Abstract
Background
Comparing a patient’s bleeding symptoms to those of healthy individuals is an important component of the diagnosis of bleeding disorders, but little is known about whether bleeding symptoms in healthy individuals vary by sex, race, ethnicity, age, or aspirin use.
Objectives, Patients/Methods
We developed a comprehensive, ontology-backed, Web-based questionnaire to collect bleeding histories from 500 healthy adults. Mean age was 43 (range 19–86); 63% female, 19% Hispanic, 37% African-American, 43% Caucasian, 8% Asian, 4% multiracial.
Results
18 of the 36 symptoms captured occurred with <5% frequency and 26% of participants reported no bleeding symptoms (range 0–19 symptoms). Differences in sex, race, ethnicity, aspirin use, and age accounted for only 6–13% of the variability in symptoms. Although men reported fewer symptoms than women (median 1 vs. 2, p<0.01), there was no difference when sex-specific questions were excluded (median 1 for both men and women, p=0.50). However, women reported more easy bruising (24% vs. 7%, p<0.01) and venipuncture-related bruising (10% vs. 3%, p=0.02). The number of symptoms did not vary by race or age, but epistaxis was reported more frequently by Caucasians than African-Americans (29% vs. 18%, p=0.02) and epistaxis frequency decreased with age (OR 0.97 per year, p<0.01). Paradoxically, infrequent aspirin users reported more bruising and heavy menses than frequent users (21% vs. 8%, p=0.01 and 56% vs. 38%, p=0.03, respectively).
Conclusions
Our findings provide a contemporaneous and comprehensive description of bleeding symptoms in a diverse group of healthy individuals. Our Web-based system is freely available to other investigators.
Keywords: Bleeding disorders, bleeding history, gender differences
Introduction
Obtaining a detailed bleeding history is an important component of the medical evaluation to determine whether a person: 1) has a bleeding diathesis, 2) is at increased risk of excessive hemorrhage in response to invasive procedures and/or surgery, and/or 3) should undergo laboratory evaluation and/or referral to a specialist [1–3]. Moreover, correlation of clinical hemorrhage with genetic, biochemical, and/or functional data related to platelets or coagulation factors can provide important information for better understanding the basic mechanisms of hemostasis. However, despite a number of early attempts to standardize bleeding history questionnaires (reviewed by Coller and Schneiderman [1]), no generally recognized standards for collecting a bleeding history emerged until recently, when Rodeghiero and colleagues developed bleeding questionnaires and scoring systems for evaluating patients with von Willebrand disease (VWD). By assigning point values to the severity of various bleeding symptoms, they demonstrated that a bleeding score is valuable for confirming the diagnosis of VWD [4–6] and predicting the risk of future hemorrhagic events in type 1 [5] and type 2B [7] VWD. Several groups have used the Vicenza bleeding score as is or with modifications to study bleeding phenotypes [4–10].
The value of such approaches depends on comparing the bleeding histories of affected and healthy individuals. The literature, however, reports marked variability of hemorrhagic symptoms among apparently healthy individuals (Table 1). For instance, reported symptom frequencies range from 2% to 85% for epistaxis, 20% to 44% for menorrhagia, 0% to 11% for postoperative bleeding, 0% to 35% for bleeding after tooth extraction, and 11% to 61% for gingival bleeding [11–17]. At present, it is unclear whether these variations reflect differences in the questionnaires used, the methods of administration, the sex, race, ethnicity, age, frequency of use of medications with antiplatelet effects of the populations sampled, or other factors.
Table 1.
Reported frequencies of selected bleeding symptoms in apparently healthy individuals
| Population, n | Menorrhagia | Excess bleeding at delivery |
Excess Bleeding with Surgery |
Tooth extraction bleeding |
Gum Bleeding | Epistaxis | Easy Bruising | Minor wound bleeding |
Hemarthrosis | Muscle Bleeding | Hemoptysis | Blood in Stools | Hematuria | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wahlberg 1980 [13] | Men, 23 | - | - | - | 4% | 52% | 57% | 22% | 13% | 0% | 4% | 9% | 13% | 0% |
| Wahlberg 1980 [13] | Women, 20 | - | - | - | 10% | 50% | 85% | 55% | 10% | 0% | 15% | 5% | 5% | 10% |
| Wahlberg 1984 [30] | 50 | 25% | - | - | 3% | 12% | 8% | 19% | 2% | 2% | 0% | 8% | 10% | 4% |
| Mauser Bunschoten 1988 [31] | Women, 60 | 23% | 6% | 6% | 13% | - | 5% | 17% | 2% | - | - | - | - | - |
| Nosek-Cekowska 1991 [15] | Children, 228 | - | - | 0% | <1% | 13% | 39% | 24% | - | <1% | - | - | 2% | 1% |
| Sramek 1995 [14] | 274 | 44% | 23% | 6% | 11% | 36% | 8% | 12% | 2% | 6% | 3% | - | 11% | 8% |
| Dilley 2001 [12] | Women, 227 | - | 19% | 6% | 8% | 11% | 2% | 23% | - | - | - | - | ||
| Drews 2002 [32] | Women, 88 | - | 17% | 6% | 6% | 24% | 23% | 19% | 9% | 0% | 0% | - | 1% | - |
| McKay 2004 [17] | Women, 104 | 53% | - | 9% | 35% | 12% | 56% | 34% | 3% | 0% | - | - | - | 12% |
| Plug 2006 [11] | Women, 245 | 20% | - | 11% | 12% | 61% | 44% | 17% | 9% | 5% | - | - | - | - |
| Friberg 2006 [33] | Women, 1019 | 37% | - | 3% | 3% | - | 20% | 21% | - | 3%* | 13% | 9% | 5% | |
| Quiroga 2007 [16] | 299 | 30% | 0% | 0% | 0% | 13% | 25% | 19% | 1% | 0% | - | 0% | 1% | 0% |
| Average frequency** | 35% | 13% | 4% | 6% | 28% | 23% | 20% | 5% | 2% | 3% | 10% | 7% | 5% | |
Hemarthrosis and muscle bleeding were assessed by a single question
