Abstract
Background
Anamnestic recall of stroke related deficits is a common clinical observation, especially during periods of systemic infection. The pathophysiology of this transient re-emergence of neurological dysfunction is unknown.
Methods
Male Lewis rats underwent 3 hours middle cerebral artery occlusion (MCAO) and were treated with lipopolysaccharide (LPS) or saline at the time of reperfusion. The delayed type hypersensitivity (DTH) response to myelin basic protein (MBP) was examined at 28 days after MCAO. Changes in behavioral outcomes were assessed following DTH testing and repeat administration of LPS or saline at 34 days. At the time of sacrifice (36 days), the immunological response of splenocytes to MBP, neuron specific enolase (NSE) and proteolipid protein (PLP) was determined by ELISPOT assay and the number of lymphocytes in brain determined by immunocytochemistry.
Results
Animals treated with LPS at MCAO had a greater DTH response to MBP than animals treated with saline. Among those animals that had fully recovered on a given behavioral test prior to DTH testing, those treated with LPS at MCAO displayed more neurological deterioration following DTH testing and had more CD8+ lymphocytes within the ischemic core of the brain. Further, the TH1 immune response to brain antigens in spleen was more robust among those animals that deteriorated following DTH testing and there were more CD4+ lymphocytes in the penumbral region of animals with a TH1 response to MBP.
Conclusions
Our data suggest that an immune response to brain contributes to the phenomenon of anamnestic recall of stroke related deficits following an infection. The contribution of the immune response to this phenomenon deserves further investigation.
Introduction
For many patients who have recovered from stroke, a stressor such as an infection can lead to transient reappearance of their initial stroke symptoms. Despite wide recognition of this phenomenon, which is often referred to as anamnestic recall, there is virtually nothing known about the pathophysiology of the transient re-emergence of stroke related deficits. The term anamnestic means “able to recall to mind” and is used to describe a variety of biological phenomena. In the immunology literature, anamnestic recall refers to the immune response that occurs upon reencounter with an antigen to which it has already been “educated”.1 This “memory” response occurs in both the cellular and humoral arm of the immune system and results in a much greater response than occurred with the primary antigen exposure; it is the greater efficiency of this secondary immune response that forms the rationale for vaccinations.
Animals exposed to a systemic inflammatory stimulus at the time of stroke are at increased risk of developing a TH1 type immune response to central nervous system (CNS) antigens and this response is associated with worse outcome.2, 3 We hypothesized that animals with a TH1 type immune response to CNS antigens would experience a reactivation of these CNS specific lymphocytes when exposed to a systemic inflammatory stimulus at a point in time remote from the initial stroke (similar to that which occurs during an infection). Once activated, these lymphocytes could traffic into brain, and an inflammatory response within the vulnerable parenchyma might precipitate a re-emergence of the initial stroke related symptoms.4–6 To investigate whether the clinical syndrome of anamnestic recall in stroke could be immunologically mediated, we examined whether animals predisposed to a TH1 immune response by treatment with lipopolysaccharide (LPS) at stroke onset would experience neurological worsening when exposed to a delayed inflammatory stimulus, and whether this worsening was related to the immune response to brain antigens.
Materials and Methods
Animals
Experiments were approved by the Institution’s Animal Care and Use Committee. Male Lewis rats (250 to 300 g) were used for all studies.
Middle Cerebral Artery Occlusion (MCAO)
Anesthesia was induced with 5% and maintained with 1.5% isoflurane. After midline neck incision, the right common carotid, external carotid and pterygopalatine arteries were ligated. A monofilament suture (4.0) was inserted into the common carotid artery and advanced into the internal carotid artery to occlude the origin of the middle cerebral artery (MCA). Animals were maintained at normothermia during surgery and reperfused 3 hours after MCAO. In sham-operated animals (N=4), the filament was inserted into the carotid but not advanced. Rectal temperature and body weight were assessed at set time intervals. Animals were sacrificed 36 days after surgery. See Figure 1 for details of the experimental paradigm.
Figure 1.
Experimental design.
