07315724.2008.10719705. [DOI] [PubMed] [Google Scholar]
- Moreno J, López-Miranda J, Péreez-Martίnez P, Marίn C, Moreno R, Gómez P, Paniagua J, Pérez-Jiménez F. A monounsaturated fatty acid-rich diet reduces macrophage uptake of plasma oxidised low-density lipoprotein in healthy young men. Br J Nutr. 2008;100:569–575. doi: 10.1017/S0007114508911508. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Ma DWL. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009;8:33. doi: 10.1186/1476-511X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjantähti-Maaroos H, Palomäki A, Kankkunen P, Laitinen R, Husgafvel S, Oksanen K. Circulating oxidized low-density lipoproteins and arterial elasticity: comparison between men with metabolic syndrome and physically active counterparts. Cardiovasc Diabetol. 2010;9:41. doi: 10.1186/1475-2840-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorela S, Meyer A, Heinonen M. Quantitative analysis of the main phenolics in rapeseed meal and oils processed differently using enzymatic hydrolysis and HPLC. Eur Food Res Technol. 2003;217:517–523. doi: 10.1007/s00217-003-0811-3. [DOI] [Google Scholar]
- Mattila P, Pihlava JM, Hellström J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J Agric Food Chem. 2005;53:8290–8295. doi: 10.1021/jf051437z. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsta LM, Jauhiainen M, Aro A, Katan MB, Mutanen M. Effects of a monounsaturated rapeseed oil and a polyunsaturated sunflower oil diet on lipoprotein levels in humans. Arterioscler Thromb. 1992;12:50–57. doi: 10.1161/01.atv.12.1.50. [DOI] [PubMed] [Google Scholar]
- Seppänen-Laakso T, Vanhanen H, Laakso I, Kohtamaki H, Viikari J. Replacement of margarine on bread by rapeseed and olive oils: effects on plasma fatty acid composition and serum cholesterol. Ann Nutr Metab. 1993;37:161–174. doi: 10.1159/000177765. [DOI] [PubMed] [Google Scholar]
- Gulesserian T, Widhalm K. Effect of a rapeseed oil substituting diet on serum lipids and lipoproteins in children and adolescents with familial hypercholesterolemia. J Am Coll Nutr. 2002;21:103–108. doi: 10.1080/07315724.2002.10719201. [DOI] [PubMed] [Google Scholar]
- Vermunt S, Beaufrère B, Riemersma R, Sébédio J, Chardigny J, Mensink R. TransLinE Investigators a. Dietary trans alpha-linolenic acid from deodorised rapeseed oil and plasma lipids and lipoproteins in healthy men: the TransLinE Study. Br J Nutr. 2001;85:249–250. doi: 10.1079/BJN2000270. [DOI] [PubMed] [Google Scholar]
- Fitó M, Guxens M, Corella D, Sáez G, Estruch R, de la Torre R, Francés F, Cabezas C, López-Sabater M, Marrugat J, Garcia-Arellano A, Arós F, Ruiz-Gutierrez V, Ros E, Salas-Salvadó J, Fiol M, Solá R, Covas M. PREDIMED Study Investigators. Effect of traditional Mediterranean diet on lipoprotein oxidation. Arch Intern Med. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- Egert S, Kratz M, Kannenberg F, Fobker M, Wahrburg U. Effects of high-fat and low-fat diets rich in monounsaturated fatty acids on serum lipids, LDL size and indices of lipid peroxidation in healthy non-obese men and women when consumed under controlled conditions. Eur J Nutr. 2010. in press . [DOI] [PubMed]
- Perez-Martinez P, Garcia-Quintana J, Yubero-Serrano E, Tasset-Cuevas I, Tunez I, Garcia-Rios A, Delgado-Lista J, Marin C, Perez-Jimenez F, Roche H, Lopez-Miranda. Postprandial oxidative stress is modified by dietary fat: evidence from a human intervention study. Clin Sci. 2010;119:251–261. doi: 10.1042/CS20100015. [DOI] [PubMed] [Google Scholar]
