Abstract
This study investigated the influence of direction of attention on the early detection of visual novelty, as indexed by the anterior N2. The anterior N2 was measured in young subjects (n=32) under an Attend and Ignore condition. Subjects were presented standard, target/rare, and perceptually novel visual stimuli under both conditions, but under the Ignore condition, attention was directed towards an auditory n-back task. The size of the anterior N2 to novel stimuli did not differ between conditions and was significantly larger than the anterior N2 to all other stimulus types. Furthermore, under the Ignore condition, the anterior N2 to visual novel stimuli was not affected by the level of difficulty of the auditory n-back task (3-back vs. 2-back). Our findings suggest that the early processing of visual novelty, as measured by the size of the anterior N2, is not strongly modulated by direction of attention.
We have proposed a model of visual novelty processing that involves several stages that could be identified because of the excellent temporal resolution of event-related potentials (ERPs). The stages include: relatively early detection of a mismatch between perceptually novel stimuli and stored representations (indexed by the anterior N2); voluntary allocation of resources dependent on the context in which the novel event occurs (indexed by the P3); and, when appropriate, more sustained processing of novelty (indexed by late slow wave activity) (Chong et al., 2008). There is growing consensus that the anterior N2 serves as an early marker of stimulus unfamiliarity/unusualness, or more broadly, a discrepancy between the current stimulus and stored representations (Daffner et al., 2000; Ferrari, Bradley, Codispoti, & Lang, 2010; Nittono, Shibuya, & Hori, 2007). Folstein and Van Petten (2008) summarize this concept in their review article in which they describe the anterior N2 response as reflecting a mismatch between a stimulus and available mental templates formed by either long-term or short-term experience.
There continues to be disagreement in the literature regarding the extent to which the early detection of visual novelty, as indexed by the anterior N2, is modulated by direction of attention. In their review article, Folstein and Van Petten (2008) argue that there has been no evidence that the anterior N2 response to visual stimuli is generated “in the absence of focal attention to the eliciting stimulus” (p.159). They point out that this observation distinguishes the mismatch operations of the anterior N2 component in the visual modality from those of the mismatch negativity (MMN) component in the auditory modality, which is elicited in response to ignored acoustic deviants. They review the results of several sequential matching tasks which found that change or mismatch between pairs of stimuli along task-relevant dimensions enhanced the anterior N2 more than along task-irrelevant dimensions, which at times did not elicit any discernable N2 (Fu, Fan, & Chen, 2003; Wang, Cui, Wang, Tian, & Zhang, 2004). For example, Wang and colleagues (2004) presented pairs of stimuli to subjects who were asked to determine matches by attending to designated features and found that although the anterior N2 was elicited under all mismatch conditions, its amplitude was larger when the mismatched features were task-relevant. In accordance with this theory, Folstein, Van Petten, and Rose (2008) conducted two experiments in which participants were instructed to categorize animal-like figures according to one or two stimulus dimensions (in Experiment 1 and 2 respectively). They found that when novel features were embedded in a dimension not essential for stimuli classification (Experiment 1), the N2 was not enhanced. Conversely, when novel features were relevant to the assigned categorization rule (Experiment 2) this was associated with large N2s. These results led the researchers to suggest that it is necessary for novel features to occur in a task-relevant dimension in order to see enhancements in the N2 and thus, early novelty detection in the visual modality is dependent on the focus of attention.
In contrast, Chong et al. (2008) found that the size of the anterior N2 response was not influenced by experimental manipulation of the degree to which novel visual stimuli were task relevant. Subjects participated in one of two conditions of a subject-controlled novelty oddball paradigm. Both conditions had the same formal task requirements (button press to advance to the next stimulus and foot-pedal to respond to a target), but the instructions emphasized different goals under each condition that determined the potential meaning of the novel stimuli. Under the ‘Target-Focused’ condition, subjects were told that the purpose of the experiment was to see how quickly and accurately individuals react to targets when they are exposed to a variety of distracter images. In this context, novel stimuli served as task-irrelevant distracters. Under the ‘Curiosity-Focused’ condition, subjects were informed that the purpose of the experiment was to learn how curious people are about the things in their environment. Here the novel stimuli could be understood as potential invitations to explore. They found that, in contrast to the subsequent P3 component and posterior slow wave activity, the size of the anterior N2 response to novel stimuli did not differ between conditions. Their results suggested that the anterior N2 component was not modulated by experimental context, and that early identification of stimulus novelty reflected a relatively automatic process.
