Abstract
BACKGROUND
Asthma was the most common comorbidity of patients hospitalized with 2009 H1N1 influenza.
OBJECTIVE
To assess immunogenicity and safety of an unadjuvanted, inactivated 2009 H1N1 vaccine in severe versus mild/moderate asthma.
METHODS
We conducted an open-label study involving 390 participants (age:12–79y) enrolled in October-November 2009. Severe asthma was defined as need for ≥880mcg/d of inhaled fluticasone equivalent and/or systemic corticosteroids. Within each severity group, participants were randomized to receive intramuscularly 15mcg or 30mcg of 2009 H1N1 vaccine twice, 21 days apart. Immunogenicity endpoints were seroprotection (≥40 titer in hemagglutination inhibition assay) and seroconversion (4-fold or greater titer increase). Safety was assessed through local and systemic reactogenicity, asthma exacerbations and pulmonary function.
RESULTS
In mild/moderate asthma (N=217), the 2009 H1N1 vaccine provided equal seroprotection 21 days after the first immunization at the 15mcg (90.6%,CI:83.5–95.4) and 30mcg (95.3%,CI:89.4–98.5) doses. In severe asthma (N=173), seroprotection 21 days after the first immunization was 77.9% (CI:67.7–86.1) and 94.1% (CI:86.8–98.1) at the 15mcg and 30mcg dose, respectively (p=0.004). The second vaccination did not provide further increases in seroprotection. Participants with severe asthma ≥60y showed the lowest seroprotection (44.4% at Day 21) with the 15mcg dose, but had adequate seroprotection with 30mcg. The two dose groups did not differ in seroconversion rates. There were no safety concerns.
CONCLUSION
Monovalent inactivated 2009 H1N1 pandemic influenza vaccine was safe and provided overall seroprotection as a surrogate of efficacy. In severe asthma participants over 60y, a 30mcg dose may be more appropriate.
Keywords: H1N1, asthma, influenza, vaccine, seroprotection, severe asthma
INTRODUCTION
The US Centers for Disease Control and Prevention estimate that between April 2009 and March 2010, 8,720–18,050 deaths and 193,000–398,000 hospitalizations can be attributed to the 2009 H1N1 influenza virus pandemic[1]. The most common comorbidity for patients hospitalized as a result of the 2009 H1N1 virus infection was asthma[2, 3] and the percentage of individuals with asthma hospitalized due to the 2009 H1N1 virus was 4–5 fold higher than the prevalence of asthma in the general population[3–5].
Viral infections are triggers of asthma exacerbations,[6, 7] although the role of influenza was not previously as clear as the epidemiologic data from the 2009 H1N1 pandemic now indicate. The Advisory Committee on Immunization Practices has recommended vaccination of individuals who have chronic disorders of the pulmonary system, including asthma[8]. Similarly, immunization against influenza has been recommended for asthma patients by national and international asthma management guidelines[9, 10] and has been reported to be safe[11].
As the 2009 H1N1 influenza pandemic was developing, it was expected that asthma would be in the priority list for vaccination given the limited vaccine stock that was initially available. However, knowledge regarding the efficacy and safety of the newly developed 2009 H1N1 pandemic influenza vaccine in this high-risk population was unknown. Furthermore, based on suggestive data from a previous study testing a seasonal influenza vaccine[12], the hypothesis was raised that optimal immunization rates may not be achieved in individuals with severe asthma due to long-term, high-dose corticosteroid treatment, .
This study was designed to assess the immunogenicity and safety of the 2009 H1N1 vaccine in two groups of individuals with asthma: those with severe disease (receiving high-dose inhaled corticosteroids and/or systemic corticosteroids) and those with mild/moderate disease. The design of the study included two intramuscular vaccine doses (15mcg and 30mcg), each dose administered twice (3 weeks apart).
METHODS
Study Population
A total of 390 male and non-pregnant female participants, aged 12–79y, were enrolled at seven clinical centers of the National Heart Lung and Blood Institute’s Severe Asthma Research Program (SARP). Eligibility was limited to participants who: (1) had physician-diagnosed asthma, (2) had confirmed asthma symptoms within the last 12 months and (3) did not have a known or suspected history of the 2009 H1N1 virus infection or treatment. Individuals who had received the trivalent seasonal influenza vaccine (TIV) within 2 weeks prior to the study were not eligible for participation, nor was TIV vaccination allowed until 3 weeks after the second 2009 H1N1 virus vaccination. Participants receiving high doses of inhaled corticosteroids (ICS, ≥880mcg fluticasone equivalent per day), continuous or near continuous (50% of year) systemic (oral or injectable) corticosteroids to maintain asthma control or who were uncontrolled despite this treatment were classified as having severe asthma[13]. The protocol was approved by all institutional review boards. Written informed consent was obtained from each participant or their parent or legal guardian. Adolescents aged 12–17y provided assent.
Study Design
This was a randomized, open-label study to investigate the safety and immunogenicity of two administrations of an unadjuvanted, inactivated 2009 H1N1 virus vaccine (Novartis Vaccines and Diagnostics Ltd., Speke, Liverpool, UK) delivered intramuscularly at two dose levels (15mcg or 30mcg), 21 days apart. The 15mcg dose was administered as one injection and the 30mcg dose as two injections (15mcg in each arm). A high dose formulation for single injection was not available at the time of the study. At the initial clinic visit, asthma characterization was performed and participants were randomized into one of two dose level groups stratified by asthma severity (mild/moderate and severe), age (12–17y, 18–64y and >64y), and investigative site. The study consisted of 5 clinic visits (Day 1, 8, 21, 28 and 41), as well as 2 interim, and 3 follow-up telephone contacts for safety assessment (Days 81, 141 and 201).