- Perona S, Montero E, Sánchez-Dominίguez J, Cañizares J, Garcia M, Ruiz-Gutiérrez V. Evaluation of the effect of dietary virgin olive oil on blood pressure and lipid composition of serum and low-density lipoprotein in elderly type 2 diabetic subjects. J Agric Food Chem. 2009;57:11427–11433. doi: 10.1021/jf902321x. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Miura S, Zhang B, Uehara Y, Matsuo Y, Fujino M, Sawamura T, Saku K. Low-density lipoprotein oxidized to various degrees activates ERK1/2 through Lox-1. Atherosclerosis. 2006;188:245–250. doi: 10.1016/j.atherosclerosis.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- Kotani K, Yamada S, Uurtya S, Yamada T, Taniguchi N, Sakurabayashi I. The association between blood glucose and oxidized lipoprotein(a) in healthy young women. Lipids Health Dis. 2010;9:103. doi: 10.1186/1476-511X-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favourable impact on the metabolic syndrome than low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- Leite JO, DeOgburn R, Ratliff J, Su R, Smyth JA, Volek JS, McGrane MM, Dardik A, Fernandez ML. Low-carbohydrate diets reduce lipid accumulation and arterial inflammation in guinea pigs fed a high-cholsterol diet. Atherosclerosis. 2010;209:442–448. doi: 10.1016/j.atherosclerosis.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.CIR.0000014682.14181.F2. [DOI] [PubMed] [Google Scholar]
- De Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb. 1991;11:298–306. doi: 10.1161/01.atv.11.2.298. [DOI] [PubMed] [Google Scholar]
- Schwab US, Sarkkinen ES, Lichtenstein AH, Li Z, Ordovas JM, Schaefer EJ, Uusitupa MI. The effect of quality and amount of dietary fat on the susceptibility of low density lipoprotein to oxidation in subjects with impaired glucose tolerance. Eur J Clin Nutr. 1998;52:452–458. doi: 10.1038/sj.ejcn.1600587. [DOI] [PubMed] [Google Scholar]
- Cicerale S, Lucas L, Keast R. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD. Fish oil improves arterial compliance in non-insulin-dependent diabetes mellitus. Arterioscler Thromb. 1994;14:1425–1429. doi: 10.1161/01.atv.14.9.1425. [DOI] [PubMed] [Google Scholar]
- Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- Cortés B, Núñez I, Cofán M, Gilabert R, Pérez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Leaf A. Cardiovascular effects of fish oils: beyond the platelet. Circulation. 1990;82:624–628. doi: 10.1161/01.cir.82.2.624. [DOI] [PubMed] [Google Scholar]
- Araujo MC, Yokoo EM, Pereira RA. Validation and calibration of a semiquantitative food frequency questionnaire designed for adolescents. J Am Diet Assoc. 2010;110:1170–1177. doi: 10.1016/j.jada.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Kratz M, Callahan HS, Yang PY, Matthys CC, Weigle DS. Dietary n-3-polyunsaturated fatty acids and energy balance in overweight or moderately obese men and women: a randomized controlled trial. Nutr Metab. 2009;6:24. doi: 10.1186/1743-7075-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm BJ, Lattin BL, Summer SS, Boback JA, Gilchrist GM, Jandacek RJ, D'Alessio DA. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care. 2009;32:215–220. doi: 10.2337/dc08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong XF, Mustafa MR, Das S, Jaarin K. Association of elevated blood pressure and impaired vasorelaxation in experimental Sprague-Dawley rats fed with heated vegetable oil. Lipids Health Dis. 2010;9:66. doi: 10.1186/1476-511X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Singh M, George J, Bhui K, Murari Saxena A, Shukla Y. Genotoxic and carcinogenic risks associated with the dietary consumption of repeatedly heated coconut oil. Br J Nutr. 2010. in press . [DOI] [PubMed]
- Bloomer RJ, Kabir MM, Marshall KE, Canale RE, Farney TM. Postprandial oxidative stress in response to dextrose and lipid meals of differing size. Lipids Health Dis. 2010;9:79. doi: 10.1186/1476-511X-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]