Abstract
Objective
While attentional functions are usually found to be impaired in schizophrenia, a review of the literature on the orienting of spatial attention in schizophrenia suggested that voluntary attentional orienting in response to a valid cue might be paradoxically enhanced. We tested this hypothesis with orienting tasks involving the cued detection of a laterally-presented target stimulus.
Method
Subjects were chronic schizophrenia patients (SZ) and matched healthy control subjects (HC). In Experiment 1 (15 SZ, 16 HC), cues were endogenous (arrows) and could be valid (100% predictive) or neutral with respect to the subsequent target position. In Experiment 2 (16 SZ, 16 HC), subjects performed a standard orienting task with unpredictive exogenous cues (brightening of the target boxes).
Results
In Experiment 1, SZ showed a larger attentional facilitation effect on reaction time than HC. In Experiment 2, no clear sign of enhanced attentional facilitation was found in SZ.
Conclusions
The voluntary, facilitatory shifting of spatial attention may be relatively enhanced in individuals with schizophrenia in comparison to healthy individuals. This effect bears resemblance to other relative enhancements of information processing in schizophrenia such as saccade speed and semantic priming.
Keywords: attention, schizophrenia, orienting, prefrontal cortex
The association between disturbances of attention and schizophrenia has been apparent for nearly a century (e.g., Bleuler, 1950/1911; Braff & Light, 2004; Kraepelin, 1971/1919; Luck & Gold, 2008; Nestor & O'Donnell, 1998; Nuechterlein & Dawson, 1984). In this report we present evidence for a particular abnormality of the orienting of visual attention in schizophrenia that has gone relatively unnoticed: in comparison to healthy individuals, individuals with schizophrenia tend to show greater reaction time (RT) benefits for validly-cued targets or smaller RT costs for invalidly-cued targets on visuospatial orienting tasks. This pattern of RT performance is typically viewed as evidence of rapid orienting of visual attention in schizophrenia, such that patients show increased benefits for valid cues and/or reduced cost for invalid cues. We review the literature for evidence of this enhanced attentional facilitation effect, and then investigate it in experiments comparing voluntary and involuntary orienting in chronic schizophrenia patients and healthy individuals. The results support the hypothesis that schizophrenia is associated with deficits in voluntary attentional processes, paradoxically manifested as increased benefits in voluntarily shifting spatial attention, as well as an increased effect of preparation. These effects may be related to other phenomena in schizophrenia of abnormally facilitated information processing such as semantic hyperpriming (e.g., Manschreck et al., 1988).
Visuospatial orienting in schizophrenia
Most of the studies of visuospatial orienting in schizophrenia have employed detection tasks in which participants press a button when a target stimulus is presented at one of two possible locations in the left and right visual fields (LVF and RVF). The target is preceded by a cue which indicates, with a certain probability, the location of the target. In the valid condition the cue indicates the location of the target, and in the invalid condition the cue indicates the opposite location. The neutral condition might consist of an uninformative cue or the absence of a cue, and provides a baseline from which to assess the benefits and costs of directing attention according to the cues to detect the targets (Jonides & Mack, 1984). Benefits for a response (such as reaction time [RT]) are calculated as the difference in responses between neutral and valid trials, while costs are calculated as the difference between invalid and neutral trials. Neurophysiological studies have provided evidence that benefits and costs in orienting may be subserved by distinct attentional mechanisms (e.g., Luck et al., 1994).
Following the pioneering work of Posner and colleagues on the cognitive and neural mechanisms underlying visuospatial attention (e.g., Posner, 1980; Posner, Early, Reiman, Pardo, & Dhawan, 1987), researchers examined whether schizophrenia was associated with abnormalities in shifting attention between locations. Much of the initial interest of these studies concerned whether shifting attention to the right side of space (in response to a left visual field invalid cue for which the target appears in right visual field) was abnormal in schizophrenia (e.g., Posner et al., 1988), which would be consistent with the dysfunction of left hemisphere brain areas that is prominent in this disorder (reviewed in Bruder, 1995). In the course of this effort, some studies (reviewed below) found evidence for a different abnormality in the orienting of visual attention in schizophrenia: a decrease in RT costs, and/or an increase in RT benefits, compared to healthy individuals.
Here we selectively review the literature on visuospatial orienting in schizophrenia. We focus on experiments in which a neutral cue condition was used to calculate orienting benefits and costs, and the patient group(s) was compared to healthy control group. (Studies employing the Attention Network Test [Fan, McCandliss, Sommer, Raz, Posner, 2002] are excluded because the cued locations and degree of eccentricity differ from standard orienting tasks.) The experiments are summarized in Table 1. Cue-target stimulus onset asynchrony [SOA] generally did not influence the overall patterns of interest to us, so we generalized across SOAs. However, for unpredictive, exogenous cueing (e.g., brightening of a box), only short (~100 ms) SOA data are considered, because inhibition of return influences the reaction time (RT) pattern at the longer SOAs that were typically employed. The breakdown of the experiments according to cue type (see Table 1 for further explanation) was 10/18 predictive and exogenous, 5/18 unpredictive and exogenous, and 3/18 predictive and endogenous.
Table 1.
Summary of experiments investigating visuospatial orienting in schizophrenia.