Administration of Immunologically Active Substances
Animals were treated with either LPS or saline at the time of reperfusion (3 hours after MCAO) and then randomly assigned to receive either LPS or saline again at 34 days after MCAO (“delayed” administration) resulting in four groups of animals (LPS/LPS, saline/LPS, LPS/saline, saline/saline). LPS (from Escherichia coli, serotype 026:B6) was mixed at a concentration of 1mg/mL in saline and given at a dose of 1 mg/kg intraperitoneal (IP); saline was given at the volume of 1 mL/kg IP. All sham operated animals received LPS at stroke onset and again 34 days after MCAO.
Delayed Type Hypersensitivity (DTH) Response
Ear thickness was measured 28 days after MCAO using a micrometer. MBP (Sigma; 50 μg in 0.05 mL saline) was then injected into the ear and the change in ear thickness measured 72 hours later.
Neurological Outcome
Neurological outcome was assessed at set time points. Tests included a modification of the Bederson scale (0=no deficit, 1=holds forepaw in flexed posture, 2=inability to resist lateral push, 3=circling, 4=agitated circling, 5=stupor)7, the “sticky tape test” 8, performance on the rotarod 9 and the foot fault test 10. The “sticky tape test” assesses sensorimotor function; the time the animal takes to attend to a piece of adhesive tape placed on the affected forelimb is recorded. The rotarod assesses motor coordination and fatigue; animals were trained prior to surgery until they could remain on the rotating rod at 5 rpm for 100 seconds; following surgery, the longest time animals could remain on the rotarod before falling (100 seconds maximum) was recorded (using the average of 3 trials). The foot fault test was done to test forelimb motor coordination; rats were placed on a wire grid for 3 minutes and the number of times the affected front paw slipped through the grid per total number of steps taken was recorded as a percentage.
ELISPOT Assay
Animals were sacrificed 36 days after MCAO and mononuclear cells (MNCs) isolated from the spleen using previously described methods.2,11,12 MNCs were cultured (1×105 cells/well) for 48 hours in 96-well plates (MultiScreenR-IP; Millipore) in media alone or in media supplemented with MBP (50μg/mL; Sigma), NSE (10μg/mL; Sigma) or PLP 139–151 (10μg/mL; ANASPEC). All experiments were performed in triplicate. The ratio of the relative increase in the number of cells secreting interferon (IFN)-γ when cultured with antigen instead of media alone to that of the relative increase in the number of cells secreting transforming growth factor (TGF)-β1 when cultured with antigen instead of media alone was used as an indicator of the TH1 response. Spots were counted by two independent investigators blinded to treatment status and aided by a semi-automated software system (Metamorph®). Animals were considered to have a TH1 response to the given antigen if the ratio of the IFN-γ:TGF-β1 response to that antigen was greater than the 75th percentile of sham- operated animals.
Immunocytochemistry
Following removal of the spleens, animals were perfused with 4% paraformaldehyde in phosphate buffered saline, the brains removed and stored in fixative overnight at 4°C. Cryoprotection was in 30% sucrose solutions for 24–48 hours before freezing in embedding compound at −80°C. Brains were then sectioned (20 μm) and stained for CD4, CD8, and CD45RA (present on B cells); slides were counterstained with cresyl violet. Antibodies were obtained from AbD Serotec (CD4 and CD8) and GenWay Biotech (CD45RA). The number of the cells labeled within 6 high powered fields (100x) in 8 distinct regions (4 in the infarcted hemisphere and 4 in the non-infarcted hemisphere) was determined in 3 different sections through the infarct (bregma 2.70 mm, −0.26 mm and −3.14 mm); individuals counting cells were masked to the treatment/outcome status.
Statistics
Parametric data are displayed as the mean ± SEM and compared using the t-test or ANOVA. Non- parametric data are displayed as the median (interquartile range [IQR]) and compared using the Mann-Whitney U test or the Kruskal-Wallis H test as appropriate. Categorical data were evaluated using the χ2-test statistic. Significance was set at P<0.05.