Solicited injection site and systemic reactogenicity was assessed according to conventional vaccine trials[14]. Asthma-related information was obtained from daily symptom diaries and participant reports. Specifically, in the diary, participants recorded the use of albuterol in the previous 24 hours; the number of awakenings requiring albuterol treatment; and the presence and severity (on a 4-point scale) of chest tightness, wheeze, cough and shortness of breath assessed each morning for overnight and each evening for daytime. In addition, at each study visit, information was obtained from each participant regarding asthma hospitalizations, unscheduled medical visits for asthma management and initiation of an oral corticosteroid burst or increase in the regular dose of oral corticosteroids. Pulmonary function testing (spirometry) was conducted at each visit. Asthma exacerbations were defined as any of the following: a) unscheduled medical visit for asthma management, b) initiation or increase in the dose of oral corticosteroids and c) increase by ≥ 6 puffs or 2 nebs per day of albuterol for 2 consecutive days.
The following serum assays were performed on blood samples collected at each clinic visit: a) 2009 H1N1 influenza virus hemagglutination inhibition (HAI) and b) 2009 H1N1 influenza microneutralization. Both assays were performed at a central laboratory (Southern Research Institute, Birmingham, Alabama) in accordance to WHO-recommended standards[15]. In addition, at baseline, total serum IgE and specific IgE for 9 common aeroallergens were measured. HAI titers against the viral components of the 2009 TIV were measured only at baseline in a subgroup of the study population who had received the 2009 seasonal influenza vaccine prior to entering our study. Each serum sample was tested against three influenza viruses (A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Brisbane/60/2008), which matched the 2009–2010 TIV. The assays were conducted at the Center for Vaccine Research, University of Pittsburgh[15].
Primary immunogenicity outcomes included the percentage of participants with putative seroprotection (HAI titers ≥40) and the percentage of participants with seroconversion (≥4-fold increase in HAI titers) after two administrations of the vaccine (Day 41). Secondary endpoints included the same data after the first vaccination (Day 21). HAI titers on the viral components of the 2009 TIV were exploratory endpoints.
Statistical Methods
A sample size of 150 participants in the severe asthma group (75 per dose group) was chosen to provide at least 87% power to detect seroprotection differences ≥20% between dose groups (i.e., 90% versus 70%). Categorical variables were summarized with enumerations and percentages, and exact 95% confidence intervals (Clopper-Pearson method) were computed to describe seroprotection and seroconversion rates. All participants who received at least one vaccination and provided serum at Day 1 and either Day 21 or Day 41 were included in analyses. Logistic regression modeling assessed associations between seroprotection and explanatory variables (e.g., receipt of 2009 influenza vaccine) adjusting for covariates (e.g., age, baseline HAI titers).
RESULTS
Study Participants
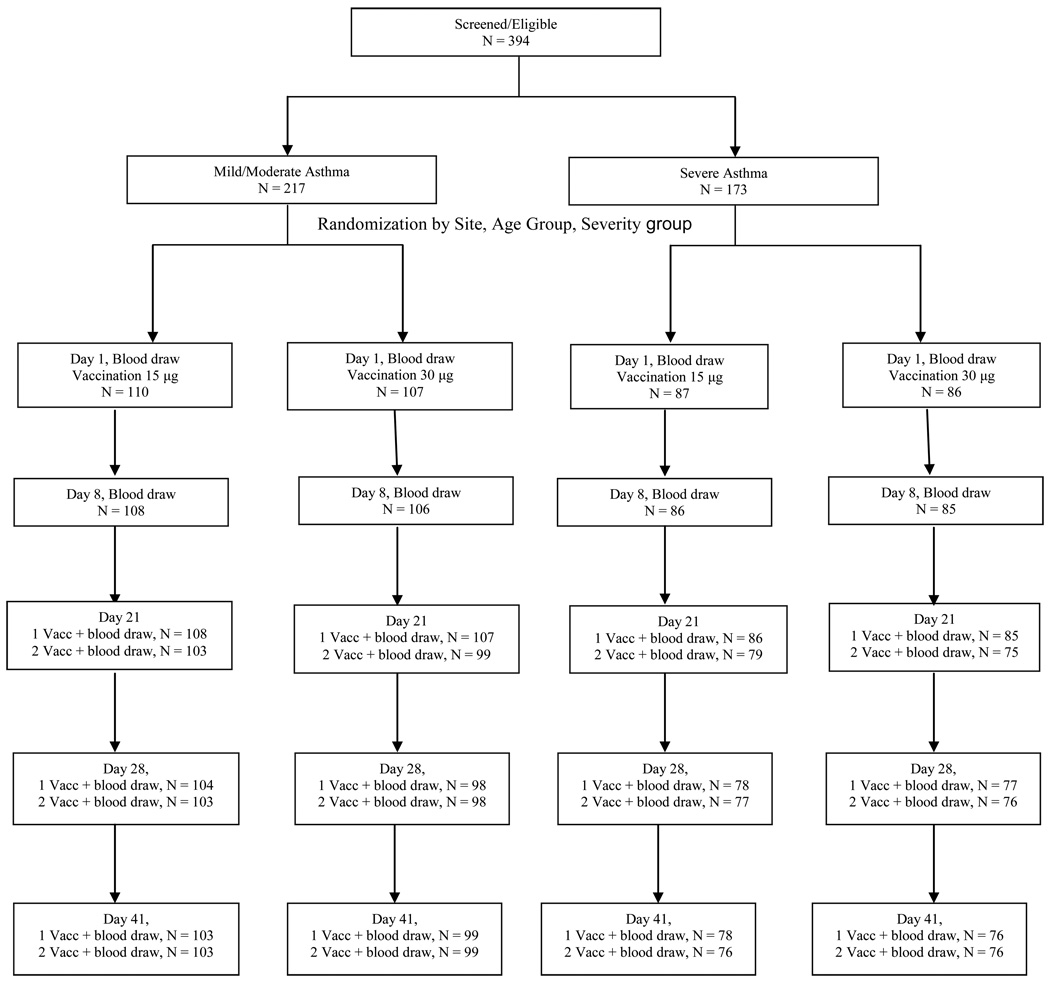
Study participants were enrolled from October 16 to November 13, 2009. Of the 394 enrolled and eligible, 390 were randomized (Figure 1) and received one (15mcg) or two (30mcg) injections of vaccine on Day 1. Of these, 357 received a second administration of the same vaccine dose on Day 21.