| Patient type | Eye movement monitoring | Cue type | Baseline | Effects | |
|---|---|---|---|---|---|
| Bustillo et al. (1997) | chronic & medicated, deficit/nondeficit subgroups | eye tracker | 1: predictive, exogenous 2: predictive, endogenous |
1: neutral 2: neutral |
1: none 2: {+B/-C in deficit subgroup, especially RVF targets} |
| Carter et al. (1992) | chronic & unmedicated | observation | 1: unpredictive, exogenous 2: predictive, endogenous |
1: neutral 2: neutral |
1: none 2: none |
| Carter et al. (1994) | chronic & unmedicated, paranoid/undifferentiated subgroups | observation | 1: unpredictive, exogenous 2: predictive, endogenous |
1: neutral 2: neutral |
1: none 2: {+B/-C for RVF targets, both subgroups} |
| Gold et al. (1992) | chronic & medicated | none reported | predictive, exogenous | null | {+B/-C} |
| Gouzoulis-Mayfrank et al. (2007) | combination of first-episode, recent-onset, and chronic patients; most unmedicated | none reported | unpredictive, exogenous | neutral | {+B/-C} |
| Liotti et al. (1993) | chronic & medicated | observation | predictive, exogenous | null | +B/-C |
| Maruff et al. (1995) * | unmedicated, recently-medicated, chronic & medicated | eye tracker | predictive, exogenous | diffuse | +B/-C for unmedicated and chronic |
| Nestor et al. (1992) | chronic & medicated | none reported | 1: predictive, exogenous | 1: null | 1: {+B}/-C |
| Oie et al. (1998) | early-onset (portions medication-naïve, unmedicated and medicated) | none | predictive, exogenous | null | {+B}/-C |
| Posner et al. (1988) | combinations of recent-onset, chronic, medicated, unmedicated | none | predictive, exogenous | null | {+B/-C especially for RVF targets} |
| Sapir et al. (2001) | mainly chronic, medicated | observation | 1: unpredictive, exogenous | 1: neutral | +B for RVF targets |
| Sapir et al. (2007) 1 | chronicity not given; medicated (low/high medication groups) | observation | 1: unpredictive, exogenous | neutral | 1: none |
| Strauss et al. (1991) | chronic & medicated | EOG | predictive, exogenous | null | none |
| Strauss et al. (1992) 2 | chronic & medicated | none | predictive, exogenous | null | {-C} |
| Wigal et al. (1997) | mainly chronic; medicated and unmedicated subgroups | none reported | predictive, exogenous | null | none |
Numbered cell entries indicate which of the multiple experiments in that study are considered. Patient type: chronicity and medication status. Eye movement monitoring: observation (experimenter visually monitoring the subject), eye tracker, EOG, none, or none reported. Cue type: predictive (valid cues more probable than other types), unpredictive (equal probability of all cues). Baseline: neutral cue, diffuse cue (increase of background luminance), null (no cue). Effects: +B = increased RT benefit; -C = decreased RT cost; none = neither increased RT benefit nor decreased cost. Brackets denote that the effect is apparent in the reported data but direct statistical tests were not reported or not significant. Abbreviations: EOG = electro-oculogram, LVF = left visual field, RVF = right visual field, RT = reaction time, SOA = cue-target stimulus onset asynchrony.
The diffuse cue RTs were considerably slower than typically found for neutral cue or null baseline conditions, in both controls and patients.
The authors reported cueing effects that could be interpreted as increased benefit/decreased costs in the patients, but these were averaged over SOA. At the 100 ms SOA, the effects were not apparent. There were significant differences in age between the control and patient groups.
There was an association between RT costs, target visual field, and low vs. high negative symptomaticity. Reduced costs were found in the RVF of the high negative symptom subgroup and in the LVF of the low negative symptom subgroup, but these were not directly compared to the controls’ data.
Abnormal patterns of orienting benefits/costs were first noted by Nestor et al. (1992), who found that chronic medicated patients had decreased RT costs compared to healthy controls. In Experiment 1 of Nestor et al., the decrease in orienting costs was greater for short (100 ms) compared to long (800 ms) SOAs. No difference was found between patients and controls in the size of RT benefits. Thus, the results of Nestor et al. suggested that the disengagement of attention from an invalidly-cued location was abnormally rapid in schizophrenia, particularly at short SOAs. This finding has been supported by other studies: Liotti, Dazzi, and Umilta (1993) found both decreased orienting costs as well as increased benefits in chronic medicated schizophrenia patients. Oie, Rund, and Sundet (1998) found that adolescent patients with early-onset schizophrenia had reduced costs at 100 ms SOA. And Sapir and colleagues (Sapir, Henik, Dobrusin, & Hochman, 2001) found increased benefits at 100 ms SOA for RVF targets in mainly chronic, medicated patients.
The results of several other studies are consistent with increased benefits and/or decreased costs, but these studies did not present analyses of such effects because they were mainly concerned with corroborating the rightward attention deficit observed by Posner et al. (1988). Orienting abnormalities at short SOAs are apparent in the data reported by Gold et al. (1992) and Bustillo et al. (1997). Furthermore, an interaction between visual field and orienting is suggested in the data from Posner et al. (1988), Strauss, Alphs, and Boekamp (1992), and Maruff, Hay, Malone, and Currie (1995). In the latter studies it appears that schizophrenia patients had increased benefits and/or decreased costs effects compared to healthy controls for RVF targets at short SOAs.
For most of the studies in which abnormal orienting patterns were reported or are apparent, patient groups consisted of mainly male, chronic medicated patients (Posner et al., 1988; Gold et al., 1992; Nestor et al., 1992; Strauss, Alphs, & Boekamp, 1992; Liotti et al., 1993; Bustillo et al., 1997; Sapir et al., 2001). However, there are exceptions. The adolescent patients in the Oie et al. (1998) study were mostly unmedicated at the time of testing. In Maruff et al. (1995) the abnormal pattern of visual orienting is apparent for their unmedicated but not long-term medicated patients. In Bustillo et al. (1997) an abnormal pattern is suggested in the data from “deficit” but not “nondeficit” patient groups. In two studies that used acute unmedicated patients (Strauss et al., 1991; Carter, Robertson, Chaderjian, Celaya, & Nordahl, 1992), increased benefits and/or decreased costs are not apparent. Hence, the evidence for abnormal orienting in schizophrenia is most closely associated with the chronic, medicated state.
In sum, there is evidence from several studies for increased benefits and/or decreased costs of visuospatial attentional orienting in schizophrenia. These effects are often found at short cue-target SOAs (~200 ms or less), suggesting that “automatic” mechanisms are involved. However, the interpretation of much of the above evidence is problematic because most studies used “no-cue” neutral trials as a baseline from which to measure RT benefits and costs. The no-cue condition differs from the valid and invalid conditions not only in the absence of information about target location, but also in the absence of an alerting signal (Jonides & Mack, 1984). Thus, it is not clear whether the differences between schizophrenia patients and healthy individuals in orienting benefits/costs reflect solely the time to shift attention to or from the cued location. In addition, since the neutral baseline RT is used to calculate both benefits and costs, these measures have a reciprocal relationship and cannot be measured independently of each other.
Present study
The goal of the present study was to directly investigate whether chronic schizophrenia patients have enhanced visuospatial orienting benefits compared to healthy individuals. In Experiment 1 (Fig. 1A) we used an orienting task that avoided some of the problems with the versions employed by other investigators. By using only valid and neutral symbolic cues with equal probability of presentation, we obtained a purer measure of voluntary attentional facilitation than tasks used in prior experiments with infrequent invalid and neutral cue conditions. It is important to note that the valid cues were completely informative about the location of the upcoming target stimulus in this task. By contrast in typical versions of the orienting task in which invalid cues are presented infrequently, a non-neutral directional cue is not completely predictive because the validity of the cue is not known until after target presentation. Therefore, subjects may attend to the uncued location on some trials, and/or divide their attention between the cued and uncued locations on other trials. These differences in strategies are better controlled in the current experiment by using only valid and neutral symbolic cues.