However, inferences from this study were limited by the fact that under both conditions attention was directed to stimuli in the visual modality. This left open the possibility that enhancement of the anterior N2 component simply requires that novel stimuli are presented under attend conditions, regardless of their degree of task relevance. The current study provided an opportunity to further investigate the extent to which the early detection of novelty, as indexed by the N2, is dependent on direction of attention. The anterior N2 was measured in young adult subjects under two conditions that manipulated attention. Under both conditions, subjects were presented standard, target/rare, and perceptually novel visual stimuli. Under the Attend condition, subjects responded to designated visual targets. Under the Ignore condition, subjects performed a difficult n-back task in the auditory modality while passively being exposed to the visual stimuli. We compared the anterior N2 to novel stimuli under both conditions to help distinguish between competing theories concerning whether it is influenced by direction of attention.
If early novelty processing, as indexed by the anterior N2, is modulated by the direction of attention, one would hypothesize that its amplitude in response to novel visual stimuli would be larger under the Attend condition than under the Ignore condition. Moreover, if the attentional demands of the primary auditory task were increased, one would expect that the size of the N2 to novel stimuli in the ignored channel would be diminished. The most robust version of this hypothesis would suggest that early novelty processing, as indexed by the N2, is entirely dependent on the focus of attention, and would lead to the expectation of an extremely attenuated or absent anterior N2 response under the Ignore condition. In addition, one would predict that under this condition, the N2 response to novel stimuli would not differ from that in response to repetitive standard stimuli. By contrast, if early novelty processing is not modulated by direction of attention, one would expect that the anterior N2 to novel visual stimuli would not differ between the Attend and the Ignore condition.
The P3 response to novel and target visual stimuli also was investigated to determine if the pattern was different from that seen in the anterior N2 across conditions. The P3a, an anteriorly oriented P3 component, has been viewed as reflecting the process of reorienting attention to deviant events (Escera, Alho, Winkler, & Naatanen, 1998; Naatanen, 1990), or as indexing the call of an executive control system to shift mental set (Barcelo, Escera, Corral, & Perianez, 2006; Barcelo, Perianez, & Knight, 2002). The P3b, a posteriorly oriented P3 component, has been interpreted as reflecting the process of updating representations in working memory (Donchin, 1981; Donchin & Coles, 1988), or as indexing the process of categorizing an event (Chao, Nielsen-Bohlman, & Knight, 1995; Ford, 1978; Kok, 2001; Squires, Hillyard, & Lindsay, 1973). Since it has been shown that direction of attention strongly modulates both the P3a and P3b components (Duncan-Johnson & Donchin, 1977; Hillyard & Kutas, 1983; Holdstock & Rugg, 1995; Johnson, 1986; Woods, Knight, & Scabini, 1993), we hypothesized that the P3 to novel visual stimuli would be larger under the Attend condition than under the Ignore condition.
Methods
Participants
Thirty-two English-speaking subjects participated in this study (mean age = 21.6 ± 2.0 years; 16 males). All were free of CNS diseases or major psychiatric disorders based on DSM-IV criteria (American Psychiatric Association, 1994). They had a mean of 15.1 ±1.3 years of education. Written informed consent was obtained from all subjects before the study. Subjects were paid for their time.1
Experimental Procedures
The experimental procedures used were analogous to the ones described in Riis et al. (2009). Each subject participated in two experimental conditions, the Attend condition and the Ignore condition, whose order was counterbalanced. Under both conditions, 250 line drawings, white on black background, were presented in 5 blocks of 50, each at the center of a high-resolution computer monitor. All stimuli subtended a visual angle of approximately 2.75° along their longest dimension. Each task included three categories of visual stimuli: 1) a repetitive standard stimulus (a square under Attend, a rectangle with the long side vertically oriented under Ignore)—70% frequency, 2) an infrequent target/rare stimulus (a diamond under Attend, a rectangle with the long side horizontally oriented under Ignore)—approximately 15% frequency, and 3) novel stimuli, randomly drawn from a set of unusual/unfamiliar line drawings (e.g., impossible or fragmented objects) shown only one time each—approximately 15% frequency, many of which came from the collection of drawings that have been used by Kroll and Potter (1984) and Kosslyn et al. (1994). All visual stimuli appeared within a fixation box subtending a visual angle of approximately 3.5° x 3.5° that remained on the screen at all times. Three different sets of novel stimuli were used, counterbalanced across subjects and conditions. The standard and target/rare stimuli were varied across conditions to help prevent subjects from responding to rare stimuli as they had to targets under the Attend condition. Under both conditions, each visual stimulus was presented for 600 ms and was followed by a period in which only the fixation box remained on the screen (1000–1900 ms; mean (SD): 1310 (192) ms).