Results
Neurological Outcome to Day 28
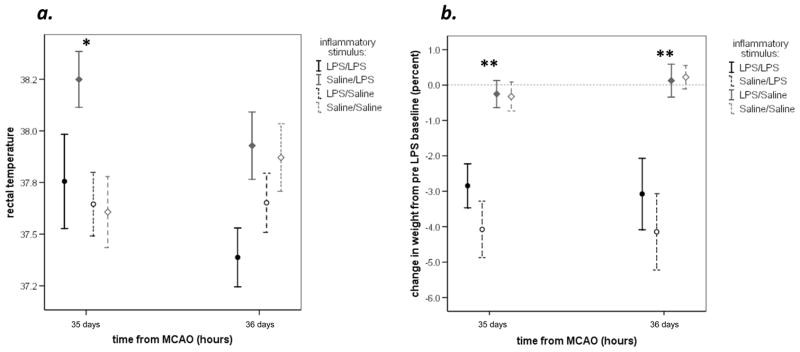
Mortality was identical 8/34 (24%) among animals that received LPS at stroke onset and those that did not; there was no mortality among sham operated animals. The body temperatures did not differ among animals treated with LPS or saline at any time point after MCAO (Figure 2a), but the amount of weight lost following MCAO was greater in LPS treated animals (Figure 2b). The temperatures of LPS treated animals undergoing MCAO were higher than that of LPS treated animals undergoing sham surgery from 3 hours through 48 hours after stroke onset (data not shown). Weight loss in LPS treated animals undergoing MCAO was greater than that of LPS treated sham operated animals throughout the duration of the study (data not shown).
Figure 2.
Rectal temperatures did not differ significantly following MCAO in LPS and saline treated animals to the point of DTH testing (a). Animals treated with LPS, however, lost significantly more body weight than those treated with saline (b). Data are depicted as the mean with standard errors of the mean; statistics are by student t-test. *P<0.05; **P<0.001
Stroke severity, as assessed by the neurological score, was identical at 3 hours after MCAO in animals that went on to receive either LPS or saline at reperfusion; following reperfusion, the neurological score was higher among LPS treated animals until 21 days after MCAO, at which time stroke severity was similar in both groups. At 28 days after MCAO, just prior to DTH testing, there were no differences in the performances on any of the behavioral tests between LPS and saline treated animals. Further, there was no difference in the proportion of LPS and saline treated animals that had fully recovered on any of these tasks up to this time point. LPS treated sham operated animals performed better on all behavioral tests throughout the duration of the study than animals treated with LPS and subjected to MCAO (data not shown). Similarly, more sham operated animals had complete recovery on the behavioral tasks at this time point (3/4 on the tape test, 4/4 on the foot fault test, and 2/4 on the rotarod).
DTH Response
At 72 hours after intradermal injection of MBP into the ear, animals treated with LPS at MCAO had more ear swelling than those treated with saline at MCAO; the increase in ear thickness was 0.048±0.008 mm in LPS treated animals compared to 0.029±0.005 mm in saline treated animals (P=0.004). In sham-operated animals, the increase was 0.038±0.013 mm, which is not significantly different than the ischemic animals.
Six days after DTH testing (and just prior to re-exposure to LPS or saline), 18/25 (72%) of animals treated with LPS at MCAO and 11/26 (42%) of animals treated with saline at stroke onset displayed worsening performance on the foot fault test (P=0.032). Those animals that worsened had a strong trend towards a more significant DTH response (0.045±0.008 mm vs. 0.029±0.004 mm; P=0.084). The deterioration in performance on the sticky tape test and rotarod during this same time period did not differ between LPS and saline treated animals. Among sham-operated animals, 1/4 (25%) worsened on the foot fault test after DTH testing, which is less than that of ischemic animals treated with LPS (P=0.066) and similar to that of animals treated with saline (P=NS) at stroke onset.
Response to Delayed LPS Injection
Animals treated with saline at stroke onset and LPS at 34 days had higher temperatures than animals in other treatment groups at 35 days (Figure 3); this difference was no longer seen at 48 hours after delayed LPS administration. Administration of LPS at 34 days led to rapid and significant weight loss, as evidenced in Figure 3. The increase in temperatures among sham operated animals that received the second dose of LPS was similar to that of animals with stroke receiving a dose of LPS at this time point; ironically, the decrease in body weight following the second exposure to LPS was more in sham-operated animals (P=0.005).
Figure 3.
