Abstract
Glioblastoma multiform (GBM) is by far the most malignant glioma. We have introduced a new treatment for GBMs that comprises the inhalation of a naturally occurring terpene with chemotherapeutic properties known as perillyl alcohol (POH). Clinical trial results on recurrent GBM patients showed that POH extends the average life by more than eight months, temporarily slows tumor growth, and in some cases even decreases tumor size. After approximately seven months the tumor continues to grow and leads to a dismal prognosis. To investigate how these tumors become resistant to POH we generated an A172 human glioblastoma cell culture tolerant to 0.06 mM of POH (A172r). We used Multidimensional Protein Identification Technology (MudPIT) to compare the protein expression profile of A172r cells to the established glioblastoma A172 cell line. Our results include a list of identified proteins unique to either the resistant or the non-resistant cell line. These proteins are related to cellular growth, negative apoptosis regulation, Ras pathway, and other key cellular functions that could be connected to the underlying mechanisms of resistance.
Keywords: Glioblastoma, MudPIT, perillyl alcohol, A172 cells, resistant, protein profile
Introduction
Glioblastoma multiform (GBM) is by far the most common and malignant glioma. During the last 25 years, no significant advances in the treatment of glioblastoma multiform (GBM) have been developed. Currently, patients with surgical resection who are treated by radiation or chemotherapy have an approximate survival time of 12 months.1 In such a scenario, the quest for more effective chemotherapeutic agents is of great importance. Perillyl alcohol (POH), a naturally occurring terpene, is a new and promising chemotherapeutic agent. It has cytostatic and cytotoxic effects 2 and induces apoptosis in lung, 3 leukocyte, 4 prostate, 5 and breast cancer cell lines. 6 In vivo, POH has anti-metastatic effects and is a potent inhibitor of angiogenesis.7,8 Its chemotherapeutic effects are under evaluation in several clinical trials, including patients with colorectal, breast, or ovarian cancer. 9-11
In particular, we previously reported an ongoing clinical trial (phase I) comprising GBM patients treated by a new method of administration through intranasal delivery that has statistically shown to increase survival time.1 The side effects of treatment with POH are almost none, even in patients that survived for more than a year. Our results showed POH slows tumor growth and in some cases even decreases tumor size as shown in Figure 1. However, after approximately seven months the tumor becomes resistant to POH, continues to grow, and leads to an adverse prognosis. 1
Figure 1.
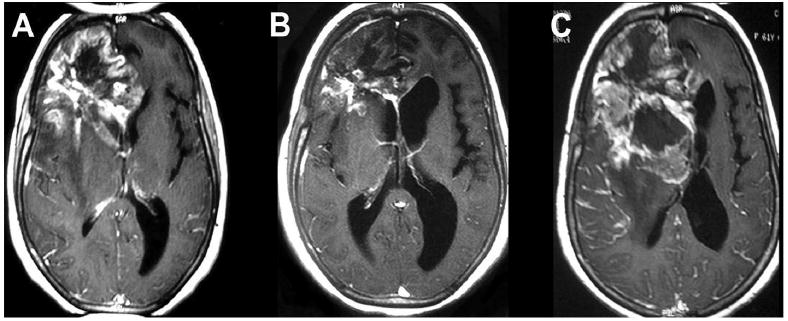
Magnetic resonance imaging (MRI) follow-up on a patient with recurrent primary glioblastoma multiform (GBM) in the lobar location under treatment with perillyl alcohol (POH) by intranasal delivery. A: MRI before starting the treatment with POH. B: MRI after 5 months of treatment with POH. C: MRI after 7 months of treatment with POH.
In a previous report, we used Differential Gel Electrophoresis (DIGE) to compare the plasma protein profile of these patients before and after 4 months of treatment with POH. Our protocol included depleting the six most abundant plasma proteins, excising spots of interest and analyzing them by MALDI-TOF-TOF.12 We identified candidate prognostic proteins (e.g., anti-thrombin) that correlated with the progression of the disease; however, they were common plasma proteins that were not specific to the disease. In this regard, the results shed little light on the effects of the POH treatment at a molecular level. Moreover, few proteins were identified because of the nature of the technique employed. 12
To continue evolving the use of POH as a treatment we need to identify the molecular changes that lead to tumor resistance to POH. Identifying pathways activated by treatment with POH could provide insights on how to combine chemotherapeutic agents to synergistically treat the disease or in developing new treatments. To increase the number of identified proteins we employed the state-of-the-art proteomic methodology known as Multidimensional Protein Identification Technology (MudPIT). The later uses two-dimensional liquid chromatography (LC/LC) online with tandem mass spectrometry and is capable of identifying thousands of proteins in complex mixtures. To overcome the problem of finding specific tumor proteins, we focused our experiments on human glioblastoma cells instead of plasma.
To tackle the problem of investigating tumor resistance to POH, we generated a new glioblastoma cell culture, referred heretofore as A172r, which can tolerate up to 0.06 mM of POH; in contrast, the commercial A172 cell line dies when exposed to this concentration. The A172r protein profile was compared with the established human glioblastoma multiform cell line A172. Relative protein quantitation was performed using spectral counting 13,14 and the profile comparisons were carried out using the PatternLab for proteomics suite. 15
Experimental section
1. A172 cell culture
A172 cells were obtained from the American Type Culture Collection and grown as monolayers in 25 cm2 tissue culture flasks in Dulbecco's modified Eagle medium (D-MEM) supplemented with 0.2 mM nonessential amino acids, 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (fungizone, 2.5 mg/ml). For sub-cultivations, confluent monolayers were gently washed with phosphate-buffered saline (PBS 1×) pH 7.2, and after a short period of trypsin treatment, the cells were suspended in the previous culture medium for the control cells.
2. A172 resistant cell culture
The A172r were generated by gradually increasing the concentration of POH in the medium, starting with 0.003% (v/v) during three passages, followed by 0.006% (v/v) during four passages, 0.0075% (v/v) throughout 5 passages, then 0.0085% (v/v) through 5 passages and finally 0.01% (0.06 mM) during 8 passages. We then tested the effects of 0.06 mM on normal A172 cells. One passage of A172 cells directly to a medium containing 0.06 mM of POH kills these cells in 36 hours.