Under the Attend condition, subjects focused their attention on the visual stimuli and responded to rare stimuli, designated as targets, with a foot pedal press. Left/right foot pedal press was counterbalanced across subjects. Under the Ignore condition, subjects performed a difficult n-back task in the auditory modality in which a series of letters were binaurally presented (digitized voice) for 200 ms by computer via headphones during the period when only the fixation box appeared on the screen. To reduce the influence of the ERPs elicited by auditory stimuli on the ERPs elicited by visual stimuli (Woldorff, 1993), the time between the onset of the auditory stimuli and the onset of the visual stimuli was varied between 500 and 900 ms. Subjects performed a 3-back task during blocks 1 and 2, and a 2-back task during blocks 3–5, and responded to targets by foot pedal press (left/right foot pedal press was counterbalanced across subjects). N-back auditory target letters were presented at approximately 12% frequency and occurred randomly with respect to the presentation of rare, novel, or standard visual stimuli.
While performing the auditory n-back task, subjects were instructed that, in order to minimize artifacts from eye activity, they should keep their gaze at the center of the fixation box on the computer monitor in which the visual stimuli were presented. Thus, under the Ignore condition, subjects passively viewed the same types of visual stimuli as those responded to in the Attend condition. However, in the Ignore condition, these visual stimuli were irrelevant to the assigned task. Under both conditions, subjects were asked to respond quickly while trying to be as accurate as possible.
Although the types of visual stimuli presented were physically the same under both conditions, their roles differed as a function of the task assigned to subjects. Under the Attend condition, the infrequent target/rare stimuli required a response (foot pedal press) and are therefore referred to as target stimuli. Under the Ignore condition, the same kinds of stimuli used as targets under the Attend condition were task-irrelevant, as the paradigm required attending to the auditory modality. They are referred to as rare, and not target, stimuli because subjects were not instructed to generate a behavioral response to the visual events.
ERP Recordings
An electrode cap (Electro-Cap International, Eaton, OH, USA) was used to hold 35 tin electrodes to the scalp, whose locations were based on the International 10–20 system. Electrodes were arranged in 5 columns (midline, 2 inner lateral, 2 outer lateral), each with 7 antero-posterior sites. All sites were referenced to the left mastoid, and the impedance between each recording site and reference was reduced to less than 5K ohms. An electrode was placed beneath the left eye (referenced to an electrode placed above the left eye) to monitor for blinks. Another electrode was placed to the right of the subjects’ right eye (referenced to an electrode placed to the left of the left eye) to monitor for lateral eye movement. A final electrode was placed over the right mastoid (referenced to the left one) to monitor for asymmetrical mastoid activity. (None was identified.) The EEG was amplified by an SA Instrumentation (San Diego, CA, USA) system, using a band filter with negative 3dB cutoffs of 0.01 and 40 Hz, and continuously digitized (200 Hz) by a computer yielding 1280 ms of data from each electrode site, beginning 100 ms before stimulus onset.
Data Analysis
Behavioral data were collected under both conditions. In the Attend condition, target hit rate, false alarm rate, accuracy rate (target hit rate – false alarm rate), and median reaction time were calculated in the 200–1800 ms interval post-stimulus presentation. In the Ignore condition, auditory n-back target hit rate, false alarm rate, accuracy rate, and median reaction time were calculated in the 200–1800 ms interval post-stimulus presentation for the 2-back and 3-back tasks separately. Although the same measures were obtained under the Attend and Ignore conditions, behavioral findings were not compared across conditions as the task in the visual modality under the Attend condition was much easier (0-back) than the task in the auditory modality under the Ignore condition (2 or 3-back).