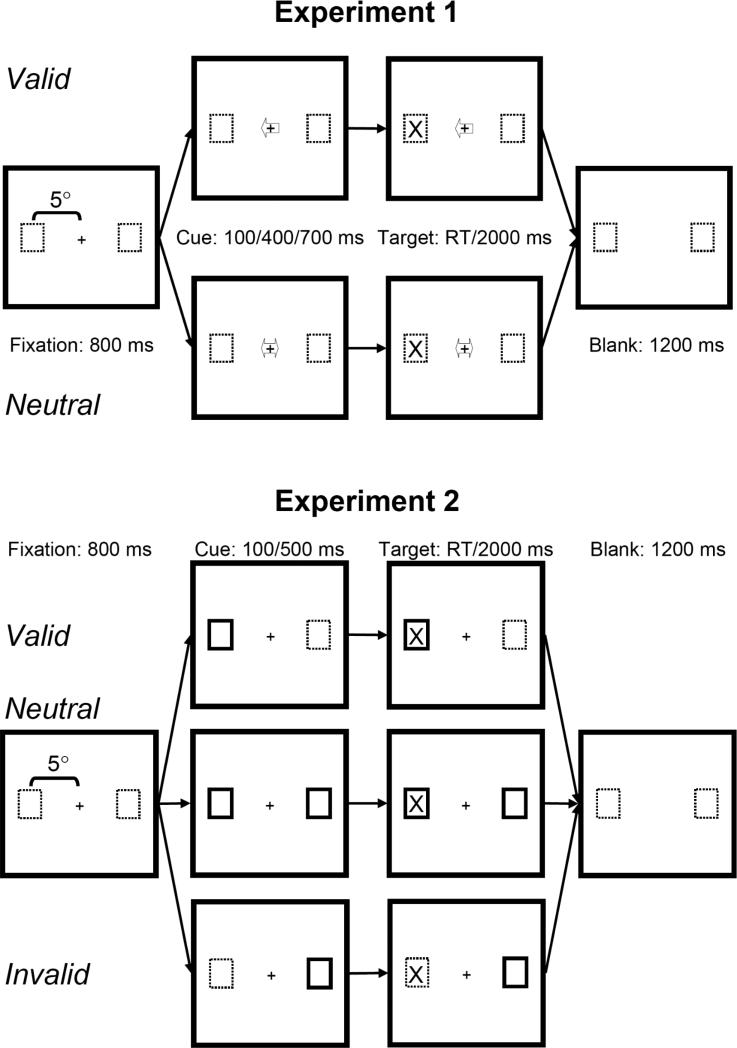
Figure 1.
Trial structures for Experiments 1 (A) and 2 (B). In both tasks, trials began with the presentation of the fixation cross for 800 ms, followed by the cue (variable duration) and target stimuli. Target stimuli remained visible until the response button was pressed or for a maximum duration of 2000 ms. A “blank” screen containing only the outlines of the potential target locations was presented for 1200 ms until the next trial began.
In Experiment 2 (Fig. 1B), we examined automatic attentional facilitation in a standard paradigm with unpredictive exogenous cues (valid, neutral, and invalid) to compare with voluntary attentional facilitation in Experiment 1. Making the cues unpredictive necessitated the addition of an invalid cue condition, which was not included in Experiment 1. Had we used only valid and neutral cues, the predictability of the valid cues could have been detected by subjects, and they could have used this predictability to voluntarily shift their attention.
In both experiments, horizontal eye movements were monitored with the horizontal electro-oculogram (HEOG), and trials on which subjects did not maintain central fixation were rejected from analysis. Furthermore, rather than having subjects respond with only their dominant (right) hand and possibly induce an action-related bias (cf., Danielmeier, Zysset, Müsseler, & von Cramon, 2004), subjects performed the task with each hand in a blocked design.
Methods
Subjects
The study was approved by the Institutional Review Boards of the Veterans Affairs Boston Healthcare System and Harvard Medical School. Informed consent was obtained from all subjects, who were paid for their participation. All subjects were male. Healthy control subjects (HC) were recruited from the community and were free of Axis I or II disorders (First, Gibbon, Spitzer, & Williams, 1997; Spitzer, Williams, Gibbon, & First, 1990), as well as a history of Axis I disorders in first-degree relatives. Patients were diagnosed with schizophrenia (SZ) according to DSM-IV criteria (First, Spitzer, Gibbon, & Williams, 1995). Subjects were selected without regard for ethnicity and met our standard inclusion/exclusion criteria: 1) right-handed (Oldfield, 1971); 2) no history of electroconvulsive treatment or neurological illness; 3) no alcohol or drug dependence within the last 5 years (DSM-IV criteria); 4) no present medication for medical disorders that would have deleterious EEG, neurological, or cognitive consequences; 5) estimated verbal IQ above 75; and 6) normal or corrected-to-normal vision.
Twenty HC and 19 SZ participated in Experiment 1. The data from 3 subjects in each group were excluded due to failure to maintain central fixation on at least 75% of the trials (see HEOG procedures below). In addition, the data from 1 HC were excluded because he did not follow the task instructions, and the data from 1 SZ were excluded due to excessively high error rates (missing all of the trials in one condition, and missing >40% of the trials in 5 other conditions). The final samples consisted of 16 HC and 15 SZ.
Twenty-two HC and 22 SZ participated in Experiment 2. The data from 5 HC and 6 SZ were excluded due to failure to maintain central fixation on at least 75% of the trials. In addition, 1 HC was excluded due to a poor HEOG recording. The final samples consisted of 16 HC and 16 SZ. Seven HC and 5 SZ participated in both experiments.
Demographic and clinical data and inter-group comparisons for Experiments 1 and 2 are summarized in Tables 2 and 3, respectively. All patients were receiving antipsychotic medication at the time of testing. None of the results of this study were correlated with antipsychotic dosage, calculated as chlorpromazine equivalents (Stoll, 2001; Woods, 2003).
Table 2.
Experiment 1: comparison of demographic and clinical variables for the HC and SZ groups. Mean +/- standard deviation are given for each variable.
| HC (N=16) | SZ (N=15) | Statistic | p | |
|---|---|---|---|---|
| Age (years) | 44.2 +/- 5.3 | 45.8 +/- 7.5 | t[29] = -0.692 | 0.494 |
| Parental socio-economic status (Hollingshead, 1965) | 2.56 +/- 1.1 | 3.0 +/- 1.2 | t[29] = -1.064 | 0.296 |
| Age of onset (years) | 23.3 +/- 4.5 | |||
| Medication dosage (CPZ equivalents) | 507 +/- 369 range 100-1250 |
Table 3.
Experiment 2: comparison of demographic and clinical variables for the HC and SZ groups. Mean +/- standard deviation are given for each variable.
| HC | SZ | Statistic | p | |
|---|---|---|---|---|
| Age (years) | 43.2 +/- 7.4 | 43.5 +/- 7.7 | t[28] = -0.097 | 0.924 |
| Parental socio-economic status (Hollingshead, 1965) | 3.1 +/- 1.4 | 2.8 +/- 0.9 | t[27] = 0.635 | 0.530 |
| Age of onset (years) | 22.3 +/- 5.1 | |||
| Medication dosage (CPZ equivalents) | 522 +/- 374 range 100-1250 |
Stimuli and procedures
Subjects were seated in a comfortable chair in a quiet room. They were instructed to fixate a cross in the center of the monitor during each trial, and to press a button on the response box as soon as they detected the presentation of a target stimulus.