3. Protein solubilization and trypsin digestion
Each culture medium (A172 & A172r) was discarded and the pellets were obtained by centrifugation at 700 RCF. After washing three times with PBS, the pellets were incubated on ice for 45 minutes with 100 μL of 10 mM Tris (pH 7.5), 1 mM EDTA for enrichment of the membrane fraction. Cells were lysed in a 1cc U-100 insulin syringe filled with cell suspension by pushing and compressing the plunger several times. The soluble fraction was separated from the membrane fraction by ultra-centrifugation (18,000 RCF). The proteins pellets were then re-suspended with RapiGest and trypsinization was carried out as described by Fischer et al.16
4. Protein identification by MudPIT
Seventy micrograms of the digested peptide mixture were loaded onto a biphasic (strong cation exchange / reversed phase) capillary column and washed with a buffer containing 5% acetonitrile, 0.1% formic acid diluted in HPLC grade water. The two-dimensional liquid chromatography separation and tandem mass spectrometry conditions were as described by Washburn et al. 17 Mass spectrometry data were collected using a Finnigan LTQ-XL (Thermo, San Jose, CA) set to the data-dependent acquisition mode with dynamic exclusion turned on. One MS survey scan was followed by nine MS/MS scans and 12 salt steps were performed. The Xcalibur data system (Thermo, San Jose, CA) was used to control the mass spectrometer scan functions and HPLC solvent gradients. The analyses were performed in triplicates.
Tandem mass spectra were extracted from the raw files using RawExtract1_9_8 18 and were searched against the Homo sapiens sequences plus common contaminant proteins; all sequences were downloaded as FASTA-formatted from the EBI-IPI protein database (version 3.23, released on November 2, 2006). 19 The confidence levels and false positive rates were calculated using a decoy database that contained the reverse sequences of the original dataset appended to the target database was used 20, and the best matching sequences from the combined database were indicated by SEQUEST. The peptide mass search tolerance was set to 3 Da. No differential modifications were considered. The search space included all candidate peptides whose theoretical mass fell within the 3 Da mass tolerance window, regardless of their tryptic status; no enzymatic cleavages were imposed.
The validity of peptide / spectrum matches was assessed in DTASelect 2.0.31 21 according to the SEQUEST 22 cross-correlation score (XCorr) and the SEQUEST normalized difference in cross-correlation score (DeltaCN). The search results were grouped by charge state (+1, +2, and +3) and tryptic status (fully tryptic, half-tryptic, and non-tryptic), resulting in nine distinct subgroups. For each subgroup, the distribution of XCorr and DeltaCN values for the direct and decoy database hits was obtained, and the two subsets were separated by quadratic discriminant analysis. Outlier points in the two distributions were discarded. The full separation of the direct and decoy subsets is not generally possible, so the discriminant score was set such that a false discovery rate of 1% was determined based on the number of accepted decoy database peptides. This procedure was independently performed on each data subset, resulting in a false-positive rate that is independent of tryptic status or charge state. Additionally, a minimum sequence length of seven amino acid residues was required, and each protein on the list was supported by at least two peptide identifications. The last filtering step results in an estimated false discovery rate lower than 1%.
5. PatternLab for Proteomics data parsing
The DTASelect-filtered files (which contain the spectral counting information) were converted to PatternLab's native data format (the index and sparse matrix files) by using PatternLab's DTASelect to Sparse Matrix parser. The PatternLab output includes an index and a sparse matrix file. The index file lists all identified proteins within all the project's assays and assigns to each a unique Protein IDentification (PID) integer. The sparse matrix file contains rows were each one corresponds to an assay and follows the schema: 〈class label〉 〈PID1〉:〈value1〉…〈PIDn〉:〈valuen〉, where n is the number of identified proteins for that assay. Here, 〈class label〉 was assigned as 1 for the A172r cells and -1 for non treated A172 cells. Accordingly, 〈PIDi〉 and 〈valuei〉 correspond, respectively, to the ith protein's identification integer and its spectral count for the respective assay. The resulting sparse matrix has 6 rows obtained from each condition in triplicates. The index and sparse matrix are available in Supplemental Data I.
6. Selection of differentially expressed proteins according to the TFold methodology
The PatternLab TFold module was used to pinpoint differentially expressed proteins between the A172 and the A172r cells. Proteins having an absolute fold change greater than 2.5 and a t-test p-value of 0.01 were considered as differentially expressed. The fold change of 2.5 was obtained through the TFold procedure as to maximize the number of proteins that satisfy the fold change, t-test and the Benjamini-Hochberg theoretical false discovery rate estimator (BH-FDR)(q-value of 0.05). 23 Additionally, only proteins present in at least five of all six MudPIT assays were considered for this analysis.
7. Selection of unique proteins
The proteins that were only identified in either the A172 or the A172r were assessed using PatternLab's Approximately Area Proportional Venn Diagram module. A stringent selection criterion was imposed to only consider proteins that were identified in all three assays of a condition and found in no assays of the other condition.
8. Gene Ontology Explorer analysis
To help interpret the set of selected proteins we used PatternLab's Gene Ontology Explorer (GOE×) module.24 Our data analysis used the gene ontology database (OBO v1.2, downloaded June 2nd, 2010) and the human annotation file (GOA, downloaded on June 2nd, 2010) from http://geneontology.org and http://www.ebi.ac.uk, respectively. The Gene Ontology Explorer specialist mode was used to search proteins in the Venn diagram and TFold results that mapped to key words such as negative apoptosis regulation and cellular growth.
9. Validation of differential expression by Western Blot
Western blot for HSP 70 was performed according to Fischer et al 16 using fifteen micrograms of the membrane-enriched fraction lysate.
Results
Selection of differentially expressed proteins according to the TFold methodology
A report listing the proteins considered for the TFold analysis (present in at least 5 out of the six assays) with their fold change and p-value for differential expression is presented on Supplemental Data II. Table 1 list fifty seven proteins that were considered differentially expressed according to the TFold methodology. Figure 2 shows the graphical representation of the TFold analysis; it maps proteins according to their p-value for differential expression and their fold change.
Table I.
