Abstract
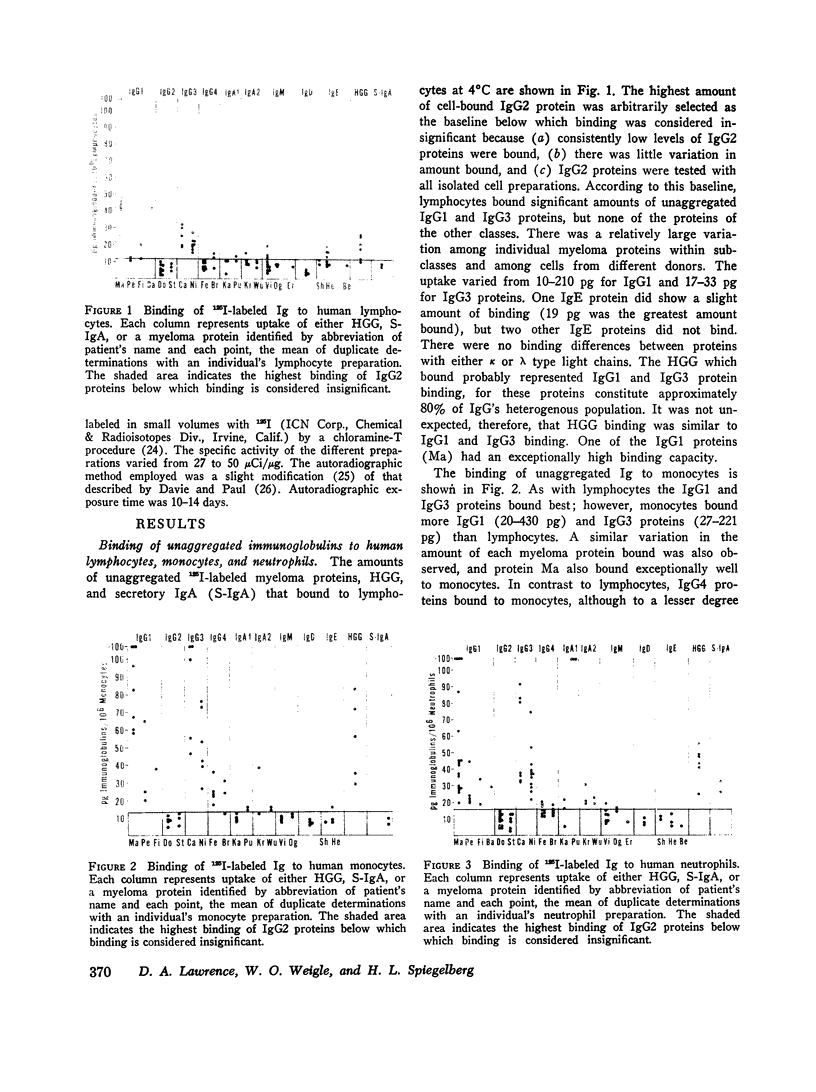
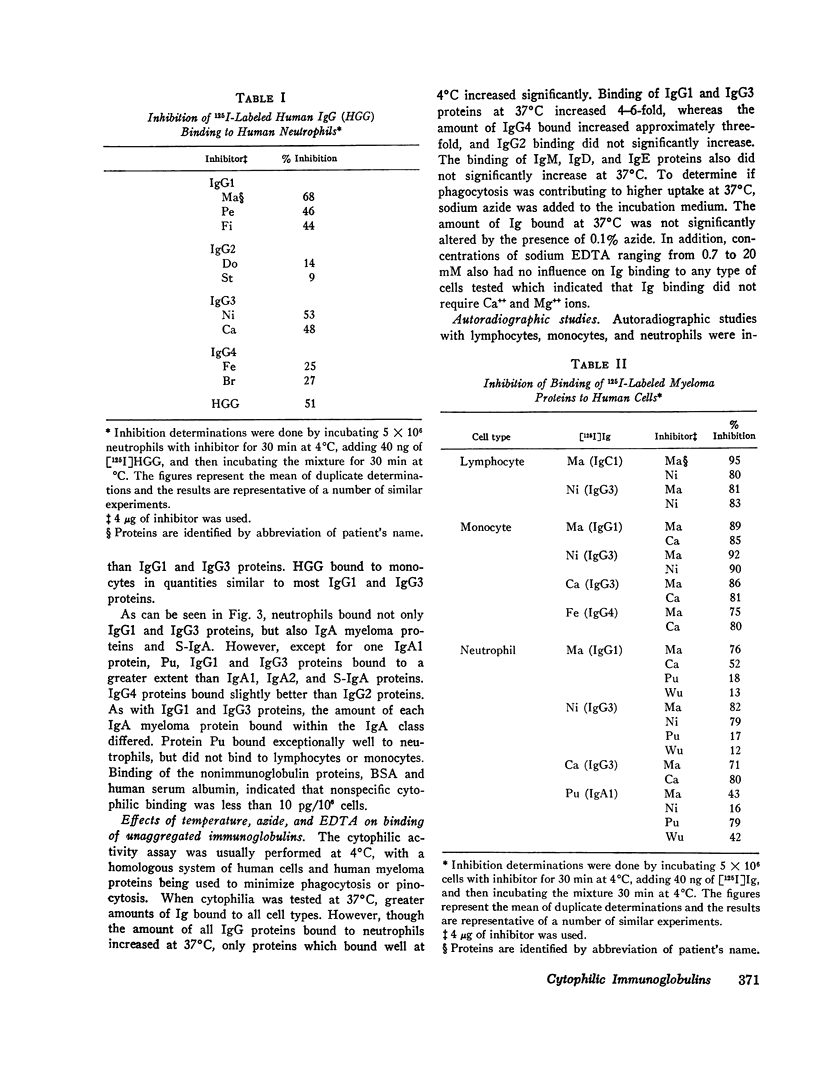
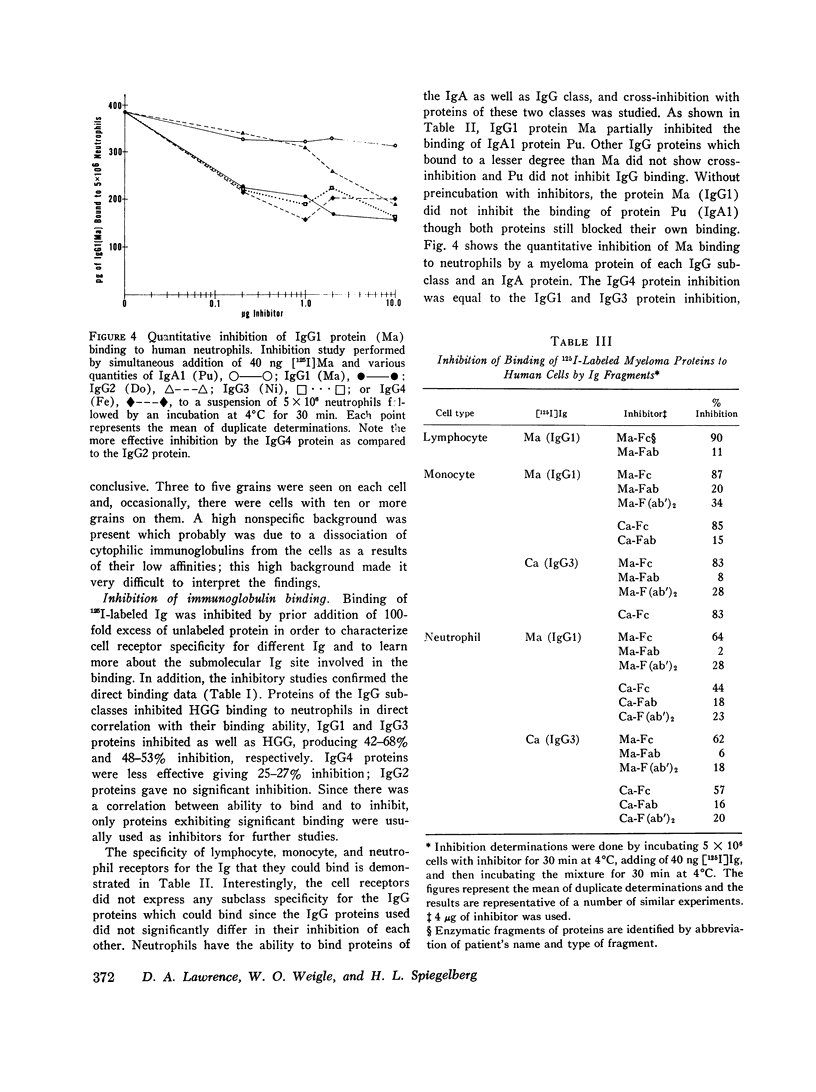
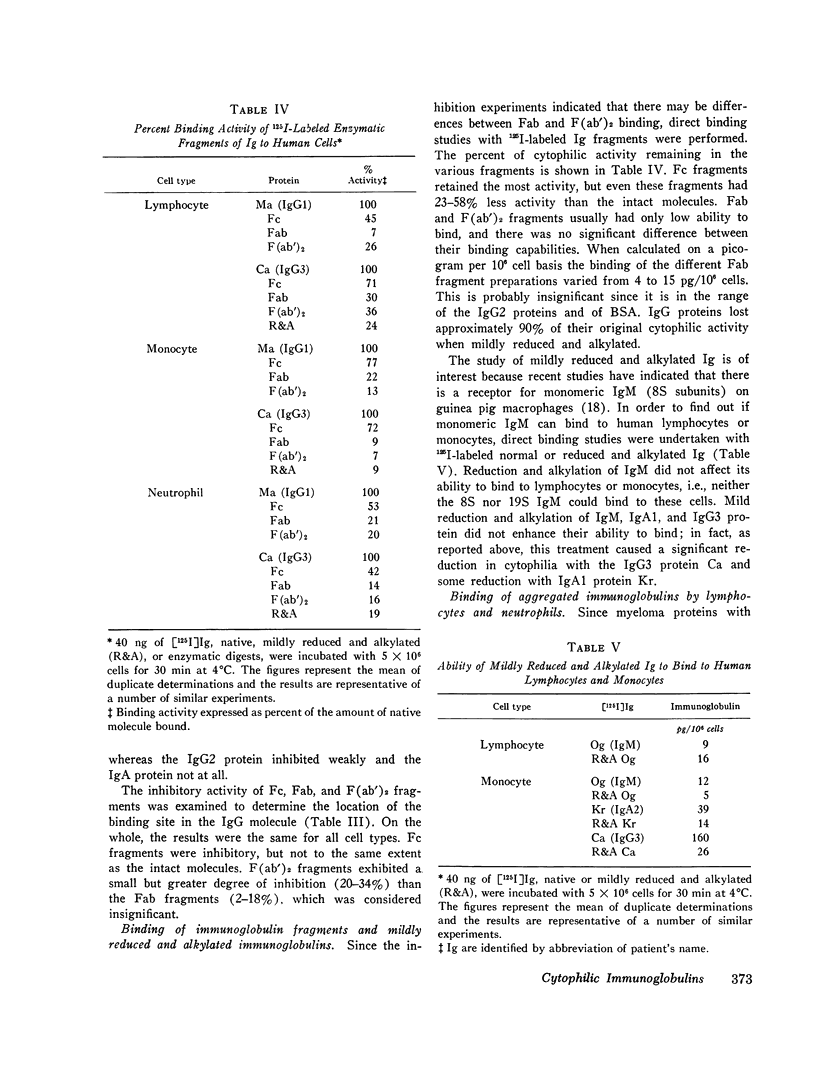
The cytophilic activity of human myeloma proteins of different classes and subclasses for lymphocytes, monocytes, and neutrophils was investigated. Binding of both unaggregated immunoglobulins (Ig) and Ig aggregated with rabbit F(ab)2 anti-Fab fragment sera was determined. Lymphocytes bound unaggregated IgG1 and IgG3 proteins, but none of the proteins of the other classes. In contrast, after aggregation, IgG of all subclasses and IgE proteins bound to lymphocytes; aggregated proteins of the other classes did not bind. Monocytes bound unaggregated IgG1 and Ig3 better than Ig4 whereas the binding of proteins of other classes was insignificant. Neutrophils bound unaggregated IgG1 and IgG3 proteins and, in addition, IgA1, IgA2, secretory IgA, and IgG4 proteins. After aggregation, the neutrophils bound more Ig of all classes; however, the differences between the amounts bound remained similar to the amounts of unaggregated proteins. The native structure of the Ig molecule is necessary for the maintenance of complete activity, because Fc fragments bound less than intact Ig, and reduction and alkylation abolished cytophilia. The Fc receptors on all cell types tested showed no specificity for any of the respective cytophilic IgG subclasses; however, neutrophils appear to have separate receptors for IgG and IgA proteins.