A continuous record of the raw EEG was stored on hard disk. Off-line, EEG epochs for the three stimulus type (visual novel, target/rare, and standard events) were averaged separately. This report will focus on the response to visual stimuli under both conditions, and only data collected from the midline electrode sites will be reviewed. Trials with eye movements or amplifier blocking were excluded from data analysis. (Of note, there were no differences across conditions for the number of events averaged for each stimulus type after trials with artifacts were rejected.) A blink correction program using principal component analysis was employed that computed the impact of the blink on the waveform in each channel (Dale, 1994).
The temporal intervals used to define the anterior N2 in each task were determined after reviewing individual and grand mean ERPs. The group’s mean local negative peak latency was determined across midline electrode sites between 210–350 ms after stimulus onset in response to each visual stimulus type for both conditions. The anterior N2 component for each subject was then computed as the mean amplitude of the 80 ms interval centered on the local negative peak latency for each visual stimulus type as determined above. The component was measured over fronto-central midline electrode sites with respect to the average of the 100 ms pre-stimulus baseline. Thus, the anterior N2 in response to standard and novel stimuli was defined as the mean amplitude between 245–325 ms after stimulus onset, and the anterior N2 in response to target/rare stimuli was defined as the mean amplitude between 255–335 ms after stimulus onset.2 To assess the effects of direction of attention on novelty processing, the anterior N2 elicited by each type of visual stimuli was measured under the Attend and Ignore conditions.
The temporal intervals used to define the P3 in each task were established in a similar fashion. After reviewing individual and grand mean ERPs, it was determined that the P3 peaked at different times under the Attend and Ignore conditions. As a result, the group’s mean local positive peak latency was determined across midline electrode sites at separate intervals for each condition: between 250–400 ms under the Ignore and between 300–500 ms under the Attend. The P3 for each subject was then computed as the mean amplitude of the 80 ms interval centered around the local positive peak latency for each visual stimulus type. The component was measured over all midline electrode sites with respect to the average of the 100 ms pre-stimulus baseline.
Data were analyzed using repeated-measures analyses of variance (ANOVAs) with condition (Attend condition and Ignore condition), stimulus type (novel, target/rare, and standard visual stimuli), and electrode site (for N2 analyses: Fz, FCz, Cz; for P3 analyses: FPz, Fz, FCz, Cz, CPz, Pz, Oz) as within-subject variables. The 2-back and 3-back tasks were collapsed in all analyses, except when investigating the impact of task difficulty in the Ignore condition. Analyses that yielded significant interactions between condition, stimulus type, or electrode site resulted in planned contrasts between the levels of the variable. The Geisser-Greenhouse correction was applied for all repeated measures with greater than 1 degree of freedom.
Results
Behavior
A summary of the behavioral findings can be found in Table 1 (including mean hit rate, mean false alarm rate, mean accuracy rate, and median reaction time under both the Attend and Ignore conditions). Under the Ignore condition, when subjects focused on the auditory n-back task, performance differed as a function of task difficulty, with subjects performing more accurately and responding more quickly on the 2-back task compared to the 3-back task (mean accuracy rate: F(1,31) = 17.33, p< 0.0005, ηp2 = 0.36; median reaction time: F(1,31) = 15.87, p< 0.0005, ηp2 = 0.34).
Table 1.