Computed as the weighted arithmetic mean of the symptom frequency across all studies in which the symptom was reported
Although only women face the hemostatic challenges of menstruation, pregnancy, and childbirth, there is only limited information on whether other bleeding symptoms differ between men and women. We found only a single study, which was limited to the evaluation of differences in epistaxis frequency between Caucasians and Asians [18], that assessed whether individuals of different racial backgrounds experience bleeding symptoms with different frequencies. Since Hispanic ethnicity is viewed as distinct from race [19], we also searched for, but did not find, reported differences in bleeding symptoms between individuals of Hispanic and non-Hispanic ethnicity. While it is recognized that some bleeding symptoms such as epistaxis occur more frequently in childhood than adulthood [1], we could not identify information on whether bleeding symptoms differ among adults of different ages. Moreover, since older individuals have had more time to sustain hemorrhagic symptoms, it is possible that age is an important factor in interpreting the bleeding history.Aspirin has significant anti-platelet properties, but the association between aspirin use and bleeding symptoms in a contemporaneous healthy population has not been systematically assessed using a standardized instrument.
To address these questions, we designed and deployed a comprehensive, Web-based bleeding history questionnaire in a study that aimed to: 1) establish the frequencies of bleeding symptoms in a diverse population of healthy adults and 2) assess whether any symptoms varied by sex, race, ethnicity, age, or aspirin use.
Patients, material, and methods
The Bleeding History Phenotyping System
The design of the bleeding history phenotyping system we used has been previously described [20]. The centerpiece is a comprehensive bleeding history questionnaire that incorporated elements from a review of the literature, one of the authors’ experience [1], and input from experts in hemostasis, questionnaire development, epidemiology, and biomedical informatics. It contains 278 questions covering 25 categories of bleeding and related covariates, with the latter including dermatologic lesions, connective tissue disorders, medications, and family history. To reduce ambiguity in terminology, 168 terms in the questionnaire and ontology were cross-referenced to the National Library of Medicine’s Unified Medical Language System (UMLS) by assigning them the corresponding code number [21].
The questionnaire was used to derive a bleeding history ontology, which is an explicit representation of the relationships among bleeding signs, symptoms, disorders, and treatments. The ontology formalizes the concepts contained in the questionnaire in an electronic format that facilitates data analysis, organization, and representation. The ontology is publicly available in the Bioportal ontology registry (http://bioportal.bioontology.org/ontologies/40546) to facilitate its critique by experts in the field and its future updating, as, for example, when new therapies for bleeding disorders are introduced.
The questionnaire is administered by a medically trained individual using a Web-based program. Studies are assigned a site identification code as well as a code to identify the person administering the questionnaire. To ensure confidentiality, each respondent is identified by a randomly generated unique personal identification number. Skip patterns were introduced into the program to speed questionnaire completion. For example, respondents who state that they have never had epistaxis are not asked questions about epistaxis frequency or duration. The program is time-stamped so that the time required to complete the study is captured. Users can log off and log on as often as they wish, allowing the questionnaire to be completed in more than one session. To help the person providing the bleeding history to better understand the questions and give accurate responses, some of the questions include visual aids, for example, photographs of petechiae. Data are stored in a secure, Web-accessible MySQL database. Investigators from other institutions can review all of the components of the system, including the database, at http://ds9.rockefeller.edu/RUBHPSR/.
Questionnaire administration
The study protocol was approved by the IRBs of both the Rockefeller University and the Clinical Directors Network (CDN, www.CDNetwork.org), a nonprofit primary care practice-based research network (PBRN) and clinician training organization that conducts clinical and translational research studies in Community Health Centers (CHCs). Bleeding symptoms were obtained from 500 healthy individuals, of whom 365 were seen at Rockefeller University and 135 were seen at two separate CDN-member CHCs (75 at Metropolitan Family Health Network, Jersey City, NJ and 60 at Newark Community Health Center, Newark, NJ). Potential participants were eligible if they met the following criteria: age equal to or greater than 18 years; self-assessment as being generally healthy; and self-assessment as being able to accurately read and answer questions in English. Exclusion criteria included a diagnosis of any bleeding disorder; hepatic or renal disease; malignancy requiring treatment within one year prior to enrollment; use of any medications with known anticoagulant or antiplatelet properties other than aspirin or non-steroidal anti-inflammatory drugs within 30 days of enrollment; or any other medical or psychological condition that would impair the participant's ability to accurately respond to questions about bleeding symptoms.