FIGURE 1.
CONSORT Diagram. Panels representing Days 21, 28 and 41 report two groups of participants. First line: participants who had their 1st vaccination on Day 1 and blood draw on the indicated study visit. Second line: participants who had their first vaccination on Day 1, second vaccination on Day 21 and blood draw on indicated study visit. The group in the second line is a sub-group of the group in the first line. Randomization was stratified by investigative site, asthma severity group and age group.
Participant age was 12–79y, and those with severe asthma were on average older than those with mild/moderate asthma (Table 1). Of the randomized participants, 52.8% reported receiving the 2009 TIV at least two weeks prior to enrollment. Severe asthma participants had lower baseline lung function and higher body mass index.
TABLE 1. Demographic characteristics of study participants, baseline spirometry and BMI.
| Mild/Moderate Asthma N=(217) |
Severe Asthma N=(173) |
|||
|---|---|---|---|---|
| Mean ± SD unless otherwise noted | 15mcg Vaccine Dose (N=110) |
30mcg Vaccine Dose (N=107) |
15mcg Vaccine Dose (N=87) |
30mcg Vaccine Dose (N=86) |
| Current age (y) | 39.1±18.1 | 36.3±17.0 | 45.2±17.0 | 46.5±17.3 |
| Age at asthma onset (y) | 17.1±17.0 | 16.4±14.9 | 21.9±19.7 | 21.9±20.1 |
| Asthma duration (y) | 21.9±13.9 | 19.9±13.4 | 23.3±16.5 | 24.6±17.8 |
| Body Mass Index (Kg/M2) | 29.5±8.5 | 28.5±6.8 | 31.8±9.7 | 30.1±8.0 |
| FEV1 % predicted | 83.8±16.3 | 86.6±17.6 | 72.6±19.4 | 69.8±20.8 |
| FVC % predicted | 92.6±14.3 | 94.3±16.1 | 85.0±17.1 | 80.9±18.7 |
| FEV1 /FVC | 74.5±9.07 | 75.7±8.99 | 68.4±10.91 | 68.4±11.76 |
| All Numbers Below are Percentages | ||||
| Sex (% female) | 65 | 58 | 60 | 52 |
| Race (%) | ||||
| White | 66 | 76 | 72 | 65 |
| African American | 28 | 20 | 25 | 34 |
| Other | 5 | 5 | 2 | 1 |
| Ethnicity (%) | ||||
| Hispanic or Latino | 4 | 4 | 3 | 0 |
| Received 2009 seasonal TIV Prior to H1N1 Study (%) | 44 | 51 | 62 | 57 |
| Received 2009 seasonal TIV Following Vaccination (%)* | 40 | 39 | 32 | 39 |
The seasonal trivalent influenza vaccine (TIV) was offered to all participants 21 days after the 2nd vaccination with the 2009 pandemic H1N1 influenza vaccine
A total of 33 participants did not receive the second vaccination on Day 21 because of adverse events (n=18), asthma exacerbations (n=9), not meeting eligibility criteria (n=4), and participant withdrawal (n=2). The percentage of participants who missed the second vaccination did not differ between the two asthma severity groups.
Immunogenicity
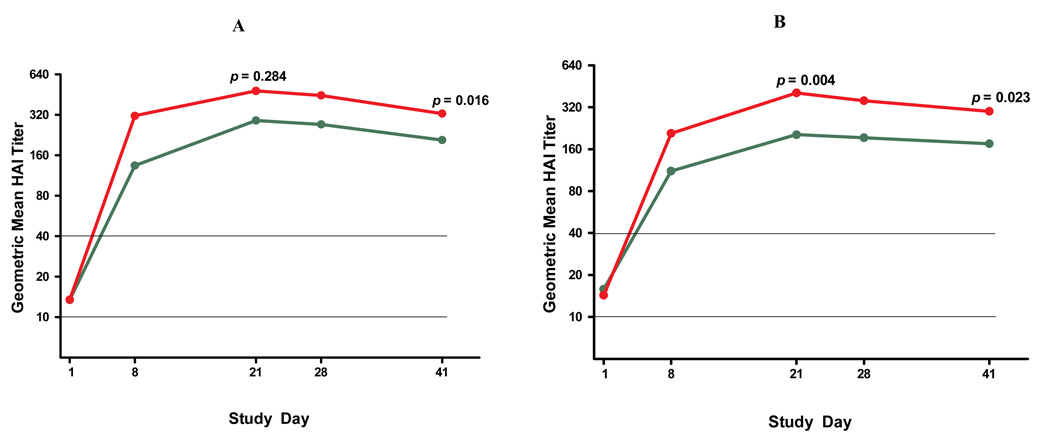
Figure 2 presents geometric mean titers (GMTs) for the HAI assay for all study participants. A single 15mcg or 30mcg dose of the 2009 H1N1 virus vaccine produced a robust immune response with significant differences between the two dose levels in the severe asthma group. The second vaccination on Day 21 produced no further increases in HAI GMTs. Consequently, other analyses presented herein focus on immunogenicity at Day 21, 3 weeks after the first vaccination.
FIGURE 2.
Geometric mean of 2009 pandemic H1N1 influenza virus serum hemagglutination inhibition (HAI) titers by asthma severity and vaccine dose groups Horizontal lines represent lower detection limit of HAI assay (1:10) as well as conventional seroprotection level (1:40). P-values represent comparisons at Day 21 and Day 41 between 15mcg and 30mcg dose groups. Panel A: mild/moderate asthma; Panel B: severe asthma); green lines represent 15 mcg; red lines represent 30 mcg.