The rectal temperatures were higher in animals that received a delayed injection of LPS 24 hours after the injection (a). The delayed injection of LPS also led to a rapid loss of weight (in comparison to the body weight at 816 hours, just prior to delayed LPS or saline injection) (b). Data are depicted as the mean with standard errors of the mean; statistics are by student t-test. *P<0.05; ** P<0.001
Behavioral changes following the second exposure to LPS (or saline) were dictated by treatment at the time of MCAO (LPS or saline); further results are therefore dichotomized by initial treatment. Table 1 depicts the changes in behavioral outcomes for each animal after DTH testing and delayed LPS or saline administration; absolute changes were computed by using the best performance to 34 days as the new “post-stroke baseline”. Animals treated with LPS at stroke onset experienced a greater increase in the latency to respond to the sticky tape at the study completion; there was no apparent effect of delayed LPS administration on the sticky tape test (Table 1). Absolute changes in the percentage of foot faults and latency to fall from the rotarod at study completion in comparison to the best performance on these tasks prior to 34 days were similar among treatment groups.
Table 1.
Changes in behavioral outcome following DTH testing and LPS treatment.
| change in tape test (seconds) median (IQR) | change in foot fault (percent total steps) median (IQR) | change in RR performance (percent of baseline) median (IQR) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RX at stroke onset: | +LPS N=25 | −LPS N=26 | P | Sham(+LPS) N=4 | +LPS N=25 | −LPS N=26 | P | Sham (+LPS) N=4 | +LPS N=25 | −LPS N=26 | P | Sham (+LPS) N=4 | |
| days from MCAO: | 35 | 3 (1,24) | 2 (0–6) | 0.095 | 0 (−1,9) | 7 (0,16) | 1 (0,5) | NS | 5 (0,10) | −15 (−35,5) | −9 (−47,1) | NS | −9 (−48,24) |
| 36 | 10 (4,30) | 2 (0–8) | 0.002 | 0 (0,3) | 10 (0,17) | 4 (0,11) | NS | 2 (0,5) | −3 (−27,12) | −2 (−28,4) | NS | 0 (−9,24) | |
| delayed RX: | LPS+ N=26 | LPS− N=25 | P | Sham (LPS+) N=4 | LPS+ N=26 | LPS− N=25 | P | Sham (LPS+) N=4 | LPS+ N=26 | LPS− N=25 | P | Sham (LPS+) N=4 | |
| days from MCAO: | 35 | 3 (0,15) | 3 (0,11) | NS | 0 (−1,9) | 0 (0,12) | 4 (0,16) | NS | 5 (0,10) | −18 (−54,0) | −5 (−37,4) | NS | −9 (−48,24) |
| 36 | 5 (0,4) | 7 (1,20) | NS | 0 (0,3) | 4 (0,13) | 10 (0,15) | NS | 2 (0,5) | −9 (−28,4) | 0 (−30,9) | NS | 0 (−9,24) | |
Statistics are non-parametric. The values for the sham-operated animals are provided for reference only; statistics are not done given the small group size.
The proportion of animals that fully recovered on the sticky tape test, the foot fault test and the rotarod prior to DTH test and delayed administration of LPS or saline was similar among treatment groups (data not shown). If one restricts analyses to only those animals who had fully recovered on each of the given tests by 34 days, LPS treatment at stroke onset was strongly associated with a decline in performance on the sticky tape test and the foot fault test following delayed administration of LPS or saline (Table 2).
Table 2.
Changes in behavioral outcome among those animals who had fully recovered on the given test prior to DTH testing and delayed LPS or saline treatment.