In both experiments (Fig. 1) each trial began with the presentation of a fixation cross for 800 ms, followed by a location cue to orient visual attention in space. A target stimulus was presented in the left or right visual field (LVF/RVF) at a variable SOA after the cue. The fixation cross, cue, and target remained on the screen for 2000 ms or until the subject responded. The target locations were outlined by boxes which were constantly presented throughout each block of trials.
In each block of trials every combination of Cue, SOA, and Target was presented 6 times. Subjects performed 4 blocks of trials, responding with their left hand in 2 blocks and with their right hand in 2 blocks. Response hand order was counterbalanced across subjects.
In Experiment 1 the cue stimuli were arrows that overlapped the fixation cross (Fig. 1A). The cue types were Valid (pointing to the target location, 50% of trials) or Neutral (pointing to both target locations, 50% of trials). Thus, Valid cues were completely informative about the location of the upcoming target stimulus, and subjects were instructed about the cue-target contingencies. There were three cue-target SOAs: 100, 400, and 700 ms.
In Experiment 2 the cue stimuli consisted of a brightening of the target location boxes (Fig. 1B). Cues were Valid (upcoming target box, 33%), Invalid (box opposite to the upcoming target, 33%), or Neutral (both boxes, 33%). Hence, the cues did not predict the location of the target stimuli, and no instructions were given to the subjects regarding cue-target contingencies. Cue-target SOAs were 100 and 500 ms.
HEOG recording and analysis
Central fixation was monitored with the HEOG, which was continuously recorded from Ag/AgCl electrodes using a Neuroscan Synamp amplifier (0.01-100 Hz filter, 250 Hz sampling rate). The HEOG electrodes were placed at the left and right outer canthi and bipolar-referenced. A ground electrode was placed on the forehead. Electrode impedances were <10 kΩ.
At the beginning of the experimental session, participants performed a HEOG calibration task in which they moved their eyes to follow a small circle on the monitor. The circle shifted position to the left or right with displacements of 1° of visual angle on each trial (25 trials for each direction). The HEOG voltage corresponding to a 1° eye movement was used as a criterion for discarding trials from further analysis. If the HEOG on a given trial deviated by more than this criterion voltage during the period from the onset of the fixation cross until 200 ms after target onset, that trial was discarded. Trials on which the subject failed to respond by 2000 ms after target onset were also discarded.
Single-trial epochs were derived from the continuous recordings for each trial, beginning 100 ms before fixation onset and ending 1000 ms after target onset. If the percentage of discarded trials exceeded 25% of the total number of trials for a condition, that participant's data were excluded.
Statistical analyses
Behavioral measures were error rate and median RT, analyzed in ANOVAs with the design Group (HC/SZ) X response Hand (Left/Right) X Cue X SOA X Target (LVF/RVF). In Experiment 1 the levels of the Cue and SOA factors were Valid/Neutral and 100/400/700 ms, respectively. In Experiment 2 the levels of Cue and SOA were, respectively, Valid/Neutral/Invalid and 100/500 ms. Attentional facilitation and inhibition were measured respectively by RT benefits (Neutral minus Valid) and costs (Invalid minus Neutral). The Greenhouse-Geisser correction for inhomogeneity of variance (Keselman & Rogan, 1980) was applied for factors with more than 2 levels, and is reflected in the reported p values. Post-hoc tests were corrected for multiple comparisons by the Bonferroni method. Effect sizes are reported as partial η2 values.
Results
Experiment 1
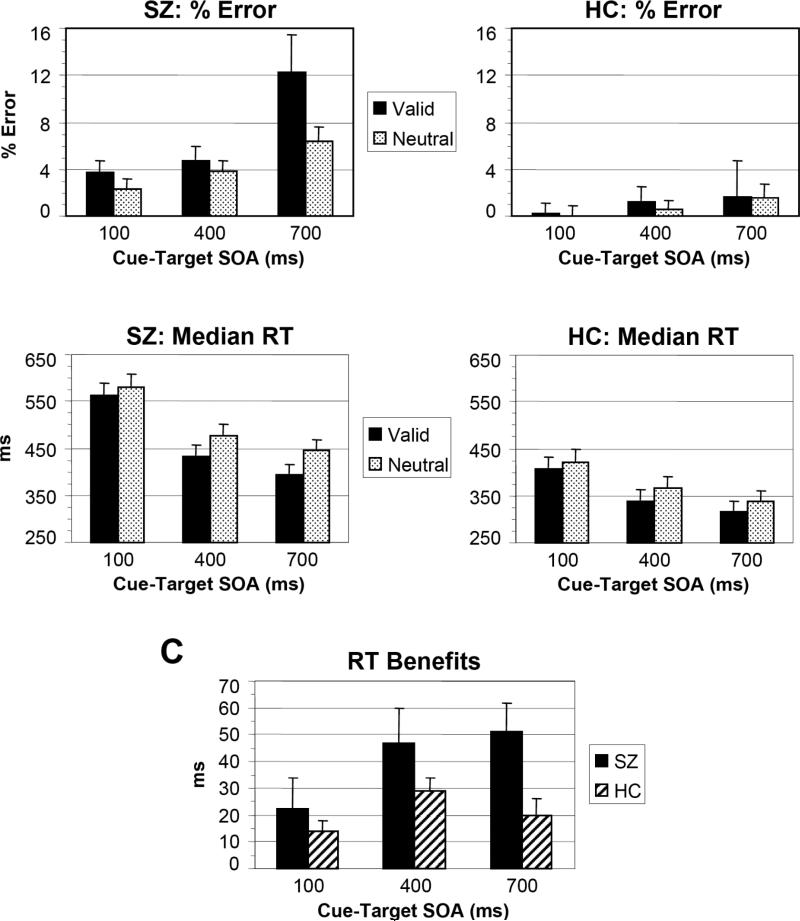
The main results for error rate and median RT are presented in Fig. 2.
Figure 2.
Results of Experiment 1 for the schizophrenia patients (SZ) and healthy control subjects (HC). Percent error (A), median reaction time (RT) (B), and RT benefits (Neutral minus Valid cue RTs) (C) are plotted as a function of cue-target stimulus onset asynchrony (SOA) and cue type.
Error rate
SZ made more errors than HC (F[1,30] = 9.3, p < 0.01, partial η2 = 0.241). Overall, subjects made more errors as SOA increased (F[2,58] = 9.37, p < 0.01, partial η2 = 0.244). This effect was larger for SZ than HC (Group X SOA: F[2,58] = 3.88, p < 0.05, partial η2 = 0.118).