Table 2 presents seroprotection rates (% of participants with HAI titers ≥40) and seroconversion rates (% of participants with ≥4-fold increase from baseline in HAI titers) as well as absolute GMT values for all participants at baseline and Day 21. At baseline, between 21.4 and 26.7% of subjects were seroprotected despite no clinical history of 2009 H1N1 virus infection; these rates were not different between study groups, but were higher in younger participants (data not shown). Following the first vaccination, seroprotection was above 90% in the mild/moderate asthma group and no difference between the 15mcg and 30mcg doses was noted. In the severe group, seroprotection was 77.9% for participants who received the 15mcg dose and 94.1% for the 30mcg dose (p=0.004). When only participants without baseline seroprotection were analyzed, the difference in vaccine-induced seroprotection between the 15mcg and the 30mcg doses became even larger in the severe asthma group (69.8% vs. 92.3%, p=0.001). The two vaccine doses did not differ in seroconversion rates. Analysis of the microneutralization assay data did not reveal any qualitative discrepancies, compared to the HAI data. Correlation coefficients between HAI GMTs and microneutralization GMTs ranged from 0.73–0.83 for Days 1 through 41. Microneutralization GMTs were overall higher than HAI GMTs, as previously reported[16, 17].
TABLE 2. Immune response at Day 21 after one dose of the 2009 pandemic influenza H1N1 vaccine.
Statistical comparisons were made on log of HAI titers, then back transformed for reporting purposes. Fisher’s exact test was used for comparison of seroprotection and seroconversion rates between dose groups. Exact binomial confidence intervals were computed to describe seroprotection and seroconversion rates as well as differences in rates between dose groups.
| Mild/Moderate Asthma (N=214) |
Severe Asthma (N=171) |
|||||
|---|---|---|---|---|---|---|
| Immunogenicity End Point |
15mcg Vaccine Dose (N=107) |
30mcg Vaccine Dose (N=107) |
30mcg-15mcg Difference 95% CI p-value |
15mcg Vaccine Dose (N=86) |
30mcg Vaccine Dose (N=85) |
30mcg-15mcg Difference 95% CI p-value |
| Baseline | ||||||
| Subjects with HAI titer ≥ 1:40 (seroprotected) % | 20.6 | 22.4 | 1.9 | 26.7 | 23.5 | −3.2 |
| 95% CI | (13.4–29.5) | (14.9–31.5) | (−11.9–15.6) | (17.8–37.4) | (15–34) | (−18.3–11.4) |
| P-value | p=0.868 | p=0.725 | ||||
| Geometric mean titer | 13.0 | 13.8 | 1.1 | 15.5 | 14.4 | 0.9 |
| 95% CI | (9.7–17.3) | (10.5–18.2) | (0.7–1.6) | (11.1–21.8) | (10.6–19.7) | (0.6–1.5) |
| P-value | p=0.747 | p=0.748 | ||||
| After vaccination at Day 21 | ||||||
| Subjects with HAI titer ≥ 1:40 (seroprotected) % | 90.7 | 95.3 | 4.7 | 77.9 | 94.1 | 16.2 |
| 95% CI | (83.5–95.4) | (89.4–98.5) | (−9.2–18.4) | (67.7–86.1) | (86.8–98.1) | (1.4–30.8) |
| P-value | p=0.284 | p=0.004 | ||||
| Subjects with seroconversion - % | 80.4 | 85.0 | 4.7 | 75.6 | 85.9 | 10.3 |
| 95% CI | (71.6–87.4) | (76.9–91.2) | (−9.2–18.4) | (65.1–84.2) | (76.6–92.5) | (−4.5–25.2) |
| P-value | p=0.470 | p=0.120 | ||||
| Geometric mean titer | 291.0 | 482.8 | 1.7 | 203.8 | 405.4 | 2.0 |
| 95% CI | (218.4–387.8) | (378.3–616.3) | (1.1–2.4) | (141.9–292.6) | (306.5–536.1) | (1.3–3.1) |
| P-value | p=0.008 | p=0.003 | ||||
| Factor increase in geometric mean titer | 22.5 | 34.9 | 1.6 | 13.1 | 28.1 | 2.1 |
| 95% CI | (16.3–31) | (25.5–47.8) | (1–2.4) | (9.2–18.6) | (19.8–39.8) | (1.3–3.5) |
| P-value | p=0.054 | p=0.002 | ||||
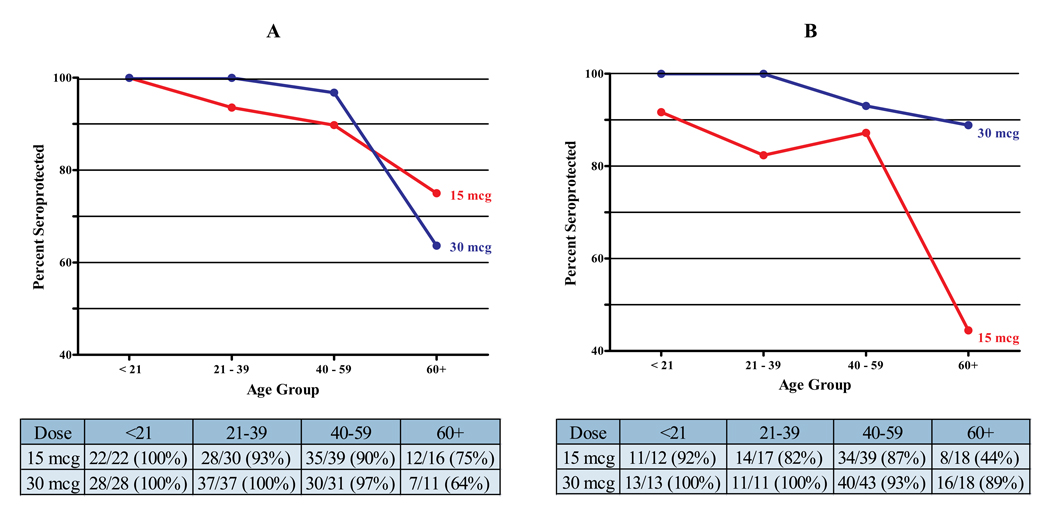
Age-related differences in HAI GMTs were observed. As previously mentioned, younger participants had higher GMTs at baseline (results not shown). Following vaccination, participants ≥60y displayed lower seroprotection rates than younger participants (Figure 3). This phenomenon was accentuated in the severe asthma group. At Day 21, the seroprotection rate in older participants with severe asthma who received the 15mcg dose was 44.4% (n=18); the rate was 88.9%, however, in those participants receiving the 30mcg dose (n=18, p=0.012).