| change in tape test (seconds) median (IQR) | change in foot fault (percent total steps) median (IQR) | change in RR performance (percent of baseline) median (IQR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RX at stroke onset: | +LPS N=13 | −LPS N=17 | P | +LPS N=14 | −LPS N=21 | P | +LPS N=8 | −LPS N=12 | P | |
| days from MCAO: | 35 | 3(1, 31) | 1 (0, 4) | NS | 8 (3, 16) | 0 (0, 9) | 0.041 | −15(−41, −3) | −30(−59, −2) | NS |
| 36 | 9(6, 30) | 1 (0, 6) | 0.007 | 13 (9, 21) | 5 (0, 11) | 0.017 | −24 (−29, −1) | −21 (−30, 0) | NS | |
| delayed RX: | LPS+ N=15 | LPS− N=15 | P | LPS+ N=16 | LPS− N=19 | P | LPS+ N=11 | LPS− N=9 | P | |
| days from MCAO: | 35 | 1 (0, 5) | 2 (0, 22) | NS | 0 (0, 12) | 5 (0, 17) | 0.080 | −24 (−61, −11) | −7(−50, 0) | NS |
| 36 | 5 (0, 14) | 5 (1, 26) | NS | 5 (0, 18) | 11 (5, 16) | NS | −23 (−32, 0) | −20 (−28, 0) | NS | |
Immunologic Outcome
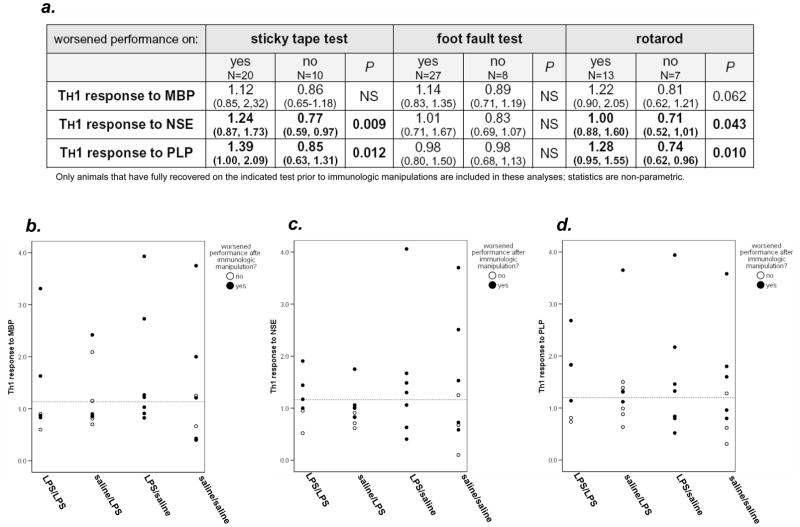
There was no difference in the degree of the TH1 response to MBP, NSE or PLP or the proportion of animals with a TH1 response to these antigens among any of the treatment groups (Figure 4). There was a strong correlation between the immune response to MBP and performance on the foot fault test, the more robust the TH1 response to MBP, the more foot faults made (r2=0.366, P=0.008). There was also an inverse relationship between the TH1 response to MBP and performance on rotarod, the more robust the TH1 response the shorter the latency to fall (r2= −0.244, P=0.084). The relationships between the TH1 responses to NSE and PLP and rotarod performance were more robust, r2= −0.347, P=0.013 and r2= −0.338, P=0.015, respectively. Further, those animals that experienced complete recovery on the rotarod had less robust responses to NSE (0.71 [0.52, 1.34] vs. 1.06 [0.83, 1.45]; P=0.070) and PLP (0.80 [0.61, 1.14] vs. 1.13 [0.88, 1.58]; P=0.010).
Figure 4.
The immune responses to MBP, NSE and PLP are shown for individual animals and depicted as the ratio of the antigen specific IFN-γ:TGF-β1 response to that antigen. The horizontal line represents the median value of the sham-operated animals; the dotted lines represent the 25th and 75th percentiles for the sham-operated animals.
To address the question of an immunologic contribution to neurological worsening, analyses were restricted to animals that had fully recovered on the given behavioral measure prior to immunologic manipulations (DTH testing and delayed LPS or saline administration). For both the sticky tape test and the rotarod, animals that demonstrated deterioration in performance by the study’s end had more robust TH1 responses to both NSE and PLP (Figure 5a). Individual animal data for each antigen and the sticky tape test are also displayed graphically in Figure 5. Among animals that experienced a full recovery on the sticky tape by day 28 after MCAO, a greater proportion of animals that worsened following the immunologic manipulations had evidence of a TH1 response to NSE (15/20 [75%] vs. 2/10 [20%]; P=0.004) and PLP (13/20 [65%] vs. 3/10 (30%); P=0.070). Graphic data for the rotarod are not shown, but among the animals that deteriorated following immunologic manipulations, the proportion of animals with a TH1 response to PLP was higher (7/13 [54%] vs. 1/7 [14%]; P=0.085). There was no relationship between deteriorating performance on the foot fault test and the degree of the immune response to the brain antigens, but as for the other behavioral tasks, those that deteriorated following the immunologic manipulations were more likely to have a TH1 response to PLP (12/17 [71%] vs. 0/8; P=0.020).