Participants recruited at Rockefeller were identified through online and print advertisements seeking healthy volunteers. Participants recruited through CHCs were patients identified in the waiting room during routine primary care visits who were willing to complete the interview. At Rockefeller, 372 individuals responded to advertisements and passed telephone screening, of which 365 (98%) were enrolled. At the CHCs, 235 individuals were approached and 135 (57%) agreed to participate. In both settings, after written informed consent was obtained, a physician or nurse trained in the use of the questionnaire conducted the interview and entered the participant’s responses directly into the database using a personal computer. Personnel conducting the interviews completed a credentialing process that included: 1) observing one of us (ACM) conduct two interviews, 2) obtaining two histories under ACM’s direct supervision, and 3) completing a checklist of skills. ACM conducted 129 interviews, trained research nurses conducted 236 interviews, and CDN PBRN staff (CK) conducted 135 interviews at CDN CHCs. All questionnaires were administered in English. Participants received a $20 honorarium for their time. The mean time to complete the questionnaire was 33 minutes (range 12–156 minutes).
Statistical analysis
All analyses were performed using PASW 18.0 (PASW Inc., Chicago, IL). The questionnaire includes both top-level screening questions and detailed follow-up questions (e.g., pertaining to the frequency or duration of bleeding symptoms). For this study, 36 top-level questions were selected for detailed analysis based on their: 1) similarity to questions reported in the existing literature, 2) reflecting the presence or absence of a bleeding symptom rather than attributes such as the frequency, duration, or severity of a symptom, and 3) eliciting dichotomous responses that were suitable for binary logistic regression analysis.
Both intra-rater and inter-rater reliabilities were evaluated. To assess the consistency of responses to these questions by the same subject over time (intra-rater reliability), 30 individuals were recalled six to nine months after the questionnaire was initially administered and the questionnaire was administered again. Of the 36 questions analyzed, 36% had complete concordance between the first and second administration, 36% had 90–99% concordance, and 28% had concordances that ranged from 56–89%. To assess whether the person administering the questionnaire had any influence on the response, one of us (ACM) reviewed 31 randomly selected audio recordings conducted by other interviewers and completed a second questionnaire for each participant based on the recording (inter-rater reliability). The recorded responses were completely concordant for 30 of the 36 questions, four questions had >90% concordance, and two questions had 82% concordance. An analysis of responses by site of administration revealed minor differences in a few symptoms, but the analysis was confounded by the different demographics at the sites and the relatively small number of individuals reporting the symptoms. Thus, no adjustments were made for the site of administration.
To test whether the prevalence of the 36 bleeding symptoms differed according to one or more demographic characteristics, a binary multiple logistic regression model was constructed for each symptom. For each model, the question response was the dependent variable and sex, race, ethnicity, age, and the frequency of aspirin use were the independent variables. For each question, individuals who answered “don’t remember” were excluded from analysis. For the aspirin analysis, “frequent” aspirin users were defined as those who used aspirin once a week or more often (10%), “infrequent” aspirin users were defined as those who used aspirin less than once a week (49%), and “never” users were defined as those who denied using aspirin (39%); 1% of respondents did not remember their frequency of aspirin use. Prior to regression analysis, multicollinearity among the independent variables was tested using the Spearman rank correlation coefficient for ratio and ordinal variables and Cramer’s V for nominal variables. Based on this analysis, Hispanic ethnicity was found to be correlated with African American and Caucasian race. Therefore, ethnicity was excluded when race was evaluated as an independent variable, and race was excluded when ethnicity was evaluated as an independent variable. Model fit was tested using the χ2 goodness-of-fit and Hosmer-Lemeshow tests [22]. For models with adequate fit, adjusted odds ratios (ORs) were calculated for independent variables. For each symptom, the proportion of variance explained by the regression model was estimated using the Nagelkerke R2 [22]. Differences with p values less than 0.05 were defined as statistically significant. No adjustments for multiple comparisons were made in these exploratory analyses.
Results
Demographic characteristics are displayed in Table 2. The mean age was 43 years (SD±13.8, range 19–86 years) and 63% of subjects were female. The racial distribution was: 37% AfricanAmerican, 43% Caucasian, 8% Asian, <1% Pacific Islander, and 4% more than one race; 8% preferred not to report race. The ethnic distribution was 19% Hispanic and 80% non-Hispanic; 1% preferred not to report ethnicity.
Table 2.