FIGURE 3.
Age effect on seroprotection rates at Day 21 by severity and dose level group (A: mild/moderate asthma; B: severe asthma). Tables below each panel provide information on the number and percentage of participants per age group that were seroprotected.
Seroprotection rates at Day 21 or 41 (3 weeks after first and second H1N1 vaccination, respectively) were significantly lower in participants who had received the 2009 TIV prior to enrolling in the trial compared to those who had not (Table E1). The effect of TIV was significant (p<0.001 by logistic regression model with seroprotection status as outcome) and remained significant after adjustment for baseline H1N1 HAI titers, age, H1N1 vaccine dose, severity group, dose x severity group interaction, BMI, gender, and baseline FEV1 % predicted (p=0.002). To test the hypothesis that antibodies against the 3 viral components of the TIV might interfere with the H1N1 HAI assay (in which case an inverse relationship between TIV and 2009 H1N1 virus HAI titers would be expected), we measured, in a subgroup of participants who had received the TIV prior to entering our study, HAI titers against the TIV viruses (H1N1 Brisbane/59/2007, H3N2 Brisbane/10/2007 and B/Brisbane/3/2007) at baseline. We computed correlations between the antibody titers against the above TIV viruses and antibody titers against the 2009 pandemic H1N1 virus at Day 21 post-vaccination. We found no inverse relationship between the HAI titers for the 2009 H1N1 virus and any of the TIV viruses; in contrast, a positive correlation between the Day 21 titers for the 2009 H1N1 and the Day 1 titers for the H1N1 Brisbane/59/2007 viruses was observed (data not shown).
Atopy, evaluated on every participant by way of total serum IgE and the number of positive (≥ Class I) serum allergen-specific IgE tests, had no influence on HAI GMTs or on seroprotection rates (data not shown). Similarly, we detected no effects of BMI, systemic corticosteroid usage for at least 6 out of the previous 12 months or ICS dose (fluticasone equivalents) at study entry (data not shown).
Safety (not asthma-related)
After the first vaccination, 64% of participants experienced systemic adverse events (solicited) and this did not differ statistically between the two dose levels within the mild/moderate and severe asthma groups nor did participants with severe asthma experience a significantly higher rate of systemic events (Table E2). After the second vaccination, less systemic reactogenicity was observed (46%). The percentage of participants who experienced injection site adverse events (solicited) within the first 8 days of the first vaccination was higher after the 30mcg dose compared to the 15mcg dose in the severe asthma group (p=0.014). A slightly higher, but statistically significant, rate of severe injection site and systemic solicited reactions occurred with the 30mcg dose in the mild/moderate asthma group. The severe asthma group tended to have less frequent severe solicited reactions with the 30mcg dose.
Unsolicited, non-serious adverse events were reported by 49% of participants with no significant differences between dose groups or between severity groups within each system organ class.
Three serious adverse events (SAEs) not associated with an asthma exacerbation occurred within 21 days of vaccination. Fifteen additional SAEs occurred during the telephone follow-up phase (Days 42 – 201). None of these SAEs were characterized as vaccine-associated and there was no predilection for the higher vaccine dose or the severe asthma group.
Safety (asthma-related)
Four asthma-related SAEs (hospitalizations) - 3 in the severe asthma group - occurred within 21 days of vaccination. Only 1 of these participants had received the 30mcg vaccine dose. Eight additional asthma-related SAEs occurred during the telephone follow-up phase of the study, all in the severe asthma group. One of the SAEs was a fatality due to status asthmaticus; the participant died at home approximately two months following the second vaccination.
Asthma exacerbations not resulting in hospitalization (non-SAEs) were recorded in 59 participants (total of 68 events) - 38 in the severe asthma group. Thirty of the 68 exacerbations occurred in participants who received the 30mcg dose.
Pulmonary function testing (FEV1 % predicted, FVC% predicted, FEV1/FVC) results did not demonstrate significant changes from Day 1 to Day 8, or from Day 21 to Day 28, within any of the asthma severity/dose groups except of FVC% predicted in the severe 30mcg group, which declined by an average of 1% from Day 1 to Day 8 (p=0.017), but returned to baseline levels on Day 21 and did not decline after the second vaccination.
DISCUSSION
The most common comorbidity in people hospitalized due to the 2009 pandemic H1N1 influenza virus was asthma[2]. This is not surprising given that viral respiratory infections are believed to be the major cause of asthma exacerbations, even though rhinovirus is the dominant virus found in association with these events[6]. Based on these experiences, asthma patients are a high priority group for H1N1 vaccination.
Our study was designed to compare the immunogenicity and safety of two doses, 15mcg and 30mcg, of an unadjuvanted 2009 H1N1 influenza virus vaccine administered intramuscularly in patients with mild/moderate and severe asthma. Severe asthma was a primary concern because of the presumed greater risk for loss of asthma control with H1N1 infection and the potential influence on vaccine immunogenicity of high doses of inhaled and, in some patients, systemic corticosteroids.
We found that both the 15mcg (standard vaccine dose) and 30mcg H1N1 vaccine doses generally provide excellent seroprotection 21 days after a single immunization in patients with mild/moderate asthma. In severe asthma, the response to 15mcg and 30mcg doses was significantly different, with 77.9% achieving seroprotection with the 15mcg, versus 94.1% with the 30mcg dose. This difference in seroprotection rates was not seen in the mild/moderate asthma group although HAI GMTs following the 15mcg dose were significantly lower than those after the 30mcg in both groups. Interestingly, in studies conducted in healthy adults, no differences between the 15mcg and the 30mcg doses of the 2009 H1N1 pandemic influenza vaccine (albeit from different manufacturers) were found[18, 19].