The main effect of Hand (F[1,29] = 3.24, p = 0.083, partial η2 = 0.100) approached significance, with subjects tending to make more errors for left hand than right hand responses. The main effect of Cue also approached significance (F[1,29] = 3.74, p = 0.063, partial η2 = 0.114), with subjects tending to make more errors for Valid than Neutral cues. However, the Group X Cue interaction was not significant (F[1,29] = 2.25, p = 0.144, partial η2 = 0.072) , and the main effect of Cue did not approach significance for either group alone (p's > 0.116). The interaction of Hand X Cue was significant (F[1,29] = 12.2, p < 0.01, partial η2 = 0.296), due to subjects making more errors for Valid than Neutral cues when responding with the left hand (5.4% vs. 3.0%) than the right hand (2.6% vs. 1.9%). The main effect of Target was not significant (F[1,30] = 0.342, p = 0.563, partial η2 = 0.012).
RT
Subjects responded more quickly to targets preceded by Valid than Neutral cues (Cue: F[1,29] = 56.3, p < 0.0001, partial η2 = 0.660). This cueing effect was larger in SZ than HC (Group X Cue: F[1,29] = 5.03, p < 0.05, partial η2 = 0.148). Since RTs were longer overall for SZ than HC (F[1,29] = 12.0, p < 0.01, partial η2 = 0.293), we examined whether the larger cueing effect in SZ was an artifact due to their longer RTs overall by correlating the size of the facilitation effect (Neutral – Valid RT) with mean RT (within the SZ group). This correlation was not significant (Pearson's r = 0.057, p = 0.840). When overall mean RT was included as a covariate there was no interaction between mean RT and Cue (F[1,28] = 0.001, p = 0.992, partial η2 = 0.000), and the Group X Cue interaction became marginally significant (F[1,28] = 3.41, p = 0.075, partial η2 = 0.109), due to the reduction in power by the covariate.
In general, RTs decreased as SOA increased (F[2,58] = 134.3, p < 0.0001, partial η2 = 0.822), and this effect was larger for SZ than HC (Group X SOA: F[2,58] = 11.1, p < 0.001, partial η2 = 0.278). The overall interaction between Cue and SOA approached significance (F[2,58] = 2.83, p = 0.073, partial η2 = 0.089), but SOA did not influence the Group X Cue interaction (Group X Cue X SOA: F[2,58] = 0.852, p = 0.423, partial η2 = 0.029).
The SOA effects on RT stand in contrast with the SOA effects on error rate. As cue-target SOA increased, error rates also increased, but RTs decreased, and both of these effects were larger for SZ than HC. Since errors in this task are misses, rather than incorrect responses, these effects cannot be attributed to a speed-accuracy tradeoff. Rather, it appears that on a small percentage of trials, subjects’ attention waned as the cue-target SOA increased, causing them to miss the target stimuli. But on trials during which their attention did not wane, longer cue-target SOAs gave the subjects more time to prepare a response, and their RTs became shorter.
There was a significant Group X Hand interaction (F[1,29] = 5.31, p < 0.05, partial η2 = 0.155) on RT. SZ responded more quickly with their left hand (462 ms) than their right hand (500 ms) (F[1,14] = 5.83, p < 0.05, partial η2 = 0.294), even though all the SZ were right-handed. For HC, however, response hand had no influence on RT (left hand: 364 ms; right hand: 363 ms) (F[1,15] = 0.006, p = 0.940, partial η2 = 0.000). There were no other significant interactions involving the Hand factor (p's > 0.192). Target visual field had no effects on RT (for main effect and interactions, p's > 0.240).
The results of Experiment 1 thus demonstrate that schizophrenia patients show larger effects of voluntary attentional facilitation than healthy individuals, an effect that is not an artifact of an overall RT difference.
Experiment 2
The main results for error rate and median RT are presented in Fig. 3.
Figure 3.
Results of Experiment 2 for SZ and HC, plotted as in Fig. 2. RT costs are calculated as Invalid minus Neutral cue RTs.
Error rate
There was a trend for SZ to make more errors than HC, but it was not significant (F[1,30] = 3.00, p = 0.093, partial η2 = 0.091). Overall, subjects made more errors as SOA increased (F[1,30] = 10.6, p < 0.01, partial η2 = 0.261), but unlike in Experiment 1, this pattern did not differ between groups (Group X SOA: F[1,30] = 2.30, p = 0.140, partial η2 = 0.071). The main effect of Cue approached significance (F[2,60] = 2.82, p = 0.071, partial η2 = 0.086), while the main effects of Hand (F[1,30] = 0.966, p = 0.333, partial η2 = 0.031) and Target (F[1,30] = 2.87, p = 0.101, partial η2 = 0.087) were not significant.
RT
There was a significant main effect of Cue (F[2,60] = 23.3, p < 0.0001, partial η2 = 0.437) and an interaction between Group and Cue (F[2,60] = 3.40, p < 0.05, partial η2 = 0.102). In HC, Invalid cues led to longer RTs than Valid and Neutral cues (F[2,30] = 5.90, p < 0.01, partial η2 = 0.282; Valid vs. Neutral: p = 1.000; Valid vs. Invalid: p < 0.05; Neutral vs. Invalid: p < 0.05). In SZ, RTs were shortest for Valid cues, longer for Neutral cues, and longest for Invalid cues (F[2,30] = 20.3, p < 0.0001, partial η2 = 0.575; Valid vs. Neutral: p < 0.05; Valid vs. Invalid: p < 0.001; Neutral vs. Invalid: p < 0.01). SZ tended to have longer RTs than HC, but in contrast to Experiment 1, the difference here was not significant (F[1,30] = 2.62, p = 0.116, partial η2 = 0.80).
To examine RT benefits, an ANOVA was conducted as above but including just the Valid and Neutral levels of the Cue factor. There was a significant Group X Cue interaction (F[1,30] = 4.56, p < 0.05, partial η2 = 0.132), confirming the comparison above that showed a facilitation effect for SZ but not HC. To examine RT costs, an ANOVA was similarly conducted with just the Neutral and Invalid levels of Cue. The main effect of Cue was significant (F[1,30] = 25.2, p < 0.0001, partial η2 = 0.456), but not the interaction with Group (F[1,30] = 0.505, p = 0.483, partial η2 = 0.017). Neither benefits nor costs were correlated with mean RT in the SZ group (Valid – Neutral effect: Pearson's r = 0.068, p = 0.803; Neutral – Invalid effect: r = 0.089, p = 0.742).