Behavioral Data
| Mean (SD) | |
|---|---|
| Attend: | |
| Mean Hit Rate | 0.99 (0.02) |
| Mean False Alarm Rate | < 0.001 (< 0.010) |
| Mean Accuracy Rate | 0.98 (0.02) |
| Median Reaction Time | 719 ms (119) |
| Ignore: | |
| 2-Back Task | |
| Mean Hit Rate | 0.81 (0.18) |
| Mean False Alarm Rate | 0.04 (0.02) |
| Mean Accuracy Rate | 0.78 (0.19) |
| Median Reaction Time | 867 ms (116) |
| 3-Back Task | |
| Mean Hit Rate | 0.66 (0.20)* |
| Mean False Alarm Rate | 0.06 (0.04)* |
| Mean Accuracy Rate | 0.59 (0.20)* |
| Median Reaction Time | 1028 ms (223)* |
Denotes significant differences between the 2-back and 3-back tasks (p < 0.001)
ERP Data
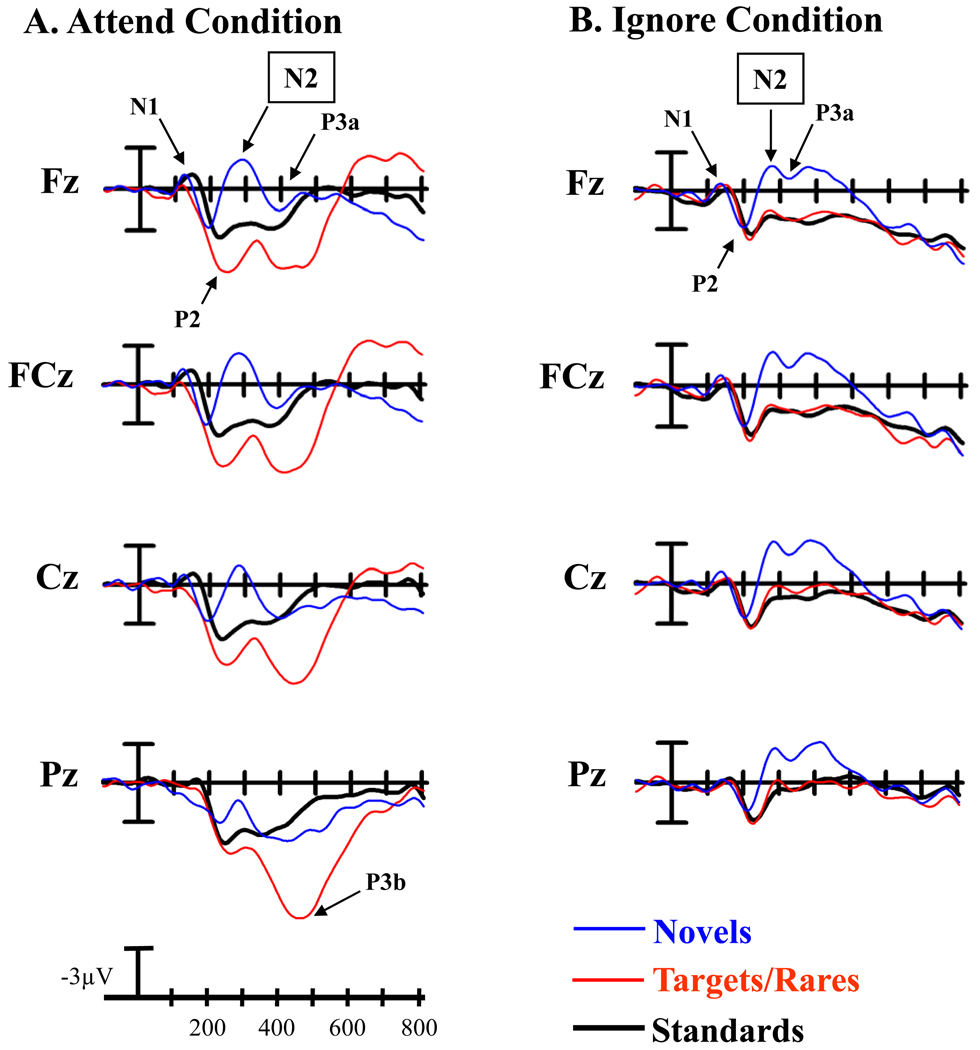
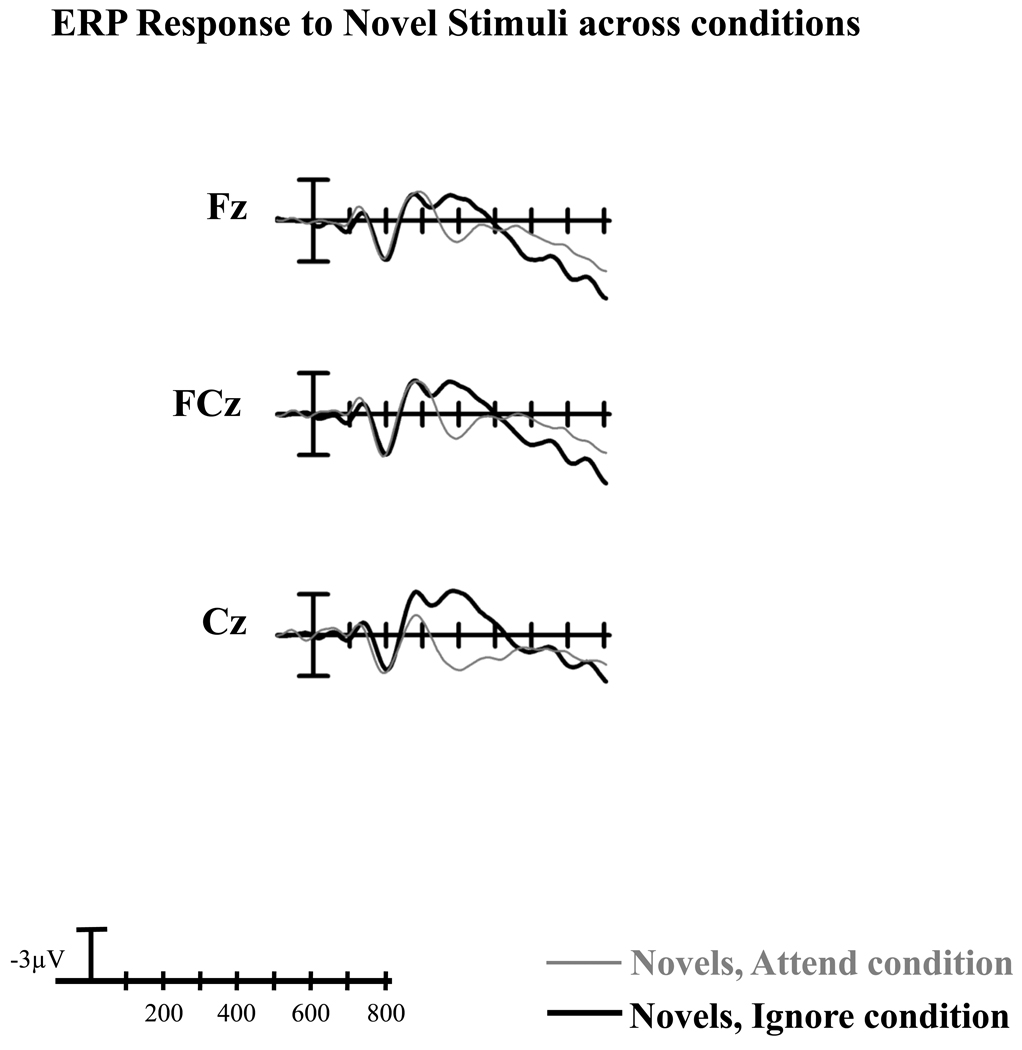
Midline ERPs in response to novel, target/rare, and standard stimuli under each condition are illustrated in Figure 1. Figure 2 compares the midline ERPs in response to novel stimuli under both conditions.
Figure 1.
Midline grand average ERPs at frontocentral sites (Fz, FCz, Cz, Pz) in response to novel, target/rare, and standard stimuli under the A) Attend condition and the B) Ignore condition. In (A) arrows illustrate the N1, P2, N2, and P3a waves at Fz, and the P3b wave at Pz under the Attend condition. In (B) arrows illustrate the N1, P2, N2, and P3a waves at Fz.
Figure 2.
Midline grand average ERPs at frontocentral sites (Fz, FCz, Cz) in response to novel stimuli under the Attend vs. Ignore conditions.
Effect of stimulus type on the anterior N2
Attend condition
There was an effect of stimulus type under the Attend condition, with novel stimuli eliciting a larger anterior N2 than either standard or target stimuli (F(2,62) = 51.48, p< 0.0001, ε = 0.90, ηp2 = 0.62; novel vs. target: F(1,31)= 73.71, p< 0.0001, ηp2 = 0.70; novel vs. standard: F(1,31) = 54.67, p< 0.0001, ηp2 = 0.64). The anterior N2 elicited by standard and target stimuli also differed, with the anterior N2 to standard stimuli being larger (target vs. standard: F(1,31) = 10.59, p< 0.005, ηp2 = 0.26). Additionally, there was an effect of electrode site, with the N2 having maximal amplitude at Fz/FCz (F(2,62) = 3.49, p< 0.05, ε = 0.79, ηp2 = 0.10; Fz ≈ FCz > Cz). The scalp distribution of the anterior N2 did not differ between stimulus types (Stimulus type x Electrode site interaction: F(4,124) = 2.14, p> 0.12, ε = 0.52, ηp2 = 0.07).