Demographic characteristics by site of enrollment
| Characteristic | Total (n=500) | Rockefeller University (n=365) | Clinical Directors Network Community Health Centers (n=135) | p* | |
|---|---|---|---|---|---|
| Mean age in years (range) | 43 (19–86) | 42 (19–86) | 47 (19–78) | <0.01** | |
| Sex | Male | 183 (37%) | 154 (42%) | 29 (22%) | <0.01† |
| Female | 317 (63%) | 211 (58%) | 106 (79%) | ||
| Race | Caucasian | 213 (43%) | 188 (52%) | 25 (19%) | <0.01‡ |
| African-American | 187 (37%) | 109 (30%) | 78 (58%) | ||
| Asian | 40 (8%) | 33 (9%) | 7 (5%) | ||
| Native Hawaiian/Pacific Islander | 1 (<1%) | 1 (<1%) | 0 (0%) | ||
| More than one race | 19 (4%) | 18 (5%) | 1 (1%) | ||
| Prefer not to answer | 40 (8%) | 16 (4%) | 24 (18%) | ||
| Ethnicity | Hispanic | 96 (19%) | 50 (14%) | 46 (34%) | <0.01‡ |
| Not Hispanic | 398 (80%) | 310 (85%) | 88 (65%) | ||
| Prefer not to answer | 6 (1%) | 5 (1%) | 1 (1%) | ||
| Frequency of aspirin use | Once a week or more often | 49 (10%) | 29 (8%) | 20 (15%) | <0.01‡ |
| Less than once a week | 247 (49%) | 219 (60%) | 28 (21%) | ||
| Never used aspirin | 197 (39%) | 115 (32%) | 82 (61%) | ||
| Don’t remember | 7 (1%) | 2 (0.5%) | 5 (4%) | ||
For Rockefeller University versus Community Health Centers
Fisher’s exact test
T-test
Chi-square
The frequencies of the 36 bleeding symptoms analyzed are displayed in Table 3. Symptom frequencies ranged from 0% (teething bleeding, hemorrhagic stroke, circumcision bleeding, and umbilical cord bleeding) to 47% (heavy menses). Of note, 18 of the 36 symptoms were reported by fewer than 5% of subjects who responded to the question.
Table 3.
Frequencies of selected bleeding symptoms
| Symptom* | Number of respondents reporting symptom/Total number of respondents | Percentage of individuals who reported symptom among those who responded to the question** |
|---|---|---|
| Epistaxis | 124/500 | 25% |
| Gum bleeding | 20/500 | 4% |
| Lip bleeding | 3/500 | <1% |
| Tongue bleeding | 5/500 | 1% |
| Easy bruising | 88/500 | 18% |
| Subcutaneous hematoma | 6/88 | 7% |
| Teething bleeding | 0/500 | 0% |
| Tooth loss bleeding | 5/500 | 1% |
| Prolonged bleeding after tooth extraction | 67/365 | 18% |
| Menorrhagia | 148/317 | 47% |
| Treatment for menorrhagia | 34/148 | 23% |
| Hematuria | 49/500 | 10% |
| Hemoptysis | 54/500 | 11% |
| Hematemesis | 17/500 | 3% |
| Razor nick bleeding | 15/377 | 4% |
| Other minor cut bleeding | 14/500 | 3% |
| Hemarthrosis | 3/500 | <1% |
| Muscle hematoma | 3/500 | <1% |
| Hematochezia | 96/500 | 19% |
| Dark stools | 73/500 | 15% |
| Hemorrhagic stroke | 0/500 | 0% |
| Subdural hematoma | 1/353 | <1% |
| Ophthalmic bleeding | 36/500 | 7% |
| Bleeding after venipuncture | 13/498 | 3% |
| Bruising after venipuncture | 36/498 | 7% |
| Circumcision bleeding | 0/127 | 0% |
| Umbilical cord bleeding | 0/500 | 0% |
| Petechiae | 27/500 | 5% |
| Bleeding during surgery | 8/268 | 3% |
| Prolonged bleeding after surgery | 2/268 | <1% |
| New onset bleeding after surgery | 6/268 | 2% |
| Bleeding during pregnancy | 19/188 | 10% |
| Bleeding at delivery | 14/188 | 7% |
| Postpartum bleeding | 11/188 | 6% |
| Trauma bleeding (self-assessed) | 43/279 | 15% |
| Trauma bleeding (per treating healthcare provider) | 19/279 | 7% |
Exact question wording is available at http://ds9.rockefeller.edu/RUBHPSR/Documentation.html
Some questions were contingent upon previous answers; therefore, not all questions were asked of all respondents.
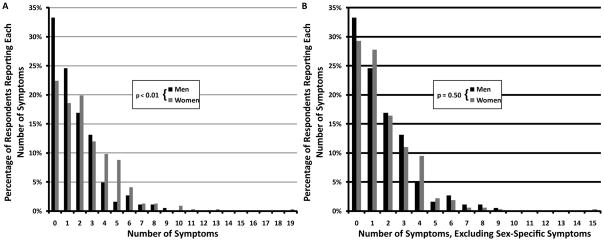
Associations with Sex
The total number of symptoms was not normally distributed (Figure 1). When all symptoms were considered, women reported more bleeding symptoms than men (p<0.01 by Mann-Whitney U, Figure 1A). Men reported a median of one symptom (interquartile range [IQR] 0–3) and women reported a median of two symptoms (IQR 1–4). However, after removing sex-specific bleeding symptoms (heavy menses, treatment for heavy menses, bleeding during pregnancy, bleeding at delivery, postpartum bleeding, and, for men, circumcision bleeding), men and women both reported a median of one symptom (IQR 0–3 for both men and women, p=0.50 by Mann-Whitney U, Figure 1B). When analyzing individual symptoms by logistic regression, however, differences by sex were noted (Table 4). Thus, easy bruising was more common among women than men (24% vs. 7%, OR 4.78, 95% CI 2.50–9.16, p<0.01), as was venipuncture bruising (10% vs. 3%, OR 3.04, 95% CI 1.22–7.59, p=0.02).