Full text
PDF

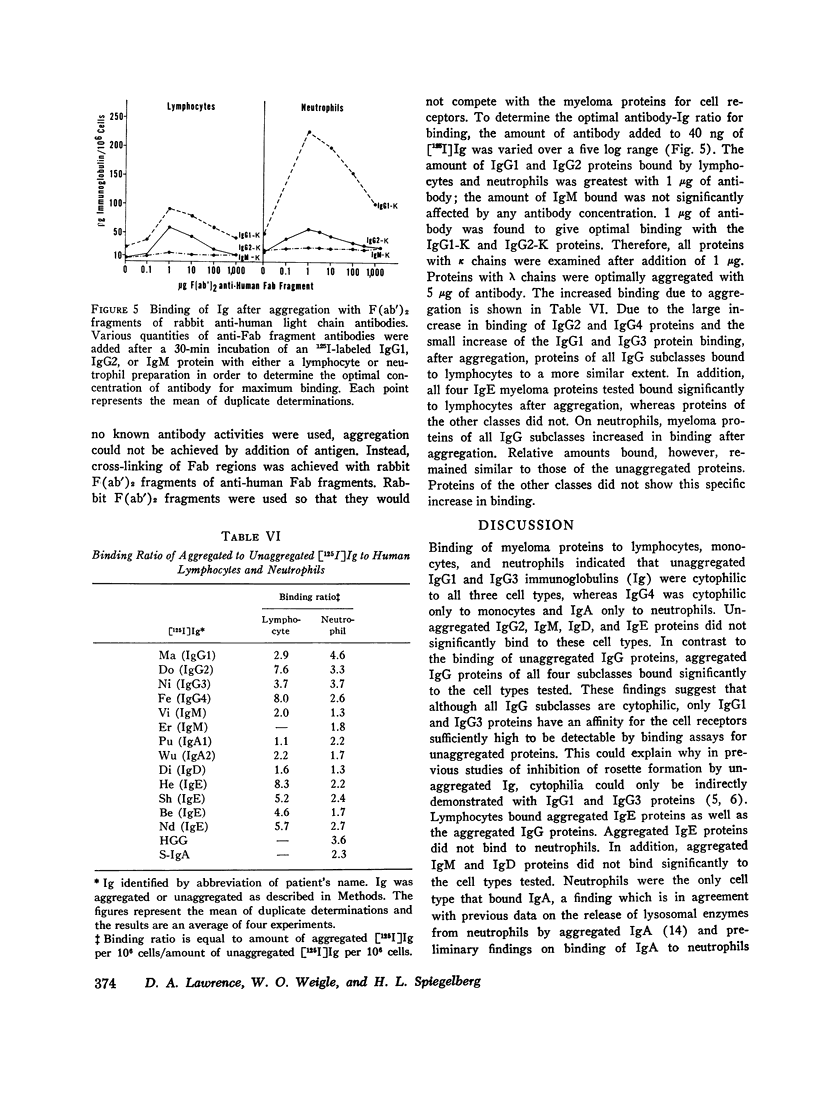


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Grey H. M. Receptors for aggregated IgG on mouse lymphocytes: their presence on thymocytes, thymus-derived, and bone marrow-derived lymphocytes. J Exp Med. 1974 May 1;139(5):1175–1188. doi: 10.1084/jem.139.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V., SORKIN E. The adsorption of antigen by spleen cells previously treated with antiserum in vitro. Immunology. 1960 Jul;3:272–283. [PMC free article] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. II. Specificity and nature of receptors on dinitrophenylated guinea pig albumin- 125 I-binding lymphocytes of normal guinea pigs. J Exp Med. 1971 Aug 1;134(2):495–516. doi: 10.1084/jem.134.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Hudson L., Shen L., Roitt I. M. Antibody-dependent cell-mediated cytotoxicity due to a "null" lymphoid cell. Nat New Biol. 1973 Mar 28;242(117):111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Inchley C., Grey H. M., Uhr J. W. The cytophilic activity of human immunoglobulins. J Immunol. 1970 Aug;105(2):362–369. [PubMed] [Google Scholar]
- Ishizaka K. Human reaginic antibodies. Annu Rev Med. 1970;21:187–200. doi: 10.1146/annurev.me.21.020170.001155. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Tomioka H., Ishizaka T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. J Immunol. 1970 Dec;105(6):1459–1467. [PubMed] [Google Scholar]
- Lawrence D. A., Spiegelberg H. L., Weigle W. O. 2,4-Dinitrophenyl receptors on mouse thymus and spleen cells. J Exp Med. 1973 Feb 1;137(2):470–482. doi: 10.1084/jem.137.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Ca++-dependent binding of antigen-19 S antibody complexes to macrophages. J Immunol. 