Figure 5.
The table (a) shows the immune responses to CNS antigens among animals that experienced “anamnestic recall” of stroke related deficits on each of the behavioral measures at study end. Data are presented as median (IQR). These data represent only the animals that exhibited complete recovery on the sticky tape test prior to the immunologic manipulations (DTH testing ± LPS) 34 days after MCAO. The immune responses to MBP, NSE and PLP are depicted as the ratio of the antigen specific IFN-γ:TGF-β1 response to that antigen. The dotted horizontal line represents the 75th percentiles for the sham-operated animals (the “threshold” for the TH1 immune response).
Immunocytochemistry
There were no differences in the total number of CD4+, CD8+ or CD45+ cells in the infarcted hemispheres (regions 1–4) among the different treatment groups (Figure 6); virtually no lymphocytes were found in the non-ischemic hemisphere (regions 5–8). There was a general trend for more CD4+ cells to be found in the penumbral regions (regions 1 and 3) and more CD8+ cells to be found within the cortical region of the infarct (region 2); CD45+ cells were seen throughout these regions. Animals treated with LPS at stroke onset had higher numbers of CD8+ cells in region 2 (P=0.019; Figure 6b); delayed treatment with LPS (at day 34) did not influence the number of CD8+ lymphocytes in brain. For animals that experienced a full recovery on the foot fault test prior to immunological manipulations and subsequently worsened, there were more CD4+ cells in the infarcted hemisphere (48 [32,65] vs. 26 [20,35]; P=0.027), and this increase in CD4+ cells was primarily seen in region 2 (31 [19,42] vs. 12 [5,16]; P=0.011). No differences were seen in the numbers of cells in brain among those animals that experienced worsening on the sticky tape test or the rotarod. Finally, animals with a TH1 response to MBP, NSE or PLP tended to have more CD4+ cells in the penumbral regions; this reached significance for a TH1 response to MBP in region 3 (15 [8,63] vs. 8 [4,20]; P=0.045)
Figure 6.
ICC was done in both the infarcted hemisphere (regions 1–4) and the non-infarcted hemisphere (regions 5–8) at predefined levels (a). The most abundant inflammatory cell infiltrates were seen in region 2. Within region 2, there were more CD8+ cells among animals treated with LPS at stroke onset (b). Examples of cells labeled for CD4, CD8 and CD45 in region 2 are seen in panel c.
Discussion
In this study we attempted to model anamnestic recall of stroke related deficits following experimental MCAO. Based on prior data, we hypothesized that the transient clinical worsening seen in stroke patients when infected at a remote time from the initial stroke could be due to a reactivation of a CNS autoimmune response. Specifically, we’ve demonstrated that LPS exposure at the time of MCAO results in an increased likelihood of developing a TH1 response to brain antigens2; it was thus anticipated that a second exposure to LPS (to mimic an infection) would result in reactivation of these cells and lead to clinical worsening as a result of the ensuing CNS specific immune response. In actuality, we found that the second exposure to LPS wasn’t as important as the first. The fact that animals treated with LPS at stroke onset and with saline at 34 days deteriorated similarly to those treated with LPS at both time points can probably be explained by the exposure to MBP during DTH testing, with reactivation of the immune response following the antigenic challenge. Given that animals treated with LPS at MCAO demonstrated a significant response to intradermal MBP injection, indicating the presence of a memory response to this antigen, reactivation of the MBP specific response during DTH testing may have precipitated the decline in neurological function.