Ignore condition
Under the Ignore condition, there was an effect of stimulus type, with novel stimuli eliciting a larger anterior N2 than either standard or rare stimuli (F(2,62) = 29.55, p< 0.0001, ε = 0.92, ηp2 = 0.49; novel vs. rare: F(1,31) = 31.24, p< 0.0001, ηp2 = 0.50; novel vs. standard: F(1,31) = 45.56, p< 0.0001, ηp2 = 0.60). The anterior N2 elicited by standard and rare stimuli did not differ (rare vs. standard: F(1,31) = 1.37, p> 0.24, ηp2 = 0.04). Additionally, there was an effect of electrode site, with the largest amplitude for the N2 at Cz (F(2,62) = 9.99, p< 0.001, ε = 0.77, ηp2 = 0.24; Cz > FCz ≈ Fz). There was no difference in the scalp distribution across stimulus types (Stimulus type x Electrode site interaction: F(4,124) = 1.03, p> 0.37, ε = 0.61, ηp2 = 0.03).
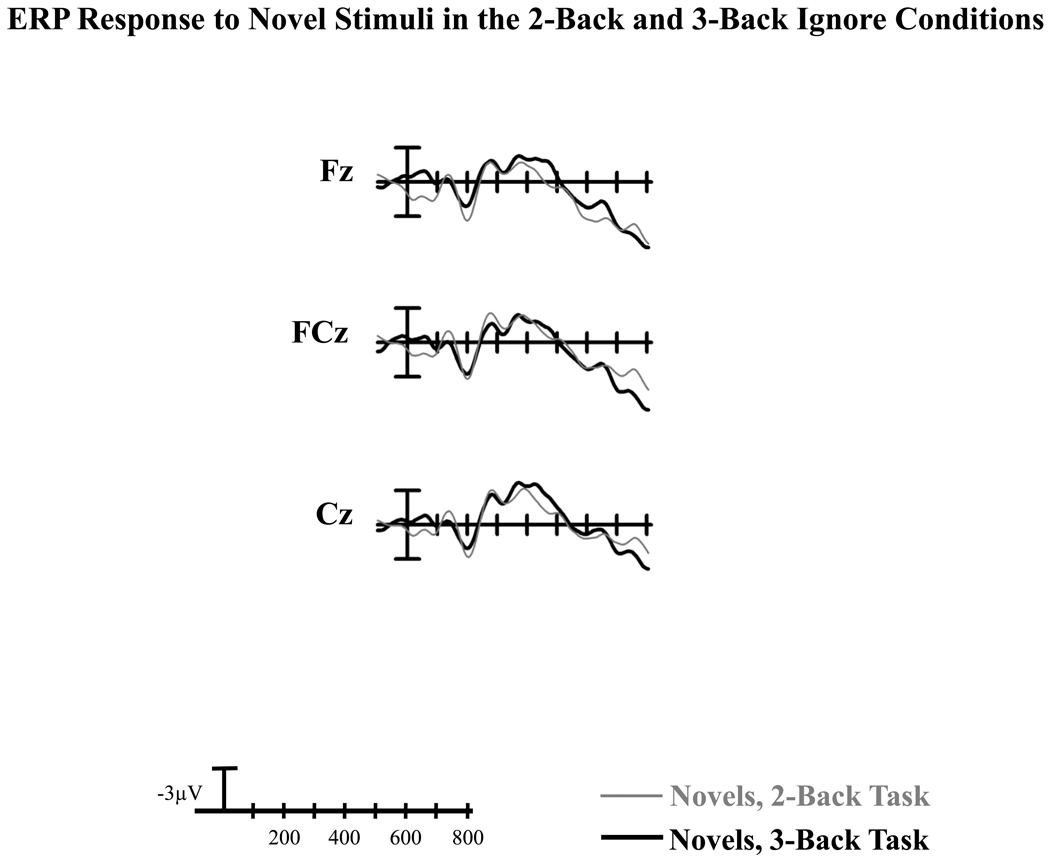
As illustrated in Figure 3, the amplitude of the anterior N2 to novel stimuli was not modified by n-back task difficulty (effect of 2-back vs. 3-back: F(1,31) = 0.19, p> 0.66, ηp2 < 0.01). Furthermore, there was no difference in the anterior N2 scalp distribution between the 2-back and 3-back tasks (N-back x Electrode site interaction: F(2,62) = 0.80, p> 0.44, ε = 0.96, ηp2 = 0.03).