RTs were shorter overall at the 500 ms than the 100 ms SOA (F[1,30] = 23.3, p < 0.0001, partial η2 = 0.438), but this effect did not differ between groups (Group X SOA: F[1,30] = 0.350, p = 0.558, partial η2 = 0.012). However, while the interaction in the omnibus ANOVA between Group, Cue, and SOA was not significant (F[2,60] = 0.643, p = 0.528, partial η2 = 0.021), inspection of the data in Figs. 3B and 3C suggests that at the 500 ms SOA, the HC group showed evidence of inhibition of return: RT benefits were negative rather than positive. Therefore, an ANOVA was conducted on just the 100 ms SOA data. In this analysis there was a significant main effect of Cue (F[2,60] = 39.6, p < 0.0001, partial η2 = 0.569), but the Group X Cue interaction was not significant (F[2,60] = 1.70, p = 0.192, partial η2 = 0.054). Exploratory analyses of RT benefits at the 100 ms SOA found a significant main effect of Cue (F[2,60] = 7.20, p < 0.05, partial η2 = 0.194), but no Group X Cue interaction (F[2,60] = 1.57, p = 0.220, partial η2 = 0.050). Therefore, the evidence for greater RT benefits in SZ in this experiment may be confounded by reduced benefits in HC at the 500 ms SOA due to inhibition of return.
In the omnibus ANOVA there was an overall Hand X Target interaction (F[1,30] = 6.47, p < 0.05, partial η2 = 0.177), indicating that responses to LVF targets were faster than responses to RVF targets when subjects responded with their left hand (F[1,30] = 5.61, p < 0.05, partial η2 = 0.158), but RTs did not differ between target visual fields for right hand responses (F[1,30] = 0.810, p = 0.375, partial η2 = 0.026).
The results of Experiment 2 do not provide convincing evidence of larger attentional facilitation effects in SZ than HC when automatic orienting mechanisms are engaged because of the apparent influence of inhibition of return. SZ and HC did not differ in the size of their attentional inhibition effects. As in Experiment 1, as cue-target SOA increased, error rates also increased and RTs decreased, but these effects did not differ between groups.
Discussion
Our review of the literature suggested that abnormally enhanced attentional facilitation might be present in schizophrenia. These studies showed that in comparison to healthy participants, patients with schizophrenia show faster RT to validly-cued targets, in relation to baseline, uncued targets. However, in these prior experiments, baseline uncued target trials differed on several dimensions in addition to the absence of a cue. These differences in task parameters have long been thought to present potential experimental confounds (see Jonides & Mack, 1984).
Accordingly then, we designed Experiment 1 using an orienting task that avoided some of the problems with the versions employed by other investigators. By using only valid and neutral symbolic cues with equal probability of presentation, we obtained a purer measure of voluntary attentional facilitation than tasks with infrequent invalid and neutral cue conditions used in prior studies. Our results provided clear evidence of abnormal orienting of attention in this sample of chronic, medicated patients with schizophrenia. That is, the data revealed a significant Group X Cue interaction as well as a significant Group X SOA interaction on RT. Follow-up comparisons indicated that the Group X Cue interaction reflected that relative to healthy controls, patients responded faster to validly-cued targets than to neutrally-cued targets. In addition, both groups showed faster RTs for longer cue-target SOAs, with the patient group showing a more pronounced effect as reflected by the Group X SOA interaction. However, there were no interactions between the Group, Cue, and SOA factors, indicating that enhanced attentional facilitation was not related to the cue-target interval. Nor could enhanced attentional facilitation be attributed to group differences in RT or eye movements, which we monitored using HEOG recordings.
In Experiment 2 we tested whether enhanced attentional facilitation would be found in a standard task that engaged automatic, rather than voluntary, orienting mechanisms by presenting subjects with exogenous, unpredictive cues. In this experiment, contrasting with Experiment 1, attentional facilitation effects did not differ between schizophrenia patients and healthy controls at a short SOA (100 ms). At a longer SOA (500 ms), schizophrenia patients did demonstrate a larger facilitation effect than controls, but this effect likely reflected the influence of inhibition of return on the RTs of the controls. The 500 ms SOA was well within the cue-target SOA range of inhibition of return (e.g., Posner & Cohen, 1984), and several studies have reported that schizophrenia patients show reduced inhibition of return compared to healthy individuals (e.g., Huey & Wexler, 1994; Sapir et al., 2001). Attentional inhibition effects did not differ between groups in this task.
In sum, larger attentional facilitation effects were found in schizophrenia patients compared to healthy control subjects when they voluntarily oriented their attention between the left and right visual hemifields. In contrast, facilitatory cueing effects did not differ between groups in Experiment 2, which engaged automatic orienting processes (although the patients did show a deficit in inhibition of return). Thus, the results of this study and the others reviewed above point to a relative enhancement of voluntary, facilitatory orienting of attention in schizophrenia, in which individuals with schizophrenia show greater benefits in utilizing cues to shift their focus of attention than healthy individuals.
It is important to note that the enhanced attentional facilitation and preparation effects are relative – the schizophrenia patients did not actually perform better than the healthy controls, but rather, they appeared to benefit more from the cues. In Experiment 1 the patients had overall deficits in RT and error rate, but not in Experiment 2. Therefore, the results of this study suggest that there may be a general deficit in voluntary attentional processes in schizophrenia (Luck & Gold, 2008), with a relative preservation of automatic processes (excepting inhibition of return). Consistent with this hypothesis, we found a marginally significant correlation between RT benefits and overall error rate in the SZ group (r = 0.465, p = 0.081), but not the HC group (r = -0.173, p = 0.521). Schizophrenia patients thus may be able to benefit relatively more from cues due to their deficit in voluntary attentional control.
One limitation of this study is that the arrows used as symbolic cues may not have engaged solely voluntary attentional processes. Ristic and Kingstone (2006) demonstrated that unpredictive arrow cues could elicit automatic orienting, and the effects of cueing with predictive arrow cues were greater than the summed cueing effects of non-predictive arrows and predictive non-directional cues. Thus, predictive arrow cues may engage both voluntary and automatic processes, which appear to interact in a superadditive manner. Therefore, we cannot state with certainty that the enhanced attentional facilitation effects seen here in schizophrenia patients are due to an abnormality in a purely voluntary process.
Basis of enhanced attentional facilitation in schizophrenia
The most obvious explanation for the enhanced attentional facilitation effect is that the speed of voluntary attentional orienting is enhanced in schizophrenia patients. Consistent with this hypothesis, studies of eye movements in schizophrenia have also reported findings of enhanced saccade speed under certain conditions. The speed of both prosaccades (Reilly, Harris, Keshavan, & Sweeney, 2005; Reilly, Harris, Khine, Keshavan, & Sweeney, 2008) and predictive saccades (McDowell, Clementz, & Wixted, 1996) have been found to be enhanced in schizophrenia patients compared to healthy individuals. In prosaccade tasks subjects make a saccade to a cued location, just as in cued attention tasks, subjects orient their attention to a cued location. In predictive saccade tasks, subjects make a saccade to an upcoming predicted target location. As the neural systems controlling eye movements and spatial attention shifts are known to overlap to a considerable degree (Corbetta et al., 1998), the present finding of enhanced attentional facilitation could share a common mechanism with those of enhanced prosaccade and predictive saccade speed in schizophrenia.