1969 May;102(5):1172–1178. [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Boyden S. V. Macrophage cytophilic antibodies and delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):15–20. doi: 10.1093/oxfordjournals.bmb.a070508. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Receptor for monomeric IgM on guinea-pig splenic macrophages. Nature. 1973 Jun 29;243(5409):527–528. doi: 10.1038/243527a0. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Lawrence D. A., Henson P. Cytophilic properties of IgA to human neutrophils. Adv Exp Med Biol. 1974;45(0):67–74. doi: 10.1007/978-1-4613-4550-3_8. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Prahl J. W., Grey H. M. Structural studies of human gamma D myeloma protein. Biochemistry. 1970 May 12;9(10):2115–2122. doi: 10.1021/bi00812a013. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Weigle W. O. The immune response to human gamma-G-immunoglobulin in rabbits unresponsive to Fc fragment and H chain protein. J Immunol. 1967 May;98(5):1020–1027. [PubMed] [Google Scholar]
- Spiegelberg H. L., Weigle W. O. The production of antisera to human gammaGlobulin subclasses in rabbits using immunological unresponsiveness. J Immunol. 1968 Aug;101(2):377–380. [PubMed] [Google Scholar]
- Tai C., McGuigan J. E. Detection of cell-absorbed protein antigen by an equilibrium diffusion method. J Immunol. 1971 Jun;106(6):1540–1544. [PubMed] [Google Scholar]
- Unanue E. R. Antigen-binding cells. I. Their idenification and role in the immune response. J Immunol. 1971 Oct;107(4):1168–1174. [PubMed] [Google Scholar]
- Webb S. R., Cooper M. D. T cells can bind antigen via cytophilic IgM antibody made by B cells. J Immunol. 1973 Jul;111(1):275–277. [PubMed] [Google Scholar]
- Zembala M., Ptak W., Hanczakowska M. The induction of delayed hypersensitivity by macrophage-associated antigen. The role of macrophage cytophilic antibody. Immunology. 1974 Mar;26(3):465–476. [PMC free article] [PubMed] [Google Scholar]