We also observed that the vaccination response was less in participants ≥60y with the lowest response (44.4% seroprotection) in the ≥60y severe asthma group who received the 15mcg dose (Figure 3). It is well known that immunogenicity of TIV is diminished in older healthy adults[20, 21]. Our data, however, suggest an interaction between age and disease severity, in that older participants with severe asthma receiving the 15mcg dose had the lowest immunogenicity to the 2009 H1N1 vaccine. Although, within the severe asthma group, 23.3% of the participants were ≥60y, their influence on the data was substantial: removal of these subjects changed the seroprotection rate of the 15mcg severe asthma group from 77.9% (95% CI: 67.7–86.1) to 86.8% (95% CI: 76.4–93.8). An encouraging finding is that older participants with severe asthma attained adequate seroprotection with the 30mcg dose suggesting that older adults with severe asthma may need to be vaccinated with a higher than standard dose. Recent studies using high dose (60mcg) TIV have demonstrated higher immunogenicity than standard dose vaccine for individuals >65y[22, 23]. In our study, older participants with mild/moderate asthma sustained acceptable seroprotection 3 weeks post-vaccination with the 15mcg dose. However, 3 weeks after the second vaccination, seroprotection declined from 75% to 56%, again suggesting that even in these individuals the 30mcg dose may need to be considered.
Limited studies have addressed the question whether inhaled or systemic corticosteroid use interferes with the antibody response to influenza immunization. The largest published study in asthma was that of Hanania et al.,[12] which compared the response to TIV in 148 subjects receiving medium to high dose ICS versus 146 subjects on no or low dose. The investigators found no difference between the two study groups in the responses to the influenza vaccine except in a post-hoc analysis where significantly lower antibody titers to influenza B were detected. In addition to employing a larger number of participants, our study of the 2009 pandemic H1N1 vaccine tested various immunization schedules (15mcg versus 30mcg dose and 1 versus 2 vaccinations) and used subjects with severe asthma who were receiving a considerably higher dose of ICS (≥880mcg/d of fluticasone) and were on average 10 years older compared to the participants in the Hanania study.
An additional observation from this study is the clear lack of a booster response by the second vaccination (Figure 2). As in many other studies examining immunogenicity of influenza vaccines, the second vaccination was included in the study design to offer a possible solution to the potential problem of low immunogenicity following a single vaccination. Indeed, in several H1N1 vaccine studies in infants or young children, a second vaccination improved seroprotection rates, which were rather low after a single vaccine dose[18, 24]. In older adults (≥61y), Zhu et al also found that the second vaccination resulted in better seroprotection rates[18]. In our study, participants ≥60y, the age group with the lowest seroprotection rates after the first vaccination, did not benefit from the second vaccine dose.
Earlier studies have reported that post-vaccination GMTs obtained with influenza vaccines can be lower in individuals reporting previous vaccination compared to those without previous vaccination[25, 26]. We have made similar observations in this study. The reasons for this vaccine interference effect are unclear. One possible explanation is that antibodies generated following TIV vaccination could bind to the 2009 H1N1 hemagglutinin vaccine component in vivo and alter its turnover and/or its uptake and presentation by antigen presenting cells. Another explanation could have been that serum antibodies against seasonal virus hemagglutinin bind to the pandemic H1N1 virus hemagglutinin protein in the HAI assay, thereby preventing the pandemic H1N1 anti-hemagglutinin antibodies in the assay from recognizing the pandemic H1N1 hemagglutinin protein. We indirectly tested this hypothesis in individuals who had received the 2009 TIV prior to entering our study by examining whether baseline HAI titers against each of the 3 viruses in the TIV vaccine were inversely correlated with the titers against the 2009 pandemic H1N1 virus at Day 21 or 41 after the first pandemic H1N1 vaccination. The lack of such correlations argues against the possibility that our observation reflects an in vitro artifact. In prior studies that reported similar vaccine interference, no differences in protection from influenza illness were observed[25]. We and others[26] also saw that this vaccine interference effect had little impact on the number of subjects achieving seroprotection against the 2009 pandemic H1N1.
We did not identify any safety concerns with the 2009 H1N1 vaccine. Injection site, but not systemic, reactogenicity was overall higher in participants with severe asthma after the 30mcg dose compared to 15mcg, this likely being secondary to the fact that the 30mcg dose was delivered with two injections, one in each arm. On the other hand, solicited reactions ranked as “severe” by study participants were more common in the mild/moderate group, regardless of vaccine dose. Overall, we can conclude that the severe asthma group tolerated the vaccine as well as the mild/moderate asthma group and that the 30mcg dose was equally well tolerated. In the severe asthma group, the rate of asthma exacerbations and hospitalizations in the 21 days following each vaccination was not higher than the expected rate from historical data obtained by the SARP investigators in individuals with asthma of similar severity[27].
Our study did not include a healthy control group as it was designed to compare the two vaccine doses in two phenotypically distinct groups of individuals with asthma. However, we had the opportunity to exchange information with the vaccine manufacturer (Novartis Vaccines and Diagnostics Ltd.) who has conducted immunogenicity and safety trials in healthy US adult populations using the same doses and vaccination intervals. Overall, the immunogenicity and safety of the 2009 H1N1 influenza virus vaccine in healthy individuals was similar to what we observed in individuals with asthma.
Our findings should not be assumed to be automatically applicable to the live attenuated intranasal vaccine. Although intranasal administration of the seasonal influenza vaccine has shown efficacy and safety in people with asthma[28, 29], individuals with severe airway disease have not been extensively tested and some concern exists as to the potential of inducing an asthma exacerbation with the intranasal vaccine in this group[30, 31]. It is also not known whether the substantial nasal mucosal disease, characteristic of the vast majority of these patients,[32] and the use of nasal corticosteroids can influence intranasal vaccine immunogenicity and safety.