Despite the DTH response to MBP among LPS treated animals, we did not see an increase in the degree of the TH1 response to this antigen (or any of the other brain antigens) among LPS treated animals at study completion (8 days after DTH testing). For those animals that had fully recovered on a given task prior to DTH testing (and delayed LPS or saline administration), the TH1 response to NSE and PLP was greater among those animals that experienced subsequent worsening on the sticky tape test and the rotarod; similar trends were seen for the TH1 response MBP. The reason that the TH1 response to MBP at study completion did not correlate as well with deteriorating performance as the other antigens is unclear, but could be related to a fundamental change in the immune response following MBP exposure, with either induction of TREG cells or apoptosis of autoreactive T cells.13–17
Animals with TH1 response to MBP (as detected by ELISPOT on splenocytes) had more CD4+ cells in the penumbral regions of brain (region3) and animals that experienced “anamnestic recall” on the foot fault test had more CD4+ cells in the ischemic hemisphere. These small differences in cell numbers, however, may not adequately represent the difference in the nature of the immune response. For instance, the CD4+ cells in the brains of animals with a TH1 response to brain antigens in spleen may truly have been TH1 effector cells while the CD4+ cells in the brains of animals without a TH1 response to brain antigens in spleen may have been TREG cells. Further histological analyses for key effector cytokines as well as non-lymphocytic inflammatory cells (ie. neutrophils and monocytes) would provide key data regarding the nature of the immune response in brain. Alternatively, ELISPOT analyses could be done to characterize the immune responses of lymphocytes extracted from brain.
The phenomenon of anamnestic recall of stroke related deficits is familiar to most clinicians who care for patients with stroke, although virtually nothing is known or written about it. The fact that this transient re-emergence of stroke related symptoms occurs primarily in the setting of systemic infection must provide a clue to its pathophysiology. In this study we show that among the animals most likely to have developed a TH1 immune response to brain antigens (ie. those treated with LPS at stroke onset), subsequent challenge with a brain antigen (ie. MBP) is associated with an “anamnestic recall” of initial stroke related dysfunction. If during an infection there is an adequate inflammatory stimulus to activate systemic lymphocytes, these cells could cross an intact blood-brain barrier (BBB).5, 18 Alternatively, a particularly robust inflammatory stimulus may compromise the BBB allowing lymphocytes to encounter CNS antigens in either the brain and periphery; these encounters could lead to lymphocyte reactivation.19, 20 Both of the situations outlined above could potentially lead to an inflammatory response in brain with transient worsening of neurological function. Indeed, systemic inflammation is capable of reactivating immune mediated lesions in the rat brain.21 Another clinically relevant scenario occurs in patients with recurrent strokes; ischemic death of brain tissue leads to antigen release into the peripheral circulation where lymphocytes previously “educated” to the antigen might re-encounter and become reactivated to the antigen allowing for transit across an intact BBB.5, 22–25 In addition, the BBB is compromised following an ischemic insult, which allows even quiescent lymphocytes access to the CNS for re-encounter to brain antigens.26, 27 The ensuing immune response may help to explain why patients with recurrent stroke are more prone to dementia and have more neurological dysfunction than might be expected for the amount of injured brain.28, 29
In summary, we have attempted to model the phenomenon of anamnestic recall of stroke related deficits following experimental stroke. We had hypothesized that delayed exposure to an inflammatory stimulus (LPS) would lead to re-emergence of stroke related deficits in animals most likely to have a TH1 response to brain antigens, but found that it was the exposure to MBP during DTH testing that precipitated the decline in performance on the behavioral tasks. These observations, along with the fact that the TH1 responses to brain antigens were greatest in the animals that experienced deterioration in performance following DTH testing, suggest that the anamnestic recall of stroke related deficits may have an immunological basis.
Table 3.