Figure 1. Number of bleeding symptoms in men and women.
(A) Men reported fewer bleeding symptoms than women (p<0.01 by Mann-Whitney U). (B) When heavy menses, treatment for heavy menses, bleeding during pregnancy, bleeding at delivery, postpartum bleeding, and circumcision bleeding were excluded, men and women reported similar frequencies of bleeding symptoms (p=0.50 by Mann-Whitney U).
Table 4.
Results of logistic regression analyses of questions with statistically significant differences in responses by one or more demographic characteristics
| Symptom | Demographic variable, if significant | Adjusted Odds Ratio | 95% Confidence Interval for Odds Ratio | p | Nagelkerke R2 |
|---|---|---|---|---|---|
| Epistaxis | Age* | 0.97 | 0.96 – 0.99 | <0.01 | 0.08 |
| African-American race† | 0.55 | 0.34 – 0.90 | 0.02 | ||
| Easy bruising | Female sex‡ | 4.78 | 2.50 – 9.16 | <0.01 | 0.13 |
| Infrequent aspirin use§ | 4.18 | 1.39–12.58 | 0.01 | ||
| Venipuncture bruising | Female Sex‡ | 3.04 | 1.22–7.59 | 0.02 | 0.07 |
| Heavy Menses | Infrequent aspirin use§ | 3.03 | 1.25–7.32 | 0.01 | 0.08 |
| Dark stools | Age* | 1.02 | 1.00–1.04 | 0.02 | 0.06 |
| Ophthalmic bleeding | African-American race† | 0.29 | 0.11–0.75 | 0.01 | 0.08 |
Odds ratio per additional year of age
Odds ratio versus Caucasian race
Odds ratio versus male sex
Defined as aspirin use less than once per week; odds ratio versus aspirin use more than once per week
Associations with Race
There was a trend toward more bleeding symptoms in Caucasians (median 2, IQR 1–3) versus either African Americans (median 1, IQR 0–3) or Asians (median 1, IQR 0–3, p=0.07 by Kruskal-Wallis test). Epistaxis was less common among African-Americans than Caucasians (18% vs. 29%, OR 0.55, 95% CI 0.34–0.90, p=0.02), as was bleeding in or around the eye, which included conjunctival hemorrhage, retinal hemorrhage, and bleeding behind the eye (3% vs. 10%, OR 0.29, 95% CI 0.11–0.75, p=0.01).
Associations with Ethnicity
Hispanics reported a similar median number of symptoms (median 2, IQR 0–3) as non-Hispanics (median 2, IQR 0–3, p=0.54). When ethnicity was substituted for race as an independent variable, no symptoms were associated with Hispanic ethnicity.
Associations with Age
Age was not associated with the total number of symptoms (Spearman r=0.05, p=0.24), but the odds of reporting epistaxis decreased with age (OR 0.97 per year, 95% CI 0.96–0.99, p<0.01). Increasing age was also associated with a higher reported frequency of dark stools (OR 1.02 per year, 95% CI 1.00–1.04, p=0.02); however, among the 72 individuals who reported a history of dark stools, 68% had been told that the change in color was due to iron therapy and only 6% had been told the change in color was due to bleeding.
Associations with Aspirin Use
There was a trend toward an increased median number of symptoms in infrequent aspirin users (median 2, IQR 1–3) versus those who never used aspirin (median 1, IQR 0–3) or who used it frequently (median 1, IQR 1–2, p=0.08 by Kruskal-Wallis test). Easy bruising was more common among infrequent than frequent aspirin users (21% vs. 8%, OR 4.18, 95% CI 1.39–12.58, p=0.01), with those who reported not taking aspirin having an intermediate frequency (15%). A history of heavy menses was also more common among infrequent aspirin users than frequent aspirin users (56% vs. 38%, OR 3.03, 95% CI 1.25–7.32, p = 0.01), with those who reported not taking aspirin again having an intermediate value (40%).
Contribution of Sex, Race, Age, and Aspirin Use to Variability in Symptoms
For 30 symptoms, no significant associations with sex, race, age, or aspirin use were identified. The contribution of sex, race, age, and aspirin use to the remaining six symptoms, as defined by Nagelkerke R2, are summarized in Table 4. For instance, the combined R2 for age and race with regards to epistaxis was 0.08, that is, the two variables accounted for 8% of the observed variability in epistaxis. Sex, race, age, and aspirin use accounted for only 6–13% of the variability in reported symptoms.
Discussion
We used a novel questionnaire and Web-based system to collect comprehensive bleeding histories from 500 healthy individuals. We found that 26% of subjects reported no symptoms and that 18 of the 36 symptoms were reported by fewer than 5% of the subjects. The frequencies of epistaxis, easy bruising, bruising after venipuncture, heavy menses, dark stools, and ophthalmic bleeding exhibited variations according to sex, racial background, age, and frequency of aspirin use, but these characteristics accounted for only an estimated 6–13% of the total variability in the reported frequencies of these six symptoms. Our findings suggest that the definition of “normal” bleeding that is not sex-specific varies relatively little by sex, race, age, and aspirin use; thus, it remains to be established whether applying adjustments for these characteristics can improve the diagnostic value of the bleeding history.