In summary, adult and adolescent patients with asthma can be safely administered the 2009 H1N1 pandemic influenza vaccine (which is currently a component of the 2010 TIV) and will most likely develop seroprotection at a rate comparable to healthy individuals with a single 15mcg dose. It is noteworthy that the 30mcg dose of the vaccine led to a higher rate of seroprotection compared to the 15mcg dose in the severe asthma group, this being primarily due to the low seroprotection in older participants. Indeed, older adults with severe asthma should be considered for the 30mcg dose, which offers substantially better seroprotection.
Clinical Implications.
H1N1 vaccination is safe and produces robust antibody responses in patients with asthma. High dose ICS did not adversely affect seroprotection. Patients with severe asthma and over 60y may benefit from a higher vaccine dose.
ACKNOWLEDGMENTS
Funding
Funding for this study was provided by the US National Institutes of Health through a supplement contribution from the National Institute of Allergy and Infectious Diseases (NIAID) to the National Heart, Lung, and Blood Institute's (NHLBI)-funded grant 3 R01 HL069116-09S1. Rho Inc., the Data and Statistical Coordinating Center for this study was funded by NIAID Contract number NO1-AI-25482. Additional funds were provided by the National Institutes of Health Research Project grants RO1 HL69170 and HL081064 and by the National Center for Research Resources under grants 1UL1RR024989 and UL1RR025008. NIAID and NHLBI scientists and project managers were involved in the design of the study and the writing of the protocol, as well as in study medical monitoring, data analysis and manuscript preparation. Vaccine was purchased from Novartis Vaccines and Diagnostics LTD. by the US Government BioMedical Advanced Research and Development Authority (BARDA) that coordinated the 2009 H1N1 pandemic influenza vaccination in the United Sates. BARDA had no other role in this study.
The H1N1 vaccination study was a collaboration of the following institutions and investigators (principal investigators are indicated by asterisks):
University of Wisconsin School of Medicine and Public Health, Madison, WI – W Busse*, N Jarjour, L Frisque, M Jackson, C Swenson. Wake Forest University Health Sciences Center for Genomics and Personalized Medicine Research, Winston-Salem, NC – E Bleecker*, W Moore, S Peters, D Meyers, R Pascual, J Ohar, C Hatzis, P Lenz, V Ortega, A Wahla, R Bermudez, T Crews, E Haney, R Hmieleski, J Krings, R Smith, P Spernoga, C Wilmoth. Washington University School of Medicine, St. Louis, MO – M Castro*, K Sumino, T Koch, J Tarsi, G Gregory, D Burgdorf, B Patterson, C Christie, H YinDeClue, L Carlstrom. University of Pittsburgh Asthma Institute, Pittsburgh, PA – S Wenzel*, S Simeone, F Holguin, S Aujla, T Dill. Cleveland Clinic, Cleveland, OH – S Erzurum*, S Ratanamaneechat, D George, R Dweik, M Aronica, S Comhair, M Koo, L Peterson, K Nagle, J Baran-Smiley, E Mattox, P Parikh, R Naples, R Hughes, D Laskowski, M Baaklini, L Danziger-Isakov, A Jain and Cleveland Clinic eResearch. Emory University, Atlanta, GA – A Fitzpatrick*, M Penugonda, K DeMuth, A Stecenko, D Whitlock. University of Virginia Children’s Hospital Division of Respiratory Medicine, Charlottesville, VA – W Teague*, B Gaston, M Plapp, S Lowenhaupt, S Dwyer. Statistical and Clinical Coordinating Center, Rho, Inc, Chapel Hill, NC – H Mitchell*, G David, L Aertker, S Autry, M Boliek, R Budrevich, A Calatroni, R James, G Johnson, C Hardee, E May, H Ross, R Slater, J Schiepan, M Walter, D Zaccaro. National Institute of Allergy and Infectious Diseases, Bethesda, MD – A Togias, M Fenton, S Sigelman, M Gomez, J Poyser. National Heart Lung and Blood Institute, Bethesda, MD – R Smith, G Weinmann.
Abbreviations
- GMT
geometric mean titers
- HAI
hemagglutination inhibition
- ICA
inhaled corticosteroids
- SAE
serious adverse event
- SARP
Severe Asthma Research Program
- TIV
trivalent seasonal influenza vaccine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William W. Busse, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Stephen P. Peters, Wake Forest University Health Sciences Center for Genomics and Personalized Medicine Research, Winston-Salem, NC.
Matthew J. Fenton, Division of Allergy, Immunology and Transplantation – NIAID/NIH, Bethesda, MD.
Herman Mitchell, Rho Federal Systems Division, Inc. Chapel Hill, NC.
Eugene R. Bleecker, Wake Forest University Health Sciences Center for Genomics and Personalized Medicine Research, Winston-Salem, NC.
Mario Castro, Washington University School of Medicine, St. Louis, MO.
Sally Wenzel, University of Pittsburgh Asthma Institute, Pittsburgh, PA.
Serpil C. Erzurum, Cleveland Clinic, Cleveland, OH.
Anne M. Fitzpatrick, Emory University, Atlanta, GA.
W. Gerald Teague, University of Virginia Children’s Hospital, Division of Respiratory Medicine, Charlottesville, VA.
Nizar Jarjour, University of Wisconsin School of Medicine and Public Health, Madison, WI.
Wendy C. Moore, Wake Forest University Health Sciences Center for Genomics and Personalized Medicine Research, Winston-Salem, NC.
Kaharu Sumino, Washington University School of Medicine, St. Louis, MO.
Scott Simeone, University of Pittsburgh Asthma Institute, Pittsburgh, PA.
Suphagaphan Ratanamaneechat, Cleveland Clinic, Cleveland, OH.
Madhuri Penugonda, Emory University, Atlanta, GA.