Differentially expressed proteins selected by the TFold Analysis. All proteins were found to be statistically differentially expressed (p < 0.01) and have an absolute fold change greater than 2.5. The set of proteins also satisfies the BH-FDR of 0.05 criterion. A positive fold indicates a greater expression on the A172r.
| IPI | Fold Change | Description |
|---|---|---|
| IPI00027493.1 | 5.36 | SLC3A2;LOC442497 4F2 cell-surface antigen heavy chain |
| IPI00382470.3 | 4.69 | HSP90AA1 Heat shock protein HSP 90-alpha 2 |
| IPI00013744.1 | 4.47 | ITGA2 Integrin alpha-2 precursor |
| IPI00384280.5 | 3.98 | PCYOX1 Prenylcysteine oxidase precursor |
| IPI00334775.6 | 3.72 | HSP90AB1 85 kDa protein |
| IPI00033494.3 | 3.71 | MRLC2 Myosin regulatory light chain |
| IPI00298237.7 | 3.67 | TPP1 Isoform 1 of Tripeptidyl-peptidase 1 precursor |
| IPI00332106.2 | 3.56 | PBXIP1 Pre-B-cell leukemia transcription factor-interacting protein 1 |
| IPI00065486.2 | 3.55 | ABCB6 Isoform 4 of Mitochondrial ATP-binding cassette subfamily B member 6 |
| IPI00031357.1 | 3.43 | PPOX Protoporphyrinogen oxidase |
| IPI00304925.5 | 3.13 | HSPA1B;HSPA1A Heat shock 70 kDa protein 1 |
| IPI00029133.4 | 2.90 | ATP5F1 ATP synthase B chain, mitochondrial precursor |
| IPI00099463.2 | 2.86 | SGPL1 Sphingosine-1-phosphate lyase 1 |
| IPI00003527.5 | -2.50 | SLC9A3R1 Ezrin-radixin-moesin-binding phosphoprotein 50 |
| IPI00456940.5 | -2.50 | RPL7L1 Ribosomal protein L7-like 1 |
| IPI00106847.3 | -2.50 | MSH6 Isoform GTBP-alt of DNA mismatch repair protein MSH6 |
| IPI00186290.6 | -2.51 | EEF2 Elongation factor 2 |
| IPI00000816.1 | -2.52 | YWHAE 14-3-3 protein epsilon |
| IPI00012773.1 | -2.57 | MTA1 Isoform Long of Metastasis-associated protein MTA1 |
| IPI00029111.2 | -2.58 | DPYSL3 DPYSL3 protein |
| IPI00007207.2 | -2.60 | LIPA Isoform 1 of Lysosomal acid lipase/cholesteryl ester hydrolase precursor |
| IPI00005107.2 | -2.64 | NPC1 Niemann-Pick C1 protein precursor |
| IPI00029019.5 | -2.75 | UBAP2L Isoform 2 of Ubiquitin-associated protein 2-like |
| IPI00169383.3 | -2.77 | PGK1 Phosphoglycerate kinase 1 |
| IPI00183786.1 | -2.89 | FADS2 fatty acid desaturase 2 |
| IPI00008868.3 | -2.97 | MAP1B Microtubule-associated protein 1B |
| IPI00218918.5 | -3.00 | ANXA1 Annexin A1 |
| IPI00000875.6 | -3.07 | EEF1G Elongation factor 1-gamma |
| IPI00376798.3 | -3.10 | RPL11 Isoform 1 of 60S ribosomal protein L11 |
| IPI00221093.7 | -3.13 | RPS17 40S ribosomal protein S17 |
| IPI00020567.1 | -3.25 | ARHGAP1 Rho GTPase-activating protein 1 |
| IPI00299086.3 | -3.33 | SDCBP Syntenin-1 |
| IPI00479018.2 | -3.33 | SDCBP Syntenin isoform 3 |
| IPI00297261.3 | -3.50 | PTPN1 Tyrosine-protein phosphatase non-receptor type 1 |
| IPI00000070.1 | -3.53 | LDLR Low-density lipoprotein receptor precursor |
| IPI00739099.2 | -3.64 | COL5A2 Collagen alpha-2(V) chain precursor |
| IPI00418471.6 | -3.80 | VIM Vimentin |
| IPI00296141.3 | -3.82 | DPP7 Dipeptidyl-peptidase 2 precursor |
| IPI00008894.2 | -3.92 | CPA4 Carboxypeptidase A4 precursor |
| IPI00479186.5 | -3.98 | PKM2 Isoform M2 of Pyruvate kinase isozymes M1/M2 |
| IPI00013068.1 | -4.00 | EIF3S6 Eukaryotic translation initiation factor 3 subunit 6 |
| IPI00072377.1 | -4.24 | SET Isoform 1 of Protein SET |
| IPI00289758.6 | -4.25 | CAPN2 Calpain-2 catalytic subunit precursor |
| IPI00025491.1 | -4.27 | EIF4A1 Eukaryotic initiation factor 4A-I |
| IPI00328298.6 | -4.29 | SMC4 Isoform 2 of Structural maintenance of chromosomes protein 4 |
| IPI00007812.1 | -4.56 | ATP6V1B2 Vacuolar ATP synthase subunit B, brain isoform |
| IPI00043598.2 | -4.63 | IKIP CDNA FLJ31051 fis, clone HSYRA2000605, weakly similar to MYOSIN HEAVY CHAIN, CLONE 203 |
| IPI00027422.1 | -4.69 | ITGB4 Isoform Beta-4C of Integrin beta-4 precursor |
| IPI00396171.3 | -4.88 | MAP4 Isoform 1 of Microtubule-associated protein 4 |
| IPI00184330.5 | -5.78 | MCM2 DNA replication licensing factor MCM2 |
| IPI00465248.5 | -6.05 | ENO1 Isoform alpha-enolase of Alpha-enolase |
| IPI00021033.2 | -6.71 | COL3A1 Isoform 1 of Collagen alpha-1(III) chain precursor |
| IPI00027626.3 | -6.90 | CCT6A T-complex protein 1 subunit zeta |
| IPI00784347.2 | -8.92 | KRT18 Keratin, type I cytoskeletal 18 |
| IPI00026781.2 | -10.00 | FASN Fatty acid synthase |
| IPI00554648.3 | -11.31 | KRT8 Keratin, type II cytoskeletal 8 |
| IPI00012011.6 | -15.64 | CFL1 Cofilin-1 |
Figure 2.
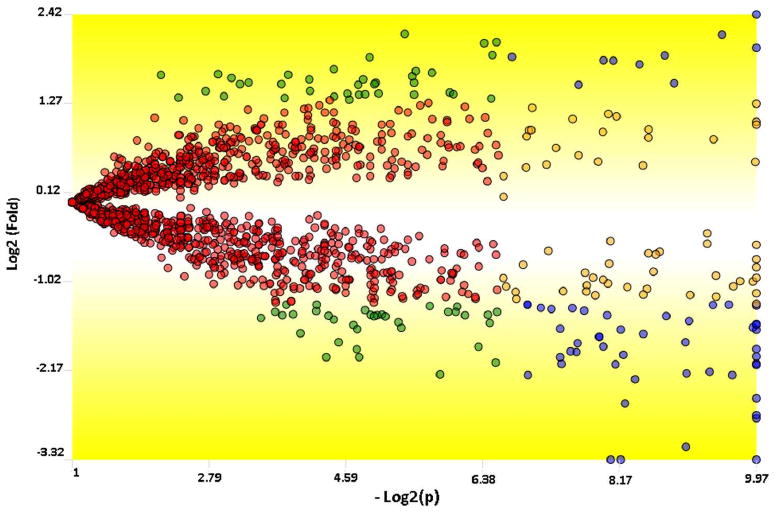
A TFold comparative plot between the A172r and the A172 cells. Only proteins found in all 6 MudPIT assays were considered for this analysis. Each protein (represented as a dot) is mapped according to its log2 (fold change) on the ordinate axis and its -log2 (t-test p-value) on the abscissa axis. The latter indicates how likely the observed fold change is a result of chance. Fold changes refer to the ratios of the average relative quantitation values obtained for each state. Accordingly, blue-dot proteins have p-value < 0.01 and an absolute fold change greater than 2.5, the established fold-change cutoff. Orange-dot proteins did not meet the fold-change cutoff but were indicated as statistically different. Green-dot proteins met the fold-change cutoff but cannot be claimed to be statistically different. Red dots did not satisfy the fold-change or the statistical cutoffs. The BH theoretical false-positive estimator (q < 0.05) indicates that all proteins selected as differentially expressed are likely to be truly differentially expressed. There are 57, 60, 72, and 1284 blue, orange, green, and red dots, respectively.
The results were further verified by confirming the differential expression of a chosen protein, in this case HSP70, with western blot (Figure 3). As shown in Figure 3 the expression of HSP70 in A172r is greater than in the A172 and is in accordance with the results obtained by spectral counting.
Figure 3.
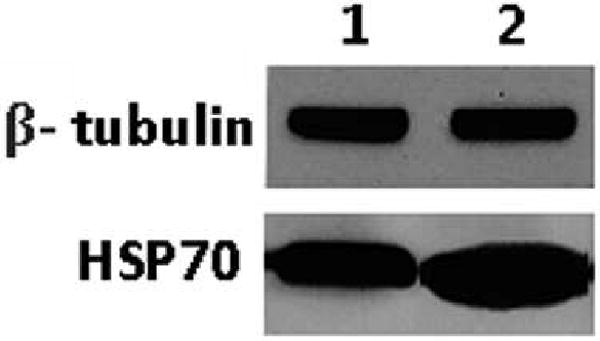
Western blot of HSP70: Slots 1 and 2 correspond to A172 and A172r, respectively. The western blot shows HSP70 to be over-expressed in A172r. Beta-tubulin, a protein not indicated as differentially expressed by the TFold, was used as an internal standard.
Selection of Unique Proteins
An approximately area proportional Venn diagram (AAPVD) analysis was generated using PatternLab to pinpoint proteins that were uniquely identified in the A172 (Table II) or in A172r (Table III). The selection criterion was stringent because it considered only proteins found in all three replicates of each state and not found in any of the other state. We identified 19 and 72 unique proteins related to the A172 and A172r cells, respectively. The Venn diagram is presented in Figure 4. A less stringent analysis considering only proteins found in at least two of the three replicates is found in Supplemental Data III; this analysis yielded 133 and 257 uniquely identified proteins for A172 and A172r, respectively.
Table II.
Uniquely identified proteins in the A172 cell culture
| IPI00384938.1 | IGHG1 Hypothetical protein DKFZp686N02209 |
| IPI00011454.1 | GANAB Isoform 2 of Neutral alpha-glucosidase AB precursor |
| IPI00008603.1 | ACTA2 Actin, aortic smooth muscle |
| IPI00015737.1 | DCAKD CDNA: FLJ22955 fis, clone KAT09907 |
| IPI00386258.1 | MTCH1 Isoform 1 of Mitochondrial carrier homolog 1 |
| IPI00003926.3 | CLN8 Protein CLN8 |
| IPI00027776.6 | FECH Ferrochelatase, mitochondrial precursor |
| IPI00016915.1 | IGFBP7 Insulin-like growth factor-binding protein 7 precursor |
| IPI00177940.2 | RDH14;NT5C1B Retinol dehydrogenase 14 |
| IPI00328383.2 | SLC25A46 48 kDa protein |
| IPI00396329.1 | BXDC1 Brix domain-containing protein 1 |
| IPI00171445.1 | ATAD1 ATPase family AAA domain-containing protein 1 |
| IPI00294380.5 | PCK2 Phosphoenolpyruvate carboxykinase [GTP], mitochondrial precursor |
| IPI00030380.1 | GTF2H1 TFIIH basal transcription factor complex p62 subunit |
| IPI00295598.4 | CBARA1 calcium binding atopy-related autoantigen 1 |
| IPI00433279.3 | SLFN5 Isoform 1 of Schlafen family member 5 |
| IPI00304331.2 | B3GAT3 Galactosylgalactosylxylosylprotein 3-beta-glucuronosyltransferase 3 |
| IPI00302966.5 | TTC17 Tetratricopeptide repeat protein 17 |
| IPI00027443.5 | CARS cysteinyl-tRNA synthetase isoform c |
Table III.
Uniquely identified proteins in the A172r cell culture
| IPI00216952.1 | LMNA Isoform C of Lamin-A/C |
| IPI00383581.3 | GANAB Isoform 1 of Neutral alpha-glucosidase AB precursor |
| IPI00021428.1 | ACTA1 Actin, alpha skeletal muscle |
| IPI00026302.3 | RPL31 60S ribosomal protein L31 |
| IPI00219910.1 | BLVRB 23 kDa protein |
| IPI00007063.5 | HDGFRP3 Hepatoma-derived growth factor-related protein 3 |
| IPI00328616.3 | MRPL52 39S ribosomal protein L52, mitochondrial precursor |
| IPI00219446.5 | PEBP1 Phosphatidylethanolamine-binding protein 1 |
| IPI00010201.4 | - 40 kDa protein |
| IPI00221091.9 | RPS15A 40S ribosomal protein S15a |
| IPI00022442.2 | NDUFAB1 Acyl carrier protein, mitochondrial precursor |
| IPI00291093.3 | POLR2E DNA-directed RNA polymerases I, II, and III subunit RPABC1 |
| IPI00215790.6 | RPL38 60S ribosomal protein L38 |
| IPI00003949.1 | UBE2N Ubiquitin-conjugating enzyme E2 N |
| IPI00419979.3 | PAK2 Serine/threonine-protein kinase PAK 2 |
| IPI00218830.1 | NMT1 Isoform Short of Glycylpeptide N-tetradecanoyltransferase 1 |
| IPI00152658.1 | NEK7 Serine/threonine-protein kinase Nek7 |
| IPI00100106.1 | RIC8A resistance to inhibitors of cholinesterase 8 homolog A |
| IPI00741973.1 | LOC131691 similar to peptidylprolyl isomerase A isoform 1 |
| IPI00031650.3 | NUDT16L1 Isoform 1 of Protein syndesmos |
| IPI00018272.3 | PNPO Pyridoxine-5′-phosphate oxidase |
| IPI00247063.3 | MME Neprilysin |
| IPI00295851.4 | COPB1 Coatomer subunit beta |
| IPI00017763.4 | NAP1L4 Nucleosome assembly protein 1-like 4 |
| IPI00037283.3 | DNM1L Isoform 5 of Dynamin-1-like protein |
| IPI00101532.5 | CEP55 Isoform 1 of Centrosomal protein of 55 kDa |
| IPI00007402.2 | IPO7 120 kDa protein |
| IPI00009982.1 | TDRKH Isoform 1 of Tudor and KH domain-containing protein |
| IPI00019901.1 | ADD1 Isoform 1 of Alpha-adducin |
| IPI00006029.2 | FOXK2 Isoform 1 of Forkhead box protein K2 |
| IPI00032957.1 | UBE2I SUMO-conjugating enzyme UBC9 |
| IPI00456635.1 | UNC13D Isoform 1 of Unc-13 homolog D |
| IPI00024364.2 | TNPO1 transportin 1 isoform 1 |
| IPI00034159.1 | ATP6V0D1 Vacuolar ATP synthase subunit d 1 |
| IPI00012726.4 | PABPC4 Isoform 1 of Polyadenylate-binding protein 4 |
| IPI00220030.1 | PXN Isoform Alpha of Paxillin |
| IPI00009943.2 | TPT1 Tumor protein, translationally-controlled 1 |
| IPI00004962.2 | GOLPH4 Golgi phosphoprotein 4 |
| IPI00165357.4 | MTA3 Isoform 1 of Metastasis-associated protein MTA3 |
| IPI00052885.7 | LOC729611;LOC731796 similar to 60S ribosomal protein L29 |
| IPI00032137.1 | ACTN3 Alpha-actinin-3 |
| IPI00008380.1 | PPP2CA Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform |
| IPI00008339.5 | C14orf173 hypothetical protein LOC64423 isoform 1 |
| IPI00419847.3 | SORBS3 Isoform Alpha of Vinexin |
| IPI00293073.4 | MFN1 Mitochondrial transmembrane GTPase FZO-2 |
| IPI00009822.1 | SRP54 Signal recognition particle 54 kDa protein |
| IPI00009057.2 | G3BP2 Isoform A of Ras GTPase-activating protein-binding protein 2 |
| IPI00029723.1 | FSTL1 Follistatin-related protein 1 precursor |
| IPI00185146.5 | IPO9 Importin-9 |
| IPI00005030.5 | SH3BP4 Isoform 1 of SH3 domain-binding protein 4 |
| IPI00061356.3 | CCDC99 Isoform 1 of Coiled-coil domain-containing protein 99 |
| IPI00291783.3 | GEMIN5 Gem-associated protein 5 |
| IPI00014456.4 | STRN Isoform 1 of Striatin |
| IPI00030009.4 | PAPSS2 Isoform A of Bifunctional 3′-phosphoadenosine 5′-phosphosulfate synthetase 2 |
| IPI00375843.2 | KRT80 Keratin-80 |
| IPI00152701.7 | PLCD3 phospholipase C delta 3 |
| IPI00742944.2 | FMN2 Formin-2 |
| IPI00030781.1 | STAT1 Isoform Alpha of Signal transducer and activator of transcription 1-alpha/beta |
| IPI00339269.1 | HSPA6 Heat shock 70 kDa protein 6 |
| IPI00003843.1 | TJP2 Isoform A1 of Tight junction protein ZO-2 |
| IPI00013871.1 | RRM1 Ribonucleoside-diphosphate reductase large subunit |
| IPI00023339.2 | CREBBP CREB-binding protein |
| IPI00014897.4 | PLCB4 Isoform 2 of 1-phosphatidylinositol-4,5-bisphosphate phosphodiesterase beta 4 |
| IPI00296388.4 | BAZ2A Isoform 1 of Bromodomain adjacent to zinc finger domain protein 2A |
| IPI00107531.1 | RAD50 Isoform 3 of DNA repair protein RAD50 |
| IPI00479125.3 | SRGAP2 SLIT-ROBO Rho GTPase-activating protein 2 |
| IPI00034006.1 | PTPN23 Tyrosine-protein phosphatase non-receptor type 23 |
| IPI00409601.1 | KIAA1219 Isoform 1 of Protein KIAA1219 |
| IPI00166010.6 | CNOT1 CCR4-NOT transcription complex, subunit 1 isoform a |
| IPI00297655.4 | NOTCH2 Neurogenic locus notch homolog protein 2 precursor |
| IPI00159322.4 | TCF20 Isoform 1 of Transcription factor 20 |
| IPI00019447.5 | FANCI Isoform 1 of Fanconi anemia group I protein |
Figure 4.

Approximately proportional Venn diagram of the identified proteins presents in all three replicates of each state: The dark green represents the proteins unique of the A172 cells; the yellow represents the proteins unique of the resistant cells (A172r). The number in parentheses represents the unique identified proteins in each condition. Only proteins present in all three replicates of a state were considered.
Discussion and Conclusions
Prior pharmacological studies have generated several clues on the mechanism of action of POH. It has been demonstrated that POH acts by inhibiting the isoprenylation of the small GTPase Ras proteins by blocking their tethering in the cytoplasmic membrane; this leads to inhibiting Ras signal transduction. 25, 26 Moreover, POH was shown to induce apoptosis, 4,27,28 causes G0/G1 arrest in several types of cancers, 29,30 instigates transitory G2/M arrest, and induce enhanced Fas-mediated apoptosis. 31 The ability of POH to disrupt protein anchorage to cell membrane suggests it may interfere with other signaling processes.
A more detailed understanding of the effects of POH on A172 could help in developing new synergic and more efficient treatments for this disease. Some key differentially expressed proteins from Table I that could be linked to the POH treatment or the underlying mechanisms of POH resistance are pinpointed below:
Cofilin (IPI00012011.6) is related to cell locomotion. This process is the one by which tumor cells spread to surrounding tissues by active invasion. In vivo, cells that have a more rapid locomotion are found to be more invasive and metastatic. 32 In vitro, cell motility has been shown to be an key part of the invasive potential of glioma tumors; moreover, the locomotory habits of particular glioma cell lines derived from tumors has been shown to be stable, and unaffected by passage number.33 Cofilin is known to be overexpressed in glioma tumor cells, 34 and in several other tumor cell types.35,36 We found a decrease in the expression of cofilin in the A172r cells as shown in Table I. This suggests that POH reduces the chances of tumor metastasis and invasiveness.
Several heat shock proteins (HSPs) were found to be up-regulated in A172r (e.g., HSP90AA1, HSP90AB1, HSPA1B, HSPA1A). HSPs, also known as stress proteins are present in cells of all life forms and appear to confer protection against a wide range of cellular and tissue injures. 37 The HSPs superfamily includes several different molecular weight class families of which the HSP70 family is the most highly conserved. HSP70 is a prominent cytoprotective factor: its downregulation can kill tumor cells and facilitate the induction of apoptosis.38,39 In contrast, its upregulation, as a consequence of either cellular stress or transfection, is linked with inhibiting apoptosis.40,41 For example, expression levels of HSP70 were shown to correlate negatively with response to chemotherapy in breast cancer. 42 Our results showed HSP70 to be over-expressed in the A172r cells suggesting a contribution to the resistance of POH.
The 4F2 cell-surface antigen heavy chain (IPI00027493.1) was found to be upregulated in A172r and is annotated in the GO to be related to cell growth. Its over-expression was previously linked with the resistance to mitoxantrone in MCF-7 cells (human breast cancer cell line). 43
ARHGAP1 Rho GTPase-activating protein 1 (IPI00020567.1), NPC1 Niemann-Pick C1 protein precursor (IPI00005107.2), ITGB4 Isoform Beta-4C of Integrin beta-4 p (IPI00027422.1) mediates the transfer of a signal from the outside to the inside of a cell by means other than the introduction of the signal molecule itself into the cell. They were found downregulated in A172r; however, ITGA2 Integrin alpha-2 precursor (IPI00013744.1), that serves the same purpose, was found to be overexpressed suggesting major changes in signal transduction. The expression of the latter has been noted to be correlated with resistance in malignant mesotheliomas. 44
The PatternLab Venn diagram analysis pinpointed proteins that were uniquely identified in either the A172 or A172r. It should be noted that “uniquely identified” does not necessarily mean that the protein is non-existent in a given condition; however, it was below the detection limits of our technique. Among these proteins we pinpoint a few found in the A172r that have been linked with tumor resistance or cell growth:
NOTCH2 Neurogenic locus notch homolog protein 2 precursor (IPI00297655.4) is linked with a negative regulation of apoptosis and programmed cell death. Recently, Wang et al. linked this protein with promoting resistance of glioma stem cells to radiotherapy.45 Moreover, the authors showed that inhibition of Notch pathway with gamma-secretase inhibitors (GSIs) renders the glioma stem cells more sensitive to radiation at clinically relevant doses.
G3BP2 Isoform A of Ras GTPase-activating protein-binding protein 2 (IPI00009057.2) is linked with signal transduction. Auger et al., has also linked the over-expression of this protein with resistance to temozolomide in glioma cell lines.46
HSPA6 Heat shock 70 kDa protein 6 (Hsp70-6) was identified among the unique proteins. The human cytosolic Hsp70s are composed of six canonical members, named Hsp70-1a, Hsp70-1b, Hsp70-1t, Hsp70-2, Hsp70-6, and Hsc70. In particular, HSP 70-6 is, most likely, the least studied of this family. It usually shows almost no basal expression and is only expressed in cases of extreme stresses.47 Such appearance suggests a link with the glioblastoma cell line and resistance to POH.
TPT1 Tumor protein, translationally-controlled 1 (IPI00009943.2). The expression of this protein is up-regulated by growth stimuli and repressed when growth arrest is induced. This protein has was shown to be correlated with glioma growth in previous studies.48
PXN Isoform Alpha of Paxillin (IPI00220030.1) is a target of a number of oncogenes involved in key signal transduction and important in cell motility and migration. The up-regulation of this protein was linked with an increase in tumor growth and cell proliferation in lung cancer.49
RIC8A resistance to inhibitors of cholinesterase 8 homolog A (IPI00100106.1). This protein is a candidate marker for tumor aggressiveness or tamoxifen resistance in estrogen receptor -positive breast cancers. 50
It is too much to expect that Ras therapies alone can be successful against high-grade malignant gliomas in the clinic. Our results mark a step forward in understanding the resistance mechanisms and candidates that could be target for knock-down experimentation. The results include lists of candidate protein markers that are representative of either the resistant or the non-resistant state of the A172 in response to POH, thus providing a good starting point for future investigations. Even though the linkage of several proteins have been made to the context of cancer disease states current knowledge only allows us to at best link the presence of these proteins to a phenotype of a disease. Therefore, these proteins are not necessarily causative of the disease and could just as likely be proteins associated to a cellular state occurring during the progression of the tumor without special consequences to the actual process of the disease. Among the challenges and limitations of this work, we should point out a few: a) the great complexity and dynamic range of proteins in the biological sample analyzed limits our analysis only to the most abundant proteins; b) The study did not target differential expression of post-translational modifications; c) The results obtained by using a human glioblastoma cell line do not necessarily reflect the same changes that occur in patients' tumors, but our expectation is that there should be many common markers. We note that disease biology encompasses the interplay of interacting proteins within the entire cellular proteome. Tailoring experiments to better understand how these multiple gene products interact and how they are linked to each level of the disease development could provide further molecular insights on how to treat such pathologies.
Supplementary Material
Acknowledgments
This work was supported by CAPES-Fiocruz 30/2006, CNPq, the Rio de Janeiro Proteomic Network, PDTIS, Fundação do Câncer, and NIH P41 RR011823 (JRY).
References
- 1.Da Fonseca CO, Schwartmann G, Fischer JSG, Nagel J, Futuro D, Quirico-Santos T, Gattass CR. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol. 2008;70:259–67. doi: 10.1016/j.surneu.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Clark SS, Zhong L, Filiault D, Perman S, Ren Z, Gould M, Yang X. Anti-leukemia effect of perillyl alcohol in Bcr/Abl-transformed cells indirectly inhibits signaling through Mek in a Ras- and Raf-independent fashion. Clin Cancer Res. 2003;12:4494–504. [PubMed] [Google Scholar]
- 3.Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007;2:216–26. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Clark SS. Perillyl alcohol induces c-Myc-dependent apoptosis in Bcr/Abl-transformed leukemia cells. Oncology. 2006;1:13–18. doi: 10.1159/000091181. [DOI] [PubMed] [Google Scholar]
- 5.Chung BH, Lee HY, Lee JS, Young CY. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2006;2:222–28. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Yuri T, Danbara N, Tsujita-Kyutoku M, Kiyozuka Y, Senzaki H, Shikata N, Kanzaki H, Tsubura A. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2004;3:251–60. doi: 10.1023/B:BREA.0000019966.97011.4d. [DOI] [PubMed] [Google Scholar]
- 7.Loutrari H, Hatziapostolou M, Skouridou V, Papadimitriou E, Roussos C, Kolisis FN, Papapetropoulos A. Perillyl alcohol is an angiogenesis inhibitor. J Pharmacol Exp Ther. 2004;2:568–75. doi: 10.1124/jpet.104.070516. [DOI] [PubMed] [Google Scholar]
- 8.Teruszkin BI, Ves de PS, Henriques SN, Curie CM, Gibaldi D, Bozza M, O Da Fonseca CO, Carvalho MGC. Effects of perillyl alcohol in glial C6 cell line in vitro and anti-metastatic activity in chorioallantoic membrane model. Int J Mol Med. 2002;6:785–8. doi: 10.3892/ijmm.10.6.785. [DOI] [PubMed] [Google Scholar]
- 9.Meadows SM, Mulkerin D, Berlin J, Bailey H, Kolesar J, Warren D, Thomas JP. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. Int J Gastrointest Cancer. 2002;2-3:125–8. doi: 10.1385/IJGC:32:2-3:125. [DOI] [PubMed] [Google Scholar]
- 10.Stearns V, Coop A, Singh B, Gallagher A, Yamauchi H, Lieberman R, Pennanen M, Trock B, Hayes DF, Ellis MJ. A pilot surrogate end point biomarker trial of perillyl alcohol in breast neoplasia. Clin Cancer Res. 2004;22:7583–91. doi: 10.1158/1078-0432.CCR-04-0295. [DOI] [PubMed] [Google Scholar]
- 11.Bailey HH, Levy D, Harris LS, Schink JC, Foss F, Beatty P, Wadler S. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern Cooperative Oncology Group Study E2E96. Gynecol Oncol. 2002;3:464–8. doi: 10.1006/gyno.2002.6647. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JGS, Carvalho PC, Neves-Ferreira AGC, Da Fonseca CO, Perales J, Carvalho MGC, Domont GB. Anti-thrombin as prognostic biomarker candidate for patients with recurrent glioblastoma multiform under treatment with perillyl alcohol. J Exp Therap Oncol. 2008;7:285–90. [PubMed] [Google Scholar]
- 13.Liu H, Sadygov RG, Yates JR., III A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;14:4193–201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho PC, Hewel J, Barbosa VC, Yates JR., III Identifying differences in protein expression levels by spectral counting and feature selection. Genet Mol Res. 2008;2:342–56. doi: 10.4238/vol7-2gmr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho PC, Fischer JSG, Chen EI, Yates JR, III, Barbosa VC. PatternLab for proteomics: a tool for differential shotgun proteomics. BMC Bioinformatics. 2008;9:316. doi: 10.1186/1471-2105-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer JSG, Liao L, Carvalho PC, Barbosa VC, Domont GB, Carvalho MGC, Yates JR., III Dynamic proteomic overview of glioblastoma cells (A172) exposed to perillyl alcohol. J Proteomics. 2010;73:1018–27. doi: 10.1016/j.jprot.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washburn MP, Wolters D, Yates JR., III Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;3:242–7. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 18.McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR., III MS1, MS2, and SQT-three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun Mass Spectrom. 2004;18:2162–68. doi: 10.1002/rcm.1603. [DOI] [PubMed] [Google Scholar]
- 19.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;7:1985–88. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Elias JE, Thoreen CC, Licklider LJ, Gygi SP. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J Proteome Res. 2003;1:43–50. doi: 10.1021/pr025556v. [DOI] [PubMed] [Google Scholar]
- 21.Tabb DL, McDonald WH, Yates JR., III DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1:21–6. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng JKL, McCormack A, Yates JR., III An Approach to Correlate Tandem Mass Spectral Data of Peptides with Amino Acid Sequences in a Protein Database. J Am Soc Mass Spectrom. 1994;5:976–89. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 24.Carvalho PC, Fischer JSG, Chen EI, Domont GB, Carvalho MGC, Degrave WM, Yates JR, III, Barbosa VC. GO Explorer: A gene-ontology tool to aid in the interpretation of shotgun proteomics data. Proteome Sci. 2009;7:6. doi: 10.1186/1477-5956-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardcastle IR, Rowlands MG, Barber AM, Grimshaw RM, Mohan MK, Nutley BP, Jarman M. Inhibition of protein prenylation by metabolites of limonene. Biochem Pharmacol. 1999;7:801–9. doi: 10.1016/s0006-2952(98)00349-9. [DOI] [PubMed] [Google Scholar]