The frequencies of most bleeding symptoms in our population were within the ranges previously reported by other investigators (Table 1). For instance, 25% of our respondents reported epistaxis, as compared with a weighted average of 23% in previous studies, and 18% of our respondents reported easy bruising, as compared with a weighted average of 20% in previous studies. Of the 13 symptoms reported in Table 1, 9 symptoms were within 5 percentage points of the reported weighted average. Greater differences were observed for menorrhagia (47% in our study vs. 35% in the literature), tooth extraction bleeding (18% vs. 6%), gum bleeding (4% vs. 28%), and hematochezia (19% vs. 7%). It is likely that some of the variation is due to differences in question wording. For instance, the lower frequency of gum bleeding we found probably reflects the requirement in our questionnaire, but not in those of other authors, that the gum bleeding last at least 5 minutes. Differences in the populations studied may also contribute to the variation.
Although women reported a higher median number of symptoms than men when viewed from the standpoint of all bleeding symptoms, this difference did not persist after excluding sex-specific questions (e.g., menstrual bleeding and postpartum bleeding). Thus, bleeding scores that are based on the number of bleeding symptoms need to be adjusted for sex, as is done, for example, with the Vicenza bleeding score for VWD [4]. Easy bruising and bruising after venipuncture were, however, more common in women than men, a finding that is consistent with the study by Wahlberg et al., in which women reported a higher frequency of spontaneous bruising than men[13]. It is also consistent with studies of primary aspirin prophylaxis, where the reported frequencies of excessive bruising among the control groups not taking aspirin were 43% among women [23] and 9–13% among men [24;25].
The literature on differences in bleeding symptoms as a function of race and ethnicity is sparse. In the only study we identified that addressed these issues, the authors found a lower frequency of epistaxis among Asians than Caucasians [18], a finding that we did not observe. We found that epistaxis and ophthalmic bleeding were reported with lower frequency by African Americans than by Caucasians. It is possible that this difference can be explained by the higher von Willebrand factor levels reported in African Americans than Caucasians [26–28], since epistaxis is a cardinal symptom of von Willebrand disease [1;4]. However, the African American women in our population did not report a lower frequency of heavy menses, another common manifestation of von Willebrand disease [4;29], than Caucasian women.
Since our bleeding history questionnaire is based on the cumulative prevalence of a list of symptoms, we considered the possibility that older individuals would report more symptoms because they had more time in which to experience symptoms. Though a correlation between increasing age and higher bleeding scores has been reported in individuals with bleeding disorders, previous reports have not found this correlation in healthy individuals [4;5]. We also found few associations between older age and more bleeding symptoms in our healthy adult population. Paradoxically, we found a modest decrease in the reported frequency of epistaxis with age, suggesting either that epistaxis is becoming more common or that older individuals are less likely to remember episodes of epistaxis from their youth. We also found an increase in the reported frequency of dark stools with age. However, among the individuals who reported dark stools, the symptom could be classified with reasonable certainty as melena in only 6% of cases. This highlights the limited utility of just inquiring about dark stools and thus the importance of following up screening questions with questions that provide more details.
Although aspirin use has been reported to increase the frequency of a number of bleeding symptoms, including easy bruising (which ranged from 9–43% in controls and from 14–53% in studies on aspirin in primary prevention studies [23–25]), we did not exclude individuals taking aspirin because we wanted a sample that represented as close as possible the demographics of our community. Unexpectedly, we found that easy bruising and heavy menses were more common among infrequent aspirin users than frequent aspirin users, with non-users having intermediate values. We have no simple explanation for this finding, but frequent aspirin use was reported by only 10% of responders and it is possible that individuals with heavy menses or easy bruising who were frequent aspirin users had been counseled to reduce their aspirin intake. Unfortunately, the aspirin primary prevention studies noted above did not report the frequency of heavy menses [23].
There is an apparent contradiction between the finding that men and women reported a similar total number of non-sex-specific symptoms and the finding that women reported higher frequencies of easy bruising and venipuncture bruising. The reason that the increased frequency of bruising in women did not affect the median value for all symptoms was that it was counterbalanced by statistically insignificant increases in symptom frequencies among men in several categories (gum bleeding, lip bleeding, tooth extraction bleeding, hemoptysis, minor cut bleeding, hematochezia, melena, eye bleeding, surgical bleeding, and trauma bleeding). Similarly, although we observed differences in several symptoms by race or frequency of aspirin use, the total number of symptoms did not vary by race, age, or frequency of aspirin use despite statistically insignificant variations within individual categories.
The Vicenza group has demonstrated the diagnostic and prognostic utility of their questionnaire for VWD [4–6]. Based on their experience and expertise, they excluded from consideration all bleeding symptoms that they defined as “trivial,” and devised their bleeding score based on the severity of individual symptoms. Our questionnaire, in contrast, includes questions on both major and minor bleeding symptoms. This explains why we found a lower percentage of healthy individuals who did not report any bleeding symptoms (26%) than did the Vicenza group (77%) [4]. We are currently employing our questionnaire to obtain the symptoms of patients with mild bleeding disorders and will compare these data with the data we have obtained in the healthy adult cohort described in this study using several different statistical methods.