Figure 3.
Midline grand average ERPs at frontocentral sites (Fz, FCz, Cz) in response to novel visual stimuli under the Ignore condition when subjects focused on the 2-back task vs. 3-back task in the auditory modality.
Effects of direction of attention (Attend vs. Ignore condition) on the anterior N2
In comparing the Attend and Ignore conditions, the most pertinent results involved interactions between stimulus type and condition (F(2,62) = 11.47, p< 0.0001, ε = 0.95, ηp2 = 0.27), and between condition and electrode site (F(2,62) = 15.03, p< 0.0001, ε = 0.73, ηp2 = 0.33). To better understand the nature of the interaction between stimulus type and condition, each stimulus type was examined separately across conditions. The interaction between condition and electrode site was present because the anterior N2 was more centrally distributed in the Ignore condition (Cz max) and more anteriorly distributed under the Attend condition (Fz/FCz max). The magnitude of this difference was similar for all stimulus types (Stimulus Type x Condition x Electrode site interaction: F(4,124) = 0.52, p> 0.59, ε = 0.50, ηp2 = 0.02).
Novel stimuli
The size of the anterior N2 to novel stimuli did not vary with the direction of attention (main effect of Condition: F(1,31) = 0.28, p> 0.60, ηp2 < 0.01). A condition by electrode site interaction (F(2,62) = 8.19, p< 0.005, ε = 0.67, ηp2 = 0.21) was present because there was no difference in the size of the anterior N2 to novel stimuli between conditions at Fz and FCz (effect of Condition, Fz: F(1,31) = 0.13, p> 0.71, ηp2 < 0.01; FCz: F(1,31) = 0.02, p>0.89, ηp2 < 0.01), but a trend towards a difference at Cz, with the N2 to novel stimuli under the Ignore condition being slightly larger than the N2 to novel stimuli under the Attend condition (F(1,31) = 3.40, p< 0.08, ηp2 = 0.10).
Target/Rare stimuli
The amplitude in the N2 latency range to target/rare stimuli was larger under the Ignore condition than under the Attend condition (effect of Condition: F(1,31) = 23.62, p< 0.0001, ηp2 = 0.43). A condition by electrode site interaction (F(2,62) = 3.74, p= 0.05, ε = 0.63, ηp2 = 0.11) was present because the magnitude of the effect of conditions was largest at Cz (effect of Condition, Fz: F(1,31) = 13.65, p< 0.001, ηp2 = 0.31; FCz: F(1,31) = 19.20, p< 0.001, ηp2 = 0.38; Cz: F(1,31) = 25.39, p< 0.0001, ηp2 = 0.41).
Standard stimuli
The amplitude in the N2 latency range to standard stimuli also was larger under the Ignore condition than under the Attend condition (F(1,31) = 6.83, p< 0.05, ηp2 = 0.18). A condition by electrode site interaction (F(2,62) = 9.01, p< 0.005, ε = 0.74, ηp2 = 0.23) was due to the fact that the anterior N2 to standard stimuli did not differ between conditions at Fz (F(1,31) = 1.35, p> 0.25, ηp2 = 0.04), but was larger under the Ignore condition than under the Attend condition at FCz and Cz (effect of Condition, FCz: F(1,31) = 5.33, p< 0.05, ηp2 = 0.15; Cz: F(1,31) = 15.09, p< 0.001, ηp2 = 0.33).
Effects of direction of attention (Attend vs. Ignore condition) on the P3
Novel stimuli
The size of the P3 to novel stimuli was larger under the Attend condition than under the Ignore condition (effect of Condition: F(1,31) = 23.92, p< 0.0001, ηp2 = 0.44). A condition by electrode site interaction (F(6,186) = 12.65, p< 0.0001, ε = 0.40, ηp2 = 0.29) was present because the magnitude of the effect of condition was largest posteriorly (CPz-Oz).
Target/Rare stimuli
The P3 response to target stimuli under the Attend condition was larger than that to rare stimuli under the Ignore condition (F(1,31) = 37.74, p< 0.0001, ηp2 = 0.55). A condition by electrode site interaction (F(6,186) = 15.23, p< 0.0001, ε = 0.53, ηp2 = 0.33) was present because the magnitude of the effect of condition was largest at posterior electrode sites (CