Abstract
Diabetic retinopathy (DR) classically presents with micro-aneurysms, small haemorrhages and/or lipoprotein exudates. Several studies have indicated that neural loss occurs in DR even before vascular damage can be observed. This study evaluated the possible relationship between structure (spectral domain- optical coherence tomography) and function (Rarebit visual field test) in patients with type 1 diabetes mellitus and no or minimal diabetic retinopathy (DR). Results demonstrated loss of macular visual function and corresponding thinning of the ganglion cell layer (GCL) in the pericentral area of the macula of diabetic patients (Rs = 0.65, p < 0.001). In multivariable logistic regression analysis, GCL thickness remained an independent predictor of decreased visual function (OR 1.5, 95% CI 1.1 – 2.1). Early DR seems to include a neurodegenerative component.
Keywords: Diabetes Mellitus, Neurodegeneration, Ganglion cells, Visual function test, Retina
Introduction
One of the most frequent causes of blindness among adults in the Western world is diabetic retinopathy (DR) (Fong et al., 2004). The clinical hallmarks of DR are primarily vascular, including microaneurysms, hemorrhages, capillary occlusions, and lipoprotein exudates. In addition to vascular changes, neurodegenerative changes have been described including neural apoptosis, loss of ganglion cell bodies, glial reactivity and reduction in thickness of the inner retinal layers in the earliest stages of DR (Abu-El-Asrar et al., 2004; Antonetti et al., 2006; Barber et al., 1998; Barber, 2003; Barber et al., 2005; Gardner et al., 2002; Gastinger et al., 2006; Gastinger et al., 2008; Kern and Barber, 2008; Li and Puro, 2002; Martin et al., 2004; Park et al., 2003; Rungger-Brandle et al., 2000). These findings of structural neuropathy may explain the neuroretinal functional deficits that are known in patients with diabetes, even before the presence of frank retinopathy. Several studies have shown electroretinogram abnormalities, loss of dark adaptation and contrast sensitivity and colour vision disturbances independent of vascular retinopathy (Bearse, Jr. et al., 2006; Bronson-Castain et al., 2009; Di Leo et al., 1992; Dosso et al., 1996; Hardy et al., 1992; Kurtenbach et al., 2002; Lopes de Faria et al., 2001; Ng et al., 2008; Realini et al., 2004).
Conventional threshold perimetry and visual function tests are insensitive measures of minor neuro-visual damage (Frisen and Quigley, 1984; Kerrigan-Baumrind et al., 2000). The Rarebit technique, which includes Rarebit Perimetry (RBP) and the Rarebit Fovea Test (RFT), was developed to improve detection of subtle defects (Frisen, 2002). The Rarebit technique is based on the principle of detection of very small and bright stimuli. The small stimulus corresponds to half the minimum angle of resolution at the tested retinal location. The test avoids simultaneous stimulation of more than one receptive field, defined as the group of photoreceptors converging on the same ganglion cell (Fischer, 1973). In a previous study employing the Rarebit technique, Nilsson et al. detected foveal dysfunction in patients with diabetes mellitus type 1 without DR (Nilsson et al., 2007).
With optical coherence tomography (OCT) the retinal thickness (RT) can be measured with high accuracy. The retinal thickness in diabetic patients with no or minimal DR is thinner than in normals. (Asefzadeh et al., 2008; Biallosterski et al., 2007; Bronson-Castain et al., 2009; Browning et al., 2008; Nilsson et al., 2007; Oshitari et al., 2009; Van Dijk et al., 2009). The high resolution of spectral domain-OCT (SD-OCT) allows measurement of the thickness of all individual retinal layers (Garvin, 2008), especially if the layers are segmented automatically in three-dimensions (Garvin et al., 2009). Results of a recent study showed that the decreased total RT in type 1 diabetic patients with minimal DR is predominantly caused by a thinning of the ganglion cell layer (GCL) in the pericentral area and retinal nerve fiber layer (RNFL) thinning in the peripheral area of the of the macula (Van Dijk et al., 2010), i.e. both axons and nerve bodies are involved in thinning.
The purpose of the present study is to evaluate the hypothesis that GCL thickness measured with SD-OCT and the function of the macula tested with the Rarebit technique are associated in patients with type1 diabetes mellitus (DM) and no or minimal DR.
Materials and Methods
Patients
Patients with type 1 DM were recruited from the outpatient clinic of the department of Internal Medicine at the Academic Medical Center (AMC University Hospital, Amsterdam, the Netherlands) for an ongoing longitudinal observational study. In September 2008 they were invited to participate in this observational cross-sectional study. Additionally, healthy age-matched individuals served as controls. The study adhered to the tenets of the Declaration of Helsinki, and Institutional Review Board approval was obtained at both the AMC and the University of Iowa. All subjects provided written informed consent.
DR status was evaluated by a retinal specialist through indirect fundoscopy, slit-lamp stereo biomicroscopy and stereoscopic fundus photography. Patients were included if they were diagnosed with minimal or no DR. The definition of minimal DR was conform stage 2 of the International Clinical Diabetic Retinopathy Disease Severity Scale (Wilkinson et al., 2003). Control subjects did not have a diagnosis of any ocular disease, diabetes or other systemic disease, and were randomly recruited from accompanying persons of patients visiting the outpatient clinic of the department of Ophthalmology. Exclusion criteria were refractive errors over S+5, or under S-8 diopters, visual acuity below 20/25, significant media opacities, previous ocular surgery and a previous diagnosis of glaucoma, uveitis, or retinal disease.
Age, gender, duration since diagnosis of diabetes and serum glycosylated hemoglobin (HbA1c) at the time of the study examinations were gathered from the patient charts. Best corrected visual acuity was obtained conform the Early Treatment Diabetic Retinopathy Study, and recorded as Snellen equivalent. All subjects underwent Rarebit Perimetry and the Rarebit Fovea Test (Frisen, 2002). Finally, all subjects underwent papillary dilatation and an ophthalmic examination including slitlamp biomicroscopy with a handheld lens (SuperField; Volk Optical, Inc., Mentor, OH), OCT imaging (3D OCT-1000, Topcon Corporation, Tokyo, Japan) and stereoscopic fundus photography (TRC-50IX; Topcon Corporation, Tokyo, Japan).
Rarebit Perimetry and Rarebit Fovea Test
The RBP and the RFT form a computerized visual function test developed to detect subtle damage to the visual system (Frisen, 2002). The RBP evaluates the central 30° visual field, while the RFT evaluates the central 4° visual field. The test principle is to briefly (200 ms) present zero, one, or two, bright small (<0.5 min of arc) dots against a dark background in a completely dark room. Due to photopic luminance levels for both the fixation mark and the test targets, dark adaptation is not of influence for test results. The subjects are asked to focus on the fixation mark and meanwhile respond by clicking a mouse button once or twice when they detect one respectively two dots anywhere on the screen. The result of the Rarebit test is presented as mean hit rate (MHR). The MHR is a percentage of the stimuli seen by the subject of all presented stimuli. In this study we combined the RFT and RBP and present a combined MHR. The combined MHR is abnormal if below 95% (Malmer and Martin, 2005; Salvetat et al., 2007).
Optical coherence tomography imaging and layer segmentation
OCT images of the subjects were obtained with SD-OCT (3D OCT-1000, Topcon Corporation, Tokyo, Japan) using the 3D volume scan protocol (6 ×6 ×2.2 mm3), consisting of 128 (y) by 512 (x) by 650 (z) voxels. From this volume, nine intraretinal surfaces defining 8 retinal layers were segmented automatically by our algorithm, which uses an extensively validated, robust fully three-dimensional graph search approach (Garvin et al., 2009). The 8 layers were interpreted as follows (from inner to outer surface): 1/ retinal nerve fiber layer (RNFL), 2/ ganglion cell layer (GCL), 3/ inner plexiform layer (IPL), 4/ inner nuclear layer (INL), 5/ outer plexiform layer (OPL), 6/ outer nuclear layer (ONL) + inner segments (photoreceptors) (IS), 7/ outer segments (photoreceptors) (OS), 8/ retinal pigment epithelium (RPE) (Figure 1).
Figure 1.
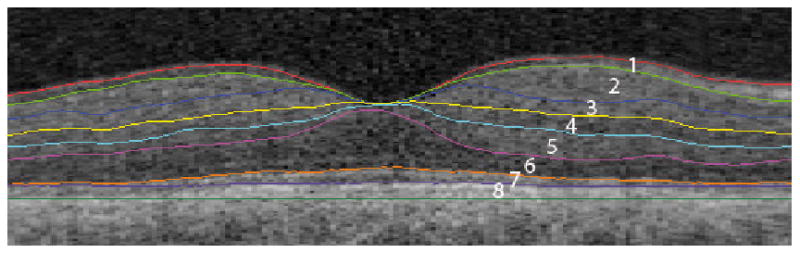
The intraretinal surfaces in a macular B-scan as indicated by the colored lines and the corresponding retinal layers in figures. The retinal surfaces are segmented fully automatically using an inherently 3D approach.
1/ retinal nerve fiber layer, 2/ ganglion cell layer, 3/ inner plexiform layer, 4/ inner nuclear layer, 5/ outer plexiform layer, 6/ outer nuclear layer + inner segments, 7/ outer segments, 8/ retinal pigment epithelium.
The pericentral area of the macula - a donut shaped ring centered on the fovea with an inner diameter of 1 mm - was defined by one of the authors (HvD), who was masked for the DR status and demographic features of the patients and controls. The mean thickness of each layer in the pericentral area was automatically calculated with the computer program ImageJ 1.41 (Abràmoff et al., 2004).
Statistical analysis
For the statistical analyses SPSS 16.0.2 for Windows (SPSS, Chicago, IL) was used. An independent t-test was used to assess differences in mean age and MHR between diabetic patients and controls. A Chi-square test was used to compare distribution of gender between patients and controls. Mean HbA1c, age, duration of diabetes and mean pericentral GCL thickness of diabetic patients with subnormal MHR and diabetic patients with normal MHR were compared using the independent t-test. The presence or absence of DR was compared between patients with subnormal and normal MHR using the Chi-square test. The possible correlation between MHR and pericentral GCL and INL thickness was analyzed using the Spearman rank test. Multivariable logistic regression analyses were performed to identify independent predictors of subnormal MHR. Confidence intervals were computed at the p = 0.05 level.
Results
Thirty-two patients type 1 diabetes with no or minimal DR and 38 controls were included. There was no significant difference in age and gender between patient groups and controls. Patients were in reasonable glycemic control (mean HbA1c = 8.1%; SD = 1.4).
The mean MHR of patients with DM and controls were 93.5 ± 5.3 and 97.1 ± 2.8, respectively. The mean MHR of the patient group was significantly decreased compared with the control group (95% CI of the difference 1.6 – 5.6).
The subjects with subnormal MHR had significantly longer duration of DM and were older than subjects with normal MHR (see Table 2). HbA1c did not differ significantly between the groups. Although the number of subjects with minimal DR differed substantially between the patient group with subnormal MHR and normal MHR this difference was not significant (Chi-square, p = 0.08). The patients with subnormal MHR had significantly lower pericentral GCL and INL thickness (see Table 3). The remaining retinal layers did not differ significantly between patients with normal and subnormal MHR.
Table 2.
Duration of diabetes, age, HbA1c and retinopathy status in patients with type 1 diabetes with subnormal MHR compared to patients with normal MHR.
| Parameters | Subnormal Rarebit (n=14) | Normal Rarebit (n=18) | Mean difference | 95% CI of the difference | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Duration of DM (yrs) | 24.6 ± 9.9 | 16.7 ± 5.3 | 7.9 | 2.3 | 13.4 |
| Age (yrs) | 39.8 ± 9.3 | 30.1 ± 7.9 | 9.6 | 3.4 | 15.8 |
| HbA1c (%) | 7.8 ± 1.3 | 8.4 ± 1.5 | - 0.59 | -1.6 | 0.5 |
| Minimal DR (%) | 64% | 33% | NA | NA | NA |
Values are the mean ± standard deviation. CI, confidence interval; DM, diabetes mellitus; DR, diabetic retinopathy; HbA1c, glycosylated hemoglobin.
Table 3.
Mean layer thickness measurements (microns) of the individual intraretinal layers in the pericentral area of the macula in patients with type 1 diabetes with subnormal Rarebit MHR compared to patients with normal Rarebit MHR.
| Parameters | Subnormal Rarebit (N=14) | Normal Rarebit (N=18) | Mean difference | 95% CI of the difference | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| RNFL | 20.3 ± 1.8 | 21.4 ± 1.9 | 1.1 | -2.4 | 0.3 |
| GCL | 46.3 ± 5.4 | 54.5 ± 4.6 | 8.2 | -11.8 | -4.6 |
| IPL | 37.0 ± 4.4 | 38.1 ± 2.9 | 1.1 | -3.8 | 1.4 |
| INL | 37.3 ± 4.7 | 40.8 ± 3.4 | 3.5 | -6.4 | -0.6 |
| OPL | 29.9 ± 4.8 | 28.3 ± 2.7 | -1.6 | -1.1 | 4.4 |
| ONL + IS | 86.9 ± 13.0 | 93.3 ± 9.0 | 6.4 | -14.4 | 1.5 |
| OS | 27.2 ± 2.0 | 26.2 ± 1.7 | -1.0 | -0.2 | 2.4 |
| RPE | 38.6 ± 1.8 | 39.6 ± 1.4 | 1.0 | -2.1 | 0.2 |
Values are the mean ± standard deviation for all subjects in each group. The bold values indicate a statistically significant difference between patients with normal and subnormal rarebit Test. DR, diabetic retinopathy; CI, confidence interval; RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium.
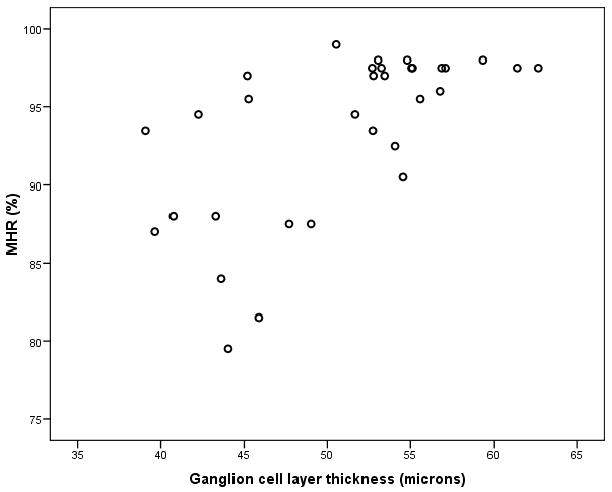
In the population of 32 DM patients, both the pericentral GCL thickness (Rs = 0.65, p < 0.001) and the pericentral INL thickness (Rs = 0.41, p = 0.02) showed a significant correlation with the MHR. However in logistic regression analysis with GCL and INL thickness as variables, the GCL thickness was the strongest independent predictor of subnormal MHR (OR 1.37, 95% CI 1.05 – 1.79) compared to the INL thickness (OR 0.99, 95% CI 0.72 – 1.37). Subsequently the GCL thickness remained the only independent predictor of subnormal MHR (OR 1.5, 95% CI 1.1 – 2.1) in a second multivariable logistic regression analysis with GCL thickness, duration of DM, DR status, HbA1c and age as variables. Duration of DM (OR 1.0, 95% CI 0.8 – 1.1), DR status (OR 0.7, 95% CI 0.04 – 10.9), HbA1c (OR 2.5, 95% CI 0.8 – 7.6) and age (OR 0.9, 95% CI 0.8 – 1.0) had no predictive value for subnormal MHR. A scatter-plot of GCL thickness versus MHR is shown in Figure 2.
Figure 2. Scatter-plot of pericentral GCL thickness versus MHR in type 1 diabetes patients.