Benjamin Gaston, University of Virginia Children’s Hospital, Division of Respiratory Medicine, Charlottesville, VA.
Ted M. Ross, University of Pittsburgh Center for Vaccine Research, Pittsburgh, PA.
Steve Sigelman, Division of Allergy, Immunology and Transplantation – NIAID/NIH, Bethesda, MD.
Joella R. Schiepan, Rho Federal Systems Division, Inc. Chapel Hill, NC.
Daniel J. Zaccaro, Rho Federal Systems Division, Inc. Chapel Hill, NC.
Corey J. Crevar, University of Pittsburgh Center for Vaccine Research, Pittsburgh, PA.
Donald M. Carter, University of Pittsburgh Center for Vaccine Research, Pittsburgh, PA.
Alkis Togias, Division of Allergy, Immunology and Transplantation – NIAID/NIH, Bethesda, MD.
REFERENCES
- 1.CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009 – March 13, 2010: Centers for Disease Control and Prevention; 2010. 2010. Apr 19, [Google Scholar]
- 2.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. The New England journal of medicine. 2009 Nov 12;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed on September 17, 2009];2009 H1N1 Flu: Underlying Health Conditions among Hospitalized Adults and Children: Centers for Disease Control and Prevention; 2010 February 24. 2010 Available at: http://www.cdc.gov/H1N1flu/eip_underlying_conditions.htm.
- 4.Bloom BCR, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2008. National Center for Health Statistics. Vital Stat. 2009;10(244) [PubMed] [Google Scholar]
- 5.Pleis JRLJ, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. National Center for Health Statistics. Vital Health Stat. 2009;10(242) [PubMed] [Google Scholar]
- 6.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. Sep 4;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears MR, Johnston NW. Understanding the September asthma epidemic. The Journal of allergy and clinical immunology. 2007 Sep;120(3):526–529. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008 Aug 8;57(RR-7):1–60. [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute. [Accessed December 7, 2009];Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma - Full Report 2007. 2007 August 28; Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 10.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2006 [cited; Available from: http://www.ginasthma.com/
- 11.The American Lung Association Asthma Clinical Research Centers. The safety of inactivated influenza vaccine in adults and children with asthma. The New England journal of medicine. 2001 Nov 22;345(21):1529–1536. doi: 10.1056/NEJMoa011961. [DOI] [PubMed] [Google Scholar]
- 12.Hanania NA, Sockrider M, Castro M, Holbrook JT, Tonascia J, Wise R, et al. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. The Journal of allergy and clinical immunology. 2004 Apr;113(4):717–724. doi: 10.1016/j.jaci.2003.12.584. [DOI] [PubMed] [Google Scholar]
- 13.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. American journal of respiratory and critical care medicine. 2000 Dec;162(6):2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. 2007. [Google Scholar]
- 15. [cited November 10, 2010];WHO Manual on Animal Influenza Diagnosis and Surveillance. [Manual] 2002 Available from: http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf.
- 16.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. The New England journal of medicine. 2009 Dec 17;361(25):2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. The New England journal of medicine. 2009 Dec 17;361(25):2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 18.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. The New England journal of medicine. 2009 Dec 17;361(25):2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 19.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. Jan 2;375(9708):41–48. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 20.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 Feb 20;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 21.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005 Jul 8;23 Suppl 1:S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. The Journal of infectious diseases. 2009 Jul 15;200(2):172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 23.Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Archives of internal medicine. 2006 May 22;166(10):1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 24.Oh CE, Lee J, Kang JH, Hong YJ, Kim YK, Cheong HJ, et al. Safety and immunogenicity of an inactivated split-virus influenza A/H1N1 vaccine in healthy children from 6 months to <18 years of age: a prospective, open-label, multi-center trial. Vaccine. Aug 16;28(36):5857–5863. doi: 10.1016/j.vaccine.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 25.Keitel WA, Cate TR, Couch RB. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. American journal of epidemiology. 1988 Feb;127(2):353–364. doi: 10.1093/oxfordjournals.aje.a114809. [DOI] [PubMed] [Google Scholar]
- 26.Treanor J, Keitel W, Belshe R, Campbell J, Schiff G, Zangwill K, et al. Evaluation of a single dose of half strength inactivated influenza vaccine in healthy adults. Vaccine. 2002 Jan 15;20(7–8):1099–1105. doi: 10.1016/s0264-410x(01)00440-6. [DOI] [PubMed] [Google Scholar]
- 27.Moore WC, Peters SP. Severe asthma: an overview. The Journal of allergy and clinical immunology. 2006 Mar;117(3):487–494. doi: 10.1016/j.jaci.2006.01.033. quiz 95. [DOI] [PubMed] [Google Scholar]
- 28.Fleming DM, Crovari P, Wahn U, Klemola T, Schlesinger Y, Langussis A, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. The Pediatric infectious disease journal. 2006 Oct;25(10):860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 29.Glezen WP. Asthma, influenza, and vaccination. The Journal of allergy and clinical immunology. 2006 Dec;118(6):1199–1206. doi: 10.1016/j.jaci.2006.08.032. quiz 207–8. [DOI] [PubMed] [Google Scholar]
- 30.Bergen R, Black S, Shinefield H, Lewis E, Ray P, Hansen J, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. The Pediatric infectious disease journal. 2004 Feb;23(2):138–144. doi: 10.1097/01.inf.0000109392.96411.4f. [DOI] [PubMed] [Google Scholar]
- 31.MedImmune, LLC. [cited November 10, 2010];FluMist Influenza Vaccine Live, Intranasal. Highlights of Prescribing Information. 2010 Available from: http://www.medimmune.com/pdf/products/flumist_pi.pdf.
- 32.Togias A. Rhinitis and asthma: evidence for respiratory system integration. The Journal of allergy and clinical immunology. 2003 Jun;111(6):1171–1183. doi: 10.1067/mai.2003.1592. quiz 84. [DOI] [PubMed] [Google Scholar]