It seems to be important that enhanced attentional facilitation is seen when voluntary, “top-down” orienting processes are engaged, rather than solely involuntary, “bottom-up” processes. With regard to the latter, Luck et al. (2006) failed to find strong evidence for slowed orienting in schizophrenia in tasks in which the focus of attention was guided by salient cues. Likewise, we did not find significant differences in orienting costs or benefits in Experiment 2 which examined automatic orienting of attention. In Experiment 1 of Luck et al. (2006), the onset latency of the N2pc component of the event-related brain potential was used as a measure of the timing of attentional selection in addition to target RT. While the schizophrenia patients in that study had longer RTs than healthy control subjects, the onset latency of the N2pc did not differ between groups. One way to test our hypothesis that the speed of voluntary attentional orienting is enhanced in schizophrenia patients would be to examine N2pc onset latency in a voluntary orienting task. We would predict that N2pc onset latency would be shortened by valid compared to neutral cues, and that this effect would be greater in schizophrenia patients than healthy control subjects.
Another possible explanation for the enhanced attentional facilitation effect is that schizophrenia patients are impaired relative to healthy individuals at dividing their attention between the two target locations in the neutral cue condition, whereas their ability to focus attention at the target location in the valid cue condition is relatively normal. Thus, the larger orienting benefits in schizophrenia patients could be due to inefficient processing in the neutral condition rather than an enhancement of facilitatory effects. However, the error rates in the valid cue condition tended to be higher than in the neutral condition for both subject groups in Experiment 1, so there is no evidence that dividing attention between the target locations was more demanding than focusing attention.
Relationship to other cognitive abnormalities
The finding of enhanced attentional facilitation stands in contrast with the attentional deficits that have been commonly described in schizophrenia (e.g., Bleuler, 1950/1911; Braff & Light, 2004; Kraepelin, 1971/1919; Luck & Gold, 2008; Nestor & O'Donnell, 1998; Nuechterlein & Dawson, 1984). However, other phenomena of abnormally enhanced information processing have been reported in schizophrenia: 1) As reviewed above, there is evidence that prosaccade and predictive saccade speed is enhanced in schizophrenia. 2) A number of studies have demonstrated enhanced semantic priming in schizophrenia patients relative to healthy individuals (e.g., Fuentes & Santiago, 1999; Manschreck et al., 1988; Niznikiewicz et al., 1997). 3) Elkins and Cromwell (1994) found that in a flanker task involving identity priming, facilitation effects were increased and inhibitory effects reduced in schizophrenia patients compared to control subjects. Thus, enhancements of attentional facilitation, prosaccade and predictive saccade speed, semantic priming, and identity priming in schizophrenia all share a common property: abnormally enhanced facilitation effects when a cue or prime stimulus provides information about the subsequent target stimulus. Future studies should examine whether these phenomena are correlated.
Neural mechanisms underlying enhanced attentional facilitation
Abnormally enhanced attentional facilitation in schizophrenia may be related to dysfunction in the network of brain regions that controls both spatial attention and oculomotor behavior, which includes the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (Corbetta et al., 1998; Moore, Armstrong, & Fallah, 2003). Given the evidence for neural circuit abnormalities in the DLPFC in schizophrenia (e.g., Lewis & Gonzalez-Burgos, 2008; Tan, Callicott, & Weinberger, 2007), and considering the crucial role of this area in the regulation of behavior (Miller & Cohen, 2001), it is possible that abnormal attentional facilitation in schizophrenia results from a dysfunction of processes in this area.
The results of studies employing transcranial magnetic stimulation (TMS) of the frontal eye fields (FEF), a subregion of the DLPFC, shed light on the possible neural circuit abnormalities responsible for enhanced attentional facilitation in schizophrenia. When a TMS pulse is delivered to the FEF prior to the arrival of target stimulus information in the visual cortex, this pulse can facilitate saccade triggering (Nyffeler et al., 2004), the execution of memory-guided saccades (Wipfli et al., 2001), attentional orienting (Grosbras & Paus, 2002; Smith, Jackson, & Rorden, 2005), and target detection (Grosbras & Paus, 2003). TMS of the DLPFC can also increase the percentage of express saccades (Müri et al., 1999). These facilitatory effects are consistent with the disruption of local circuit inhibition by TMS. One possibility is that enhanced attentional facilitation in schizophrenia may be due to dysfunctional inhibitory processes in the FEF, as there is abundant evidence for abnormalities of DLPFC inhibitory interneurons in schizophrenia, including reduced inhibitory neurotransmission (Lewis & Gonzalez-Burgos, 2008). Future studies utilizing neurophysiological methods will be necessary to test this hypothesis.