Immune responses to CNS antigens among animals that experienced “anamnestic recall” of stroke related deficits on each of the behavioral measures at study end. Data are presented as median (IQR).
| worsened performance on: | sticky tape test | foot fault test | rotarod | ||||||
|---|---|---|---|---|---|---|---|---|---|
| yes N=20 | no N=10 | P | yes N=27 | no N=8 | P | yes N=13 | no N=7 | P | |
| TH1 response to MBP | 1.12 (0.85, 2,32) | 0.86 (0.65–1.18) | NS | 1.14 (0.83, 1.35) | 0.89 (0.71, 1.19) | NS | 1.22 (0.90, 2.05) | 0.81 (0.62, 1.21) | 0.062 |
| TH1 response to NSE | 1.24 (0.87, 1.73) | 0.77 (0.59, 0.97) | 0.009 | 1.01 (0.71, 1.67) | 0.83 (0.69, 1.07) | NS | 1.00 (0.88, 1.60) | 0.71 (0.52, 1,01) | 0.043 |
| TH1 response to PLP | 1.39 (1.00, 2.09) | 0.85 (0.63, 1.31) | 0.012 | 0.98 (0.80, 1.50) | 0.98 (0.68, 1,13) | NS | 1.28 (0.95, 1.55) | 0.74 (0.62, 0.96) | 0.010 |
References
- 1.Collins FM. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7:27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- 2.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, Becker KJ. Cns immune responses following experimental stroke. Neurocrit Care. 2009 doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of t cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- 5.Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and cns inflammation: How t lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 6.Richert JR, Driscoll BF, Kies MW, Alvord EC., Jr Adoptive transfer of experimental allergic encephalomyelitis: Incubation of rat spleen cells with specific antigen. J Immunol. 1979;122:494–496. [PubMed] [Google Scholar]
- 7.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 9.Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Zhou Y, Lai Q, Li J, Park H, Diaz FG. Impaired motor activity and motor learning function in rat with middle cerebral artery occlusion. Behav Brain Res. 2002;132:29–36. doi: 10.1016/s0166-4328(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 11.Becker K, Kindrick D, McCarron R, Hallenbeck J, Winn R. Adoptive transfer of myelin basic protein-tolerized splenocytes to naive animals reduces infarct size: A role for lymphocytes in ischemic brain injury? Stroke. 2003;34:1809–1815. doi: 10.1161/01.STR.0000078308.77727.EA. [DOI] [PubMed] [Google Scholar]
- 12.Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishigami T, White CA, Pender MP. Soluble antigen therapy induces apoptosis of autoreactive t cells preferentially in the target organ rather than in the peripheral lymphoid organs. Eur J Immunol. 1998;28:1626–1635. doi: 10.1002/(SICI)1521-4141(199805)28:05<1626::AID-IMMU1626>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Tischner D, Weishaupt A, van den Brandt J, Muller N, Beyersdorf N, Ip CW, Toyka KV, Hunig T, Gold R, Kerkau T, Reichardt HM. Polyclonal expansion of regulatory t cells interferes with effector cell migration in a model of multiple sclerosis. Brain. 2006;129:2635–2647. doi: 10.1093/brain/awl213. [DOI] [PubMed] [Google Scholar]
- 15.Gordon FL, Nguyen KB, White CA, Pender MP. Rapid entry and downregulation of t cells in the central nervous system during the reinduction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;112:15–27. doi: 10.1016/s0165-5728(00)00341-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Hancock WW, Marks R, Gonnella P, Weiner HL. Mechanisms of recovery from experimental autoimmune encephalomyelitis: T cell deletion and immune deviation in myelin basic protein t cell receptor transgenic mice. J Neuroimmunol. 1998;82:149–159. doi: 10.1016/s0165-5728(97)00193-8. [DOI] [PubMed] [Google Scholar]
- 17.Furtado GC, Olivares-Villagomez D, Curotto De Lafaille MA, Wensky AK, Latkowski JA, Lafaille JJ. Regulatory t cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001;182:122–134. doi: 10.1034/j.1600-065x.2001.1820110.x. [DOI] [PubMed] [Google Scholar]
- 18.Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- 19.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L. Elevated serum levels of s-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 21.Serres S, Anthony DC, Jiang Y, Broom KA, Campbell SJ, Tyler DJ, van Kasteren SI, Davis BG, Sibson NR. Systemic inflammatory response reactivates immune-mediated lesions in rat brain. J Neurosci. 2009;29:4820–4828. doi: 10.1523/JNEUROSCI.0406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: The national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, Laskowitz DT, Valkirs GE, Buechler KF. Early biomarkers of stroke. Clin Chem. 2003;49:1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- 24.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 25.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 27.Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 28.Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: A systematic review. Stroke. 41:e41–46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 29.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]