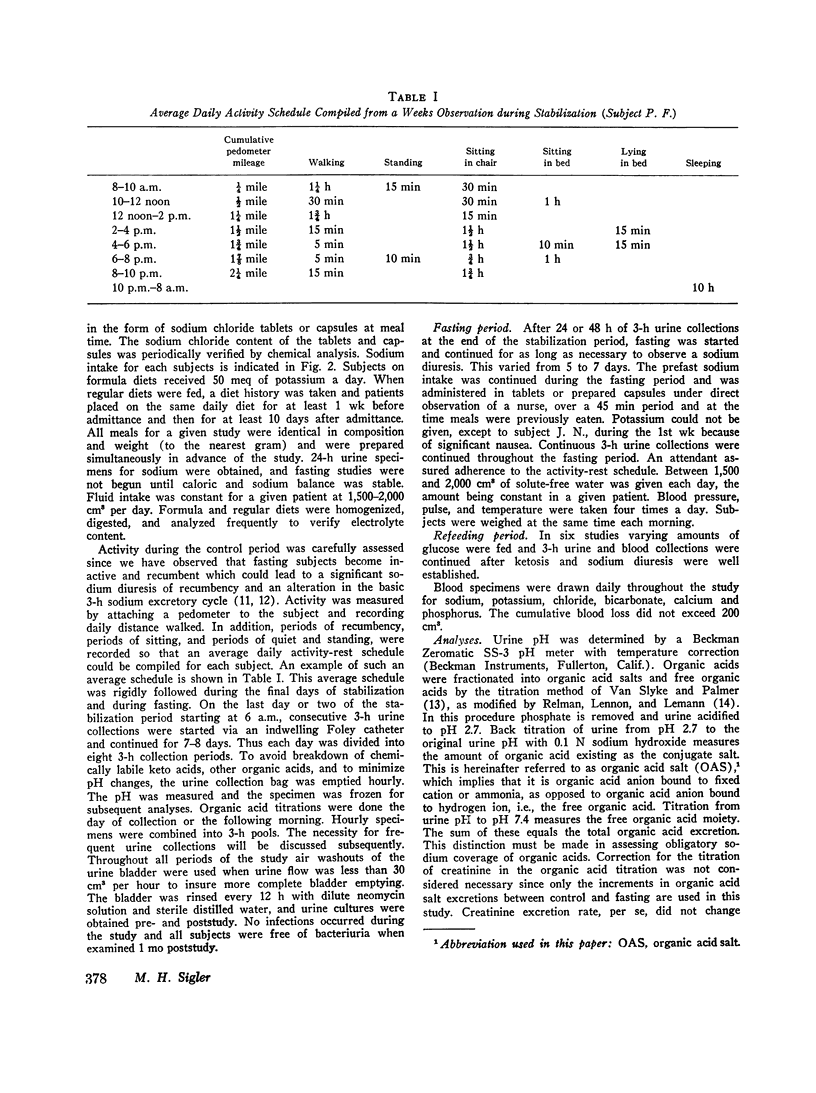
Abstract
This study tests the hypothesis than obligatory cation coverage of metabolicaly generated anions is the mechanism for the sodium diuresis of fasting. Nine obese female subjects were equilibrated on a constant sodium and caloric intake and then fasted while sodium intake was maintianed. Particular activity schedule during fasting as during control. Consecutive 3-h increases in urinary sodium , ammonium, and potassium excretion during fasting were matched against simultaneously determined increases in organic acid anions (OAS) and H2PO4 minus, which would exist in combination with the cations. The changes were significantly correlated (r equals 0.891, P less than 0.001) in the relationship y equals 0.73x plus 19 where y equals increases in organic acid salts plus H2POJ minus and x equals increases in cations. As ammonium excretion rose, sodium conservation occurred with ammonium replacing sodium at the major urinary cation. Corollaries to the hypothesis were also found to be true. They were: (a) Increases in ammonium excretion lagged considerably behind increases in OAS plus H2PO4 minus during the diuretic phase making sodium coverage necessary. (b) Sodium loss was much greater than chloride although chloride balance was minimally negative. (c) After refeeding with glucose, sodium excretion promptly decreased and appeared best correlated with simultaneous decreases in OAS. Ammonium excretion also fell but much less than sodium. The data support the hypothesis that obligatory cation coverage of metabolically generated aniuns is a major mechanism responsible for the sodium diuresis of fasting.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGLUND F., SORBO B. Turbidimetric analysis of inorganic sulfate in serum, plasma and urine. Scand J Clin Lab Invest. 1960;12:147–153. doi: 10.3109/00365516009062416. [DOI] [PubMed] [Google Scholar]
- BLOOM W. L., MITCHELL W., Jr Salt excretion of fasting patients. Arch Intern Med. 1960 Sep;106:321–326. doi: 10.1001/archinte.1960.03820030009003. [DOI] [PubMed] [Google Scholar]
- Boulter P. R., Hoffman R. S., Arky R. A. Pattern of sodium excretion accompanying starvation. Metabolism. 1973 May;22(5):675–683. doi: 10.1016/0026-0495(73)90239-4. [DOI] [PubMed] [Google Scholar]
- DOSSETOR J. B., GORMAN H. M., BECK J. C. THE DIURNAL RHYTHM OF URINARY ELECTROLYTE EXCRETION. I. OBSERVATIONS IN NORMAL SUBJECTS. Metabolism. 1963 Dec;12:1083–1099. [PubMed] [Google Scholar]
- HULET W. H., SMITH H. W. Postural naturiuresis and urine osmotic concentration in hydropenic subjects. Am J Med. 1961 Jan;30:8–25. doi: 10.1016/0002-9343(61)90060-2. [DOI] [PubMed] [Google Scholar]
- Haag B. L., Reidenberg M. M., Shuman C. R., Channick B. J. Aldosterone, 17-hydroxycorticosteroid, 17-ketosteroid, and fluid and electrolyte responses to starvation and selective refeeding. Am J Med Sci. 1967 Nov;254(5):652–658. doi: 10.1097/00000441-196711000-00009. [DOI] [PubMed] [Google Scholar]
- KESSLER G., WOLFMAN M. AN AUTOMATED PROCEDURE FOR THE SIMULTANEOUS DETERMINATION OF CALCIUM AND PHOSPHORUS. Clin Chem. 1964 Aug;10:686–703. [PubMed] [Google Scholar]