Acknowledgments
This work was supported by an Essel Foundation/NARSAD Young Investigator Award (KMS), NIH F32 MH13022 (KMS), NIH R01 MH080187 (KMS), and a US Dept. of Veterans Affairs Schizophrenia Center grant (RWM). The authors are grateful to the anonymous reviewers for their insightful comments on this paper.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Bleuler T. Dementia Praecox of the Group of Schizophrenias. International Universities Press; New York: 1950. (Original work published 1911) [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE. Cerebral laterality and psychopathology: Perceptual and event-related potential asymmetries in affective and schizophrenic disorders. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. MIT Press; Cambridge, MA: 1995. pp. 661–691. [Google Scholar]
- Bustillo JR, Thaker G, Buchanan RW, Moran M, Kirkpatrick B, Carpenter WT. Visual information-processing impairments in deficit and nondeficit schizophrenia. American Journal of Psychiatry. 1997;154:647–654. doi: 10.1176/ajp.154.5.647. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: Controlled and automatic processes. Biological Psychiatry. 1992;31:909–918. doi: 10.1016/0006-3223(92)90117-i. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, O'Shora-Celaya L, Nordahl TE. Attentional asymmetry in schizophrenia: The role of illness subtype and symptomatology. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:661–683. doi: 10.1016/0278-5846(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Zysset S, Müsseler J, von Cramon DY. Where action impairs visual encoding: an event-related fMRI study. Cognitive Brain Research. 2004;21:39–48. doi: 10.1016/j.cogbrainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, Cromwell RL. Priming effects in schizophrenia: Associative interference and facilitation as a function of visual context. Journal of Abnormal Psychology. 1994;103:791–800. doi: 10.1037//0021-843x.103.4.791. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. New York State Psychiatric Institute; New York, NY: 1995. [Google Scholar]
- Fuentes LJ, Santiago E. Spatial and semantic inhibitory processing in schizophrenia. Neuropsychology. 1999;13:259–270. doi: 10.1037//0894-4105.13.2.259. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson B, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. Journal of Abnormal Psychology. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophrenia Research. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR. Visual orienting in schizophrenia. Schizophrenia Research. 1992;7:203–209. doi: 10.1016/0920-9964(92)90013-u. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Balke M, Hajsamou S, Ruhrmann S, Schultze-Lutter F, Daumann J, Heekeren K. Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophrenia Research. 2007;97:35–42. doi: 10.1016/j.schres.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. Journal of Cognitive Neuroscience. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. European Journal of Neuroscience. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Huey ED, Wexler BE. Abnormalities in rapid, automatic aspects of attention in schizophrenia: Blunted inhibition of return. Schizophrenia Research. 1994;14:57–63. doi: 10.1016/0920-9964(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Jonides J, Mack R. On the cost and benefit of cost and benefit. Psychological Bulletin. 1984;96:29–44. [Google Scholar]
- Keselman HJ, Rogan JC. Repeated measures F tests and psychophysiological research: controlling the number of false positives. Psychophysiology. 1980;17:499–503. doi: 10.1111/j.1469-8986.1980.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox. Churchill Livingstone; New York: 1971. (Originally published 1919) [Google Scholar]
- Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology Reviews. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Liotti M, Dazzi S, Umilta C. Deficits of the automatic orienting of attention in schizophrenic patients. Journal of Psychiatry Research. 1993;27:119–130. doi: 10.1016/0022-3956(93)90056-8. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophrenia Research. 2006;85:174–195. doi: 10.1016/j.schres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisspeim C, Schneier ML. Semantic priming in thought disordered schizophrenic patients. Schizophrenia Research. 1988;1:661–666. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Maruff P, Hay D, Malone V, Currie J. Asymmetries in the covert orienting of visual spatial attention in schizophrenia. Neuropsychologia. 1995;33:1205–1223. doi: 10.1016/0028-3932(95)00037-4. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA, Wixted JT. Timing and amplitude during predictive saccadic tracking in schizophrenia. Psychophysiology. 1996;33:93–101. doi: 10.1111/j.1469-8986.1996.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Moran MJ, Thaker GK, Laporte DJ, Cassady SL, Ross DE. Covert visual attention in schizophrenia spectrum personality disordered subjects: Visuospatial cuing and alerting effects. Journal of Psychiatry Research. 1996;30:261–275. doi: 10.1016/0022-3956(96)00004-0. [DOI] [PubMed] [Google Scholar]
- Müri RM, Rivaud S, Gaymard B, Ploner CJ, Vermersch AI, Hess CW, Pierrot-Deseilligny C. Role of the prefrontal cortex in the control of express saccades. A transcranial magnetic stimulation study. Neuropsychologia. 1999;37:199–206. doi: 10.1016/s0028-3932(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Penhune V, Shenton ME, Pollak S. Attentional cues in chronic schizophrenia: Abnormal disengagement of attention. Journal of Abnormal Psychology. 1992;101:682–689. doi: 10.1037//0021-843x.101.4.682. [DOI] [PubMed] [Google Scholar]
- Nestor PG, O'Donnell BF. The mind adrift: Attentional dysregulation in schizophrenia. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Niznikiewicz MA, O'Donnell BF, Nestor PG, Smith L, Law S, Karapelou M, Shenton ME, McCarley RW. ERP assessment of visual and auditory language processing in schizophrenia. Journal of Abnormal Psychology. 1997;106:85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Bucher O, Pflugshaupt T, Von Wartburg R, Wurtz P, Hess CW, Müri RM. Single-pulse transcranial magnetic stimulation over the frontal eye field can facilitate and inhibit saccade triggering. European Journal of Neuroscience. 2004;20:2240–2244. doi: 10.1111/j.1460-9568.2004.03667.x. [DOI] [PubMed] [Google Scholar]
- Oie M, Rund BR, Sundet K. Covert visual attention in patients with early-onset schizophrenia. Schizophrenia Research. 1998;34:195–205. doi: 10.1016/s0920-9964(98)00092-9. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. X. Erlbaum; Hillsdale, NJ: 1984. pp. 531–556. [Google Scholar]
- Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Archives of General Psychiatry. 1988;45:814–821. doi: 10.1001/archpsyc.1988.01800330038004. [DOI] [PubMed] [Google Scholar]
- Posner MI, Inhoff AW, Friedrich FJ, Cohen A. Isolating attentional systems: a cognitive-anatomical analysis. Psychobiology. 1987;15:107–121. [Google Scholar]
- Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biological Psychiatry. 2005;57:145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biological Psychiatry. 2008;63:776–783. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J, Kingston A. Attention to arrows: Pointing to a new direction. Quarterly Journal of Experimental Psychology. 2006;59:1921–1930. doi: 10.1080/17470210500416367. [DOI] [PubMed] [Google Scholar]
- Sapir A, Dobrusin M, Ben-Bashat G, Henik A. Neuroleptics reverse attentional effects in schizophrenia patients. Neuropsychologia. 2007;45:3263–3271. doi: 10.1016/j.neuropsychologia.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15:361–370. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Smith DT, Jackson SR, Rorden C. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia. 2005;43:1288–1296. doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-IIIR (SCID-NP) – Non-Patient Edition. American Psychiatric Press; Washington, D.C.: 1990. [Google Scholar]
- Strauss ME, Alphs L, Boekamp J. Disengagement of attention in chronic schizophrenia. Psychiatry Research. 1992;43:87–92. doi: 10.1016/0165-1781(92)90144-r. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Novakovic T, Tien AY, Bylsma F, Pearlson GD. Disengagement of attention in schizophrenia. Psychiatry Research. 1991;37:139–146. doi: 10.1016/0165-1781(91)90071-v. [DOI] [PubMed] [Google Scholar]
- Stoll AL. The Psychopharmacology Reference Card. McLean Hospital; Belmont, MA: 2001. [Google Scholar]
- Tan H-Y, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex. 2007;17:i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Swanson JM, Potkin SG. Lateralized attentional deficits in drug-free and medicated schizophrenia patients. Neuropsychologia. 1997;35:1519–1525. doi: 10.1016/s0028-3932(97)00087-0. [DOI] [PubMed] [Google Scholar]
- Wipfli M, Felblinger J, Mosimann UP, Hess CW, Schlaepfer TE, Müri RM. Double-pulse transcranial magnetic stimulation over the frontal eye field facilitates triggering of memory-guided saccades. European Journal of Neuroscience. 2001;14:571–575. doi: 10.1046/j.0953-816x.2001.01671.x. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]