Discussion
These results support our hypothesis that GCL thickness as measured with SD-OCT and the macular sensitivity as tested with the Rarebit technique are associated. In fact, decreased GCL thickness in the pericentral area of the macula correlates highly with MHR, and is the only significant predictor of subnormal MHR, independent of other known variables. To our knowledge, this study is the first to describe the possible link between structure and function in type 1 diabetes patients.
The results support the early neurodegenerative effect of diabetes on the retina. The ganglion cells seem to be most vulnerable to diabetes related degeneration (Abu-El-Asrar et al., 2004; Antonetti et al., 2006; Barber et al., 1998; Barber, 2003; Barber et al., 2005; Gardner et al., 2002; Gastinger et al., 2008; Kern and Barber, 2008; Martin et al., 2004). This study shows a decreased MHR in diabetes patients due to loss of receptive fields which is possibly caused by apoptosis of ganglion cells throughout the retina. However, the question remains whether the function decrease is directly and solely caused by the ganglion cell loss or whether the ganglion cell loss is a proxy for a more general neuronal loss. In this study the INL was also significantly decreased in thickness. All retinal neurons - photorecoptors, bipolar cells, horizontal cells, amacrine cells and ganglion cells – are involved in the transmission of visual signals to the brain (Masland, 2001). Degeneration of the neuroretinal system is associated with architectural defects such as loss or disconnection of retinal ganglion cells or upstream connections. The results of the Rarebit test in type 1 diabetes patients indicate that such architectural defects may produce the small visual field defects as observed in this study (Frisen, 2002).
Both foveal dysfunction and GCL thinning in patients with type 1 diabetes have been described previously (Nilsson et al., 2007; Van Dijk et al., 2009; Van Dijk et al., 2010). In glaucoma, previous studies attempted to understand how functional losses may relate to structural damage. Glaucoma is an optic neuropathy characterized by a loss of retinal ganglion cells leading to characteristic changes in the structure of the optic disc and in loss of visual fields, as measured with standard automated perimetry. Recent studies detected macular thinning of the ganglion cell layer in glaucoma patients using SD-OCT. The location of ganglion cell loss colocalized with the visual field loss. The most severe loss of ganglion cells was seen in patients with the most severe loss of visual field loss. Significant thinning of the GCL could be detected in pre-perimetric glaucoma patients. These studies confirm the relationship between structural loss of ganglion cells and functional loss (Hood and Kardon, 2007; Strouthidis et al., 2006; Tan et al., 2008; Tan et al., 2009; Wang et al., 2009). In patients with DM the loss of ganglion cells is much less pronounced, and in general will not lead to frank visual field loss. The Rarebit technique tests visual function differently and is sensitive to local loss of single receptive fields.
The present study has some pitfalls. The grading of the severity of DR was done by a single reader through ophthalmologic examination and evaluation of a single set of stereoscopic central retinal photographs, instead of the gold standard, i.e. 7 field stereoscopic fundus photography assessment by independent trained graders (Early Treatment Diabetic Retinopathy Study Research Group, 1991). However, the patients definitely did not have advanced retinopathy, indicating that the results do apply to the earliest stage of DR.
Type 2 diabetes is often subclinical. It cannot be excluded that some controls actually had diabetes type 2 and elevated blood sugar levels because the HbA1c of the normal subjects was not known. The prevalence of diabetes at this age is however low. The presence of undiagnosed diabetes would most likely lead to underestimation of the difference in retinal layer thickness between patients and controls instead of overestimation.
Furthermore, the number of included patients in this study is low. Due to low power it can not be ruled out that some of the variables in the logistic regression model would have been significantly correlated with the decrease of MHR if the number of patients was higher. In a previous study concerning GCL thinning in diabetic patients, the presence of early vasculopathy was the most important explanatory variable for the observed decrease in GCL thickness. The same study showed that the duration of DM was significantly correlated with GCL thickness (Van Dijk et al., 2010). Studies by Nilsson et al. and Salvetat et al. both previously showed that age correlated negatively with MHR (Nilsson et al., 2006; Salvetat et al., 2007). However the fact that in this study solely the GCL remains an independent predictor in the multivariable logistic regression model despite the small number of included patients shows that the correlation between the GCL thinning and the visual function is strong.
In summary, this study demonstrates subtle loss of macular visual function and corresponding loss of thickness of the GCL in the pericentral area of the macula in type 1 diabetic patients. These results support the concept that early DR includes a neurodegenerative component. Though larger studies are needed, the hypothesis of diabetes causing a retinal neuropathy independent of vascular retinopathy is intriguing, and potentially links retinal neuropathy to other diabetic neuropathies.
Table 1.
Demographics of patients with type 1 diabetes and controls.
| Parameters | DM (n=32) | Controls (n=38) |
|---|---|---|
| Age (yrs) | 34 ± 10 | 35 ± 11 |
| Gender (M:F) | 12 : 20 | 19 : 19 |
| HbA1c (%) | 8.1 ± 1.4 | - |
Values are the mean ± standard deviation for all subjects in each group. DM, diabetes mellitus; -, not performed; HbA1c, glycosylated hemoglobin; MHR, mean hit rate.
Acknowledgments
This study was supported by (1) Netherlands Organization for Health Research and Development, (2) National Institutes of Health Grant R01-EY017066, (3) Research to Prevent Blindness and (4) The Edward en Marianne Blaauwfonds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- Abràmoff M, Magalhâes P, Ram S. Image Processing with ImageJ. Biophotonics. 2004;11:36–42. [Google Scholar]
- Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45:2760–2766. doi: 10.1167/iovs.03-1392. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, Kester M, Kimball SR, Krady JK, LaNoue KF, Norbury CC, Quinn PG, Sandirasegarane L, Simpson IA. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Experiment Ophthalmol. 2008;36:455–463. doi: 10.1111/j.1442-9071.2008.01769.x. [DOI] [PubMed] [Google Scholar]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearse MA, Jr, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006;25:425–448. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biallosterski C, van Velthoven ME, Michels RP, Schlingemann RO, DeVries JH, Verbraak FD. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br J Ophthalmol. 2007;91:1135–1138. doi: 10.1136/bjo.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson-Castain KW, Bearse MA, Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ. Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina. 2009;29:618–626. doi: 10.1097/IAE.0b013e31819a988b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115:533–539. doi: 10.1016/j.ophtha.2007.06.042. [DOI] [PubMed] [Google Scholar]
- Di Leo MA, Caputo S, Falsini B, Porciatti V, Minnella A, Greco AV, Ghirlanda G. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care. 1992;15:620–625. doi: 10.2337/diacare.15.5.620. [DOI] [PubMed] [Google Scholar]
- Dosso AA, Bonvin ER, Morel Y, Golay A, Assal JP, Leuenberger PM. Risk factors associated with contrast sensitivity loss in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 1996;234:300–305. doi: 10.1007/BF00220704. [DOI] [PubMed] [Google Scholar]
- Fischer B. Overlap of receptive field centers and representation of the visual field in the cat's optic tract. Vision Res. 1973;13:2113–2120. doi: 10.1016/0042-6989(73)90188-0. [DOI] [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R. Retinopathy in diabetes. Diabetes Care. 2004;27 1:S84–S87. doi: 10.2337/diacare.27.2007.s84. [DOI] [PubMed] [Google Scholar]
- Frisen L. New, sensitive window on abnormal spatial vision: rarebit probing. Vision Res. 2002;42:1931–1939. doi: 10.1016/s0042-6989(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Frisen L, Quigley HA. Visual acuity in optic atrophy: a quantitative clinicopathological analysis. Graefes Arch Clin Exp Ophthalmol. 1984;222:71–74. doi: 10.1007/BF02150634. [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47 2:S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- Garvin M, Abramoff M, Wu X, Russell S, Burns T, Sonka M. Automated 3-D Intraretinal Layer Segmentation of Macular Spectral-Domain Optical Coherence Tomography Images. IEEE Trans Med Imaging. 2009 doi: 10.1109/TMI.2009.2016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–2642. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006;47:3143–3150. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- Hardy KJ, Lipton J, Scase MO, Foster DH, Scarpello JH. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br J Ophthalmol. 1992;76:461–464. doi: 10.1136/bjo.76.8.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008 doi: 10.1113/jphysiol.2008.156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- Kurtenbach A, Flogel W, Erb C. Anomaloscope matches in patients with diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2002;240:79–84. doi: 10.1007/s00417-001-0385-3. [DOI] [PubMed] [Google Scholar]
- Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells. Invest Ophthalmol Vis Sci. 2002;43:3109–3116. [PubMed] [Google Scholar]
- Lopes de Faria JM, Katsumi O, Cagliero E, Nathan D, Hirose T. Neurovisual abnormalities preceding the retinopathy in patients with long-term type 1 diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2001;239:643–648. doi: 10.1007/s004170100268. [DOI] [PubMed] [Google Scholar]
- Malmer L, Martin L. Microdot test of foveal function. A comparison with visual acuity at high and low contrast. Ophthalmic Physiol Opt. 2005;25:81–86. doi: 10.1111/j.1475-1313.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Ng JS, Bearse MA, Jr, Schneck ME, Barez S, Adams AJ. Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci. 2008;49:1622–1628. doi: 10.1167/iovs.07-1157. [DOI] [PubMed] [Google Scholar]
- Nilsson M, von W G, Wanger P, Martin L. Early detection of macular changes in patients with diabetes using Rarebit Fovea Test and optical coherence tomography. Br J Ophthalmol. 2007;91:1596–1598. doi: 10.1136/bjo.2007.124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Wanger P, Martin L. Perception of very small visual stimuli in the fovea: normative data for the Rarebit Foveal Test. Clin Exp Optom. 2006;89:81–85. doi: 10.1111/j.1444-0938.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye (Lond) 2009;23:884–889. doi: 10.1038/eye.2008.119. [DOI] [PubMed] [Google Scholar]
- Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- Realini T, Lai MQ, Barber L. Impact of diabetes on glaucoma screening using frequency-doubling perimetry. Ophthalmology. 2004;111:2133–2136. doi: 10.1016/j.ophtha.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Rungger-Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. [PubMed] [Google Scholar]
- Salvetat ML, Zeppieri M, Parisi L, Brusini P. Rarebit perimetry in normal subjects: test-retest variability, learning effect, normative range, influence of optical defocus, and cataract extraction. Invest Ophthalmol Vis Sci. 2007;48:5320–5331. doi: 10.1167/iovs.06-1495. [DOI] [PubMed] [Google Scholar]
- Strouthidis NG, Vinciotti V, Tucker AJ, Gardiner SK, Crabb DP, Garway-Heath DF. Structure and function in glaucoma: The relationship between a functional visual field map and an anatomic retinal map. Invest Ophthalmol Vis Sci. 2006;47:5356–5362. doi: 10.1167/iovs.05-1660. [DOI] [PubMed] [Google Scholar]
- Tan O, Chopra V, Lu AT, Schuman JS, Ishikawa H, Wollstein G, Varma R, Huang D. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:2305–2314. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan O, Li G, Lu AT, Varma R, Huang D. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008;115:949–956. doi: 10.1016/j.ophtha.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk HW, Kok PH, Garvin M, Sonka M, DeVries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abramoff MD. Selective Loss of Inner Retinal Layer Thickness in Type 1 Diabetic Patients with Minimal Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, DeVries JH, Michels RP, van Velthoven ME, Schlingemann RO, Abramoff MD. Decreased Retinal Ganglion Cell Layer Thickness in Type 1 Diabetic Patients. Invest Ophthalmol Vis Sci. 2010;51:3660–3665. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hood DC, Cho JS, Ghadiali Q, De Moraes GV, Zhang X, Ritch R, Liebmann JM. Measurement of local retinal ganglion cell layer thickness in patients with glaucoma using frequency-domain optical coherence tomography. Arch Ophthalmol. 2009;127:875–881. doi: 10.1001/archophthalmol.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CP, Ferris FL, III, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]