- 26.Crowell PL, Ren Z, Lin S, Vedejs E, Gould MN. Structure-activity relationships among monoterpene inhibitors of protein isoprenylation and cell proliferation. Biochem Pharmacol. 1994;8:1405–15. doi: 10.1016/0006-2952(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 27.Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;5:979–83. [PubMed] [Google Scholar]
- 28.Fernandes J, Da Fonseca CO, Teixeira A, Gattass CR. Perillyl alcohol induces apoptosis in human glioblastoma multiforme cells. Oncol Rep. 2005;5:943–7. [PubMed] [Google Scholar]
- 29.Bardon S, Picard K, Martel P. Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer. 1998;1:1–7. doi: 10.1080/01635589809514708. [DOI] [PubMed] [Google Scholar]
- 30.Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002;2:187–94. doi: 10.1016/s0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 31.Rajesh D, Howard SP. Perillyl alcohol mediated radiosensitization via augmentation of the Fas pathway in prostate cancer cells. Prostate. 2003;1:14–23. doi: 10.1002/pros.10269. [DOI] [PubMed] [Google Scholar]
- 32.Grimstad IA. Growth and metastasis of hypermotile, hyperinvasive cancer cells selected in vitro by rapid locomotion under various conditions. Clin Exp Metast. 1988;6:257–69. doi: 10.1007/BF01753573. [DOI] [PubMed] [Google Scholar]
- 33.Hegedus B, Czirok A, Fazekas I, Babel T, Madarasz E, Vicsek T. Locomotion and proliferation of glioblastoma cells in vitro: statistical evaluation of video microscopic observations. J Neurosurg. 2000;92:428–43. doi: 10.3171/jns.2000.92.3.0428. [DOI] [PubMed] [Google Scholar]
- 34.Gunnersen JM, Spirkoska V, Smith PE, Danks RA, Tan SS. Growth and migration markers of rat C6 glioma cells identified by serial analysis of gene expression. Glia. 2000;32:146–54. [PubMed] [Google Scholar]
- 35.Stierum R, Gaspari M, Dommels Y, Ouatas T, Pluk H, Jespersen S, Vogels J, Verhoeckx K, Groten J, Van Ommen B. Proteome analysis reveals novel proteins associated with proliferation and differentiation of the colorectal cancer cell line Caco- 2. Biochim Biophys Acta. 2003;1650:73–91. doi: 10.1016/s1570-9639(03)00204-8. [DOI] [PubMed] [Google Scholar]
- 36.Unwin RD, Craven RA, Harnden P, Hanrahan S, Totty N, Knowles M, Eardley I, Selby PJ, Banks RE. Proteomic changes in renal cancer and co-ordinate demonstration of both the glycolytic and mitochondrial aspects of the Warburg effect. Proteomics. 2003;3:1620–32. doi: 10.1002/pmic.200300464. [DOI] [PubMed] [Google Scholar]
- 37.Jaattela M, Wissing D. Heat shock proteins protects cells from monocyte cytotoxicity: possible mechanism of self–protection. J Exp Med. 1993;177:231–36. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA. 2000;97(14):7871–876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burkart V, Liu H, Bellmann K, Wissing D, Jaattela M, Cavallo MG, Pozzilli P, Briviba K, Kolb H. Natural resistance of human beta cells toward nitric oxide is mediated by heat shock protein 70. J Biol Chem. 2000;275:19521–528. doi: 10.1074/jbc.M002265200. [DOI] [PubMed] [Google Scholar]
- 40.Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 41.Volloch VZ, Sherman MY. Oncogenic potential of Hsp72. Oncogene. 1999;18:3648–651. doi: 10.1038/sj.onc.1202525. [DOI] [PubMed] [Google Scholar]
- 42.Jolly C, Morimoto RIJ. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. Natl Cancer Inst. 2000;92:1564–572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 43.Rahbar AM, Fenselau C. Unbiased examination of changes in plasma membrane proteins in drug resistant cancer cells. J Proteome Res. 2005;4:2148–153. doi: 10.1021/pr0502370. [DOI] [PubMed] [Google Scholar]
- 44.Mohr S, Keith G, Galateau-Salle F, Icard P, Rihn BH. Cell protection, resistance and invasiveness of two malignant mesotheliomas as assessed by 10K-microarray. Biochim Biophys Acta. 2004;1688:43–60. doi: 10.1016/j.bbadis.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wakeman TP, Lathia JD, Hjelmeland AB, Wang XF, White RR, Rich JN, Sullenger BA. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auger N, Thillet J, Wanherdrick K, Idbaih A, Legrier ME, Dutrillaux B, Sanson M, Poupon MF. Genetic alterations associated with acquired temozolomide resistance in SNB-19, a human glioma cell line. Mol Cancer Ther. 2006;5:2182–92. doi: 10.1158/1535-7163.MCT-05-0428. [DOI] [PubMed] [Google Scholar]
- 47.Leung TK, Hall C, Rajendran M, Spurr NK, Lim L. The human heat-shock genes HSPA6 and HSPA7 are both expressed and localize to chromosome 1. Genomics. 1992;12:74–79. doi: 10.1016/0888-7543(92)90409-l. [DOI] [PubMed] [Google Scholar]
- 48.Staflin K, Zuchner T, Honeth G, Darabi A, Lundberg C. Identification of proteins involved in neural progenitor cell targeting of gliomas. BMC Cancer. 2009;9:206. doi: 10.1186/1471-2407-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, Loganathan S, Kanteti R, Reichman T, Nallasura V, Schwartz S, Faoro L, Wang YC, Girard L, Tretiakova MS, Ahmed S, Zumba O, Soulii L, Bindokas VP, Szeto LL, Gordon GJ, Bueno R, Sugarbaker D, Lingen MW, Sattler M, Krausz T, Vigneswaran W, Natarajan V, Minna J, Vokes EE, Ferguson MK, Husain AN, Salgia R. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008;68:132–42. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han W, Han MR, Kang JJ, Bae JY, Lee JH, Bae YJ, Lee JE, Shin HJ, Hwang KT, Hwang SE, Kim SW, Noh DY. Genomic alterations identified by array comparative genomic hybridization as prognostic markers in tamoxifen-treated estrogen receptor-positive breast cancer. BMC Cancer. 2006;6:92. doi: 10.1186/1471-2407-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