Because the reliability of medical histories depends on the ability of subjects to recall symptoms over their entire lifetimes and to report the data consistently, these studies will also evaluate measures of questionnaire validation such as test-retest and inter-rater reliability. In the current study we chose to use a comprehensive questionnaire, despite its length, because we did not want to prejudge which questions would have the best diagnostic and prognostic value. Going forward, we will compare the responses of the healthy adults in this study with those of patients with bleeding disorders so as to identify those questions that are most reliable and/or of the greatest diagnostic value. We will then create a shorter questionnaire that focuses on obtaining the most valuable data. The potential advantages and drawbacks of our approach remain to be determined.
The long-term goal of our research is to standardize the collection of bleeding histories by developing Web-based instruments that can be used by investigators across different sites and studies. This has the potential to aggregate large amounts of de-identified phenotypic data so as to increase the power to detect scientifically and medically important correlations with genetic and environmental data. As a first step in this process, an electronic version of our questionnaire is available to investigators at https://ds9.rockefeller.edu/RUBHPSR/.
Acknowledgments
This study was supported by grants KL2RR024142 and UL1RR024143 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research, as well as funds from Stony Brook University. Contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
We would like to thank Dr. Daniel Rubin and Dr. Mark Musen of Stanford University and Ms. Shamim Mollah of Rockefeller University for their valuable conceptual and practical contributions to the design of the Bleeding History Phenotyping System; Ms. Donna Brassil and Mrs. Diana Bernal-Messinger for their assistance in administering the BHQ; and the staff and patients of the Metropolitan Family Health Center Network (Patrick Beaty, MD, Chief Medical Officer) and the Newark Community Health Centers, Inc. (Nancy Tham, MD, MBA, Chief Medical Officer) for their participation.
Footnotes
Disclosure of Conflict of Interests
In accord with federal law and the policies of the Research Foundation of the State University of New York, Dr. Coller has a royalty interest in abciximab (Centocor), and in accord with federal law and the policies of the Mount Sinai School of Medicine, Dr. Coller has a royalty interest in the VerifyNow assay system (Accumetrics). In addition he is a consultant to Accumetrics and is an inventor of an αIIbβ3 antagonist compound identified by high throughput screening. The remaining authors have no conflicts of interest to disclose.
Reference List
- 1.Coller BS, Schneiderman PI. Clinical evaluation of hemorrhagic disorders: The bleeding history and differential diagnosis of purpura. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, et al., editors. Hematology: Basic Principles and Practice. 5. New York: Churchill Livingstone; 2004. pp. 1851–76. [Google Scholar]
- 2.Chee YL, Crawford JC, Watson HG, Greaves M. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. British Committee for Standards in Haematology. Br J Haematol. 2008;140:496–504. doi: 10.1111/j.1365-2141.2007.06968.x. [DOI] [PubMed] [Google Scholar]
- 3.Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Ann Intern Med. 2003;138:W15–W24. doi: 10.7326/0003-4819-138-3-200302040-00011-w1. [DOI] [PubMed] [Google Scholar]
- 4.Rodeghiero F, Castaman G, Tosetto A, Batlle J, Baudo F, Cappelletti A, Casana P, De Bosch N, Eikenboom JC, Federici AB, Lethagen S, Linari S, Srivastava A. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005;3:2619–26. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 5.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, Meyer D, Fressinaud E, Mazurier C, Goudemand J, Eikenboom J, Schneppenheim R, Budde U, Ingerslev J, Vorlova Z, Habart D, Holmberg L, Lethagen S, Pasi J, Hill F, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 6.Tosetto A, Castaman G, Rodeghiero F. Evidence-based diagnosis of type 1 von Willebrand disease: a Bayes theorem approach. Blood. 2008;111:3998–4003. doi: 10.1182/blood-2007-08-105940. [DOI] [PubMed] [Google Scholar]
- 7.Federici AB, Mannucci PM, Castaman G, Baronciani L, Bucciarelli P, Canciani MT, Pecci A, Lenting PJ, De Groot PG. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood. 2009;113:526–34. doi: 10.1182/blood-2008-04-152280. [DOI] [PubMed] [Google Scholar]
- 8.Bowman M, Mundell G, Grabell J, Hopman WM, Rapson D, Lillicrap D, James P. Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6:2062–6. doi: 10.1111/j.1538-7836.2008.03182.x. [DOI] [PubMed] [Google Scholar]
- 9.Bowman M, Riddel J, Rand ML, Tosetto A, Silva M, James PD. Evaluation of the diagnostic utility for von Willebrand disease of a pediatric bleeding questionnaire. J Thromb Haemost. 2009;7:1418–21. doi: 10.1111/j.1538-7836.2009.03499.x. [DOI] [PubMed] [Google Scholar]
- 10.Biss TT, Blanchette VS, Clark DS, Bowman M, Wakefield CD, Silva M, Lillicrap D, James PD, Rand ML. Quantitation of bleeding symptoms in children with von Willebrand disease: use of a standardized pediatric bleeding questionnaire. J Thromb Haemost. 2010;8:950–6. doi: 10.1111/j.1538-7836.2010.03796.x. [DOI] [PubMed] [Google Scholar]
- 11.Plug I, Mauser-Bunschoten EP, Brocker-Vriends AH, van Amstel HK, van der Bom JG, van Diemen-Homan JE, Willemse J, Rosendaal FR. Bleeding in carriers of hemophilia. Blood. 2006;108:52–6. doi: 10.1182/blood-2005-09-3879. [DOI] [PubMed] [Google Scholar]
- 12.Dilley A, Drews C, Miller C, Lally C, Austin H, Ramaswamy D, Lurye D, Evatt B. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97:630–6. doi: 10.1016/s0029-7844(00)01224-2. [DOI] [PubMed] [Google Scholar]
- 13.Wahlberg T, Blomback M, Hall P, Axelsson G. Application of indicators, predictors and diagnostic indices in coagulation disorders. I. Evaluation of a self-administered questionnaire with binary questions. Methods Inf Med. 1980;19:194–200. [PubMed] [Google Scholar]
- 14.Sramek A, Eikenboom JC, Briet E, Vandenbroucke JP, Rosendaal FR. Usefulness of patient interview in bleeding disorders. Arch Intern Med. 1995;155:1409–15. [PubMed] [Google Scholar]
- 15.Nosek-Cenkowska B, Cheang MS, Pizzi NJ, Israels ED, Gerrard JM. Bleeding/bruising symptomatology in children with and without bleeding disorders. Thromb Haemost. 1991;65:237–41. [PubMed] [Google Scholar]
- 16.Quiroga T, Goycoolea M, Panes O, Aranda E, Martinez C, Belmont S, Munoz B, Zuniga P, Pereira J, Mezzano D. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92:357–65. doi: 10.3324/haematol.10816. [DOI] [PubMed] [Google Scholar]
- 17.McKay H, Derome F, Haq MA, Whittaker S, Arnold E, Adam F, Heddle NM, Rivard GE, Hayward CP. Bleeding risks associated with inheritance of the Quebec platelet disorder. Blood. 2004;104:159–65. doi: 10.1182/blood-2003-11-4077. [DOI] [PubMed] [Google Scholar]
- 18.Daniel M, Jaberoo MC, Stead RE, Reddy VM, Moir AA. Is admission for epistaxis more common in Caucasian than in Asian people? A preliminary study. Clin Otolaryngol. 2006;31 :386–9. doi: 10.1111/j.1749-4486.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. NIH policy on reporting race and ethnicity data: subjects in clinical research. 2010 September; http://grants.nih.gov/grants/guide/notice-files/not-od-01-053.html.
- 20.Mauer AC, Barbour EM, Khazanov NA, Levenkova N, Mollah SA, Coller BS. Creating an ontology-based human phenotyping system: The Rockefeller University bleeding history experience. Clin Transl Sci. 2009;2:382–5. doi: 10.1111/j.1752-8062.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–D270. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabachnick BG, Fidell LS. Logistic Regression. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. 5. New York: Allyn & Bacon; 2007. pp. 437–505. [Google Scholar]
- 23.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 24.Steering Committee of the Physicians' Health Study Research Group Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 25.The Medical Research Council's General Practice Research Framework Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–41. [PubMed] [Google Scholar]
- 26.Miller CH, Haff E, Platt SJ, Rawlins P, Drews CD, Dilley AB, Evatt B. Measurement of von Willebrand factor activity: relative effects of ABO blood type and race. J Thromb Haemost. 2003;1:2191–7. doi: 10.1046/j.1538-7836.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 27.Green D, Ruth KJ, Folsom AR, Liu K. Hemostatic factors in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb. 1994;14:686–93. doi: 10.1161/01.atv.14.5.686. [DOI] [PubMed] [Google Scholar]
- 28.Conlan MG, Folsom AR, Finch A, Davis CE, Sorlie P, Marcucci G, Wu KK. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Thromb Haemost. 1993;70:380–5. [PubMed] [Google Scholar]
- 29.Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485–9. doi: 10.1016/S0140-6736(97)08248-2. [DOI] [PubMed] [Google Scholar]
- 30.Wahlberg TB. A method for the evaluation of clinical information, exemplified for bleeding symptoms in non-severe von Willebrand's disease type I. Methods Inf Med. 1984;23:143–6. [PubMed] [Google Scholar]
- 31.Mauser-Bunschoten EP, van Houwelingen JC, Sjamsoedin Visser EJ, van Dijken PJ, Kok AJ, Sixma JJ. Bleeding symptoms in carriers of hemophilia A and B. Thromb Haemost. 1988;59 :349–52. [PubMed] [Google Scholar]
- 32.Drews CD, Dilley AB, Lally C, Beckman MG, Evatt B. Screening questions to identify women with von Willebrand disease. J Am Med Womens Assoc. 2002;57:217–8. [PubMed] [Google Scholar]
- 33.Friberg B, Orno AK, Lindgren A, Lethagen S. Bleeding disorders among young women: a population-based prevalence study. Acta Obstet Gynecol Scand. 2006;85:200–6. doi: 10.1080/00016340500342912. [DOI] [PubMed] [Google Scholar]