- Katz A. I., Hollingsworth D. R., Epstein F. H. Influence of carbohydrate and protein on sodium excretion during fasting and refeeding. J Lab Clin Med. 1968 Jul;72(1):93–104. [PubMed] [Google Scholar]
- Lemann J., Jr, Lennon E. J., Brock J. A potential error in the measurement of urinary titratable acid. J Lab Clin Med. 1966 Jun;67(6):906–913. [PubMed] [Google Scholar]
- Malnic G., Mello Aires M., Lacaz Vieira F. Chloride excretion in nephrons of rat kidney during alterations of acid-base equilibrium. Am J Physiol. 1970 Jan;218(1):20–26. doi: 10.1152/ajplegacy.1970.218.1.20. [DOI] [PubMed] [Google Scholar]
- Maude D. L. The role of bicarbonate in proximal tubular sodium chloride transport. Kidney Int. 1974 Apr;5(4):253–260. doi: 10.1038/ki.1974.34. [DOI] [PubMed] [Google Scholar]
- North K. A., Lascelles D., Coates P. The mechanisms by which sodium excretion is increased during a fast but reduced on subsequent carbohydrate feeding. Clin Sci Mol Med. 1974 Apr;46(4):423–432. doi: 10.1042/cs0460423. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Cahill G. F., Jr Metabolic effects of exogenous glucocorticoids in fasted man. J Clin Invest. 1973 Oct;52(10):2596–2605. doi: 10.1172/JCI107452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr Liver and kidney metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):574–583. doi: 10.1172/JCI106016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPOPORT A., FROM G. L., HUSDAN H. METABOLIC STUDIES IN PROLONGED FASTING. I. INORGANIC METABOLISM AND KIDNEY FUNCTION. Metabolism. 1965 Jan;14:31–46. doi: 10.1016/0026-0495(65)90079-x. [DOI] [PubMed] [Google Scholar]
- RELMAN A. S., LENNON E. J., LEMANN J., Jr Endogenous production of fixed acid and the measurement of the net balance of acid in normal subjects. J Clin Invest. 1961 Sep;40:1621–1630. doi: 10.1172/JCI104384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB L., LOTSPEICH W. D. Renal tubular reabsorption of acetoacetate in the dog. Am J Physiol. 1954 Feb;176(2):195–200. doi: 10.1152/ajplegacy.1954.176.2.195. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Owen O. E., Cheng J. T., Ginsberg R., Boden G., Walker W. G. The effect of carbohydrates on ammonium and ketoacid excretion during starvation. J Clin Invest. 1972 Aug;51(8):2093–2102. doi: 10.1172/JCI107016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudek C. D., Boulter P. R., Arky R. A. The natriuretic effect of glucagon and its role in starvation. J Clin Endocrinol Metab. 1973 Apr;36(4):761–765. doi: 10.1210/jcem-36-4-761. [DOI] [PubMed] [Google Scholar]
- Schloeder F. X., Stinebaugh B. J. Studies on the natriuresis of fasting. II. Relationship to acidosis. Metabolism. 1966 Sep;15(9):838–846. doi: 10.1016/0026-0495(66)90176-4. [DOI] [PubMed] [Google Scholar]
- Stinebaugh B. J., Schloeder F. X. Glucose-induced alkalosis in fasting subjects. Relationship to renal bicarbonate reabsorption during fasting and refeeding. J Clin Invest. 1972 Jun;51(6):1326–1336. doi: 10.1172/JCI106929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinebaugh B. J., Schloeder F. X. Studies on the natriuresis of fasting. I. Effect of prefast intake. Metabolism. 1966 Sep;15(9):828–837. doi: 10.1016/0026-0495(66)90175-2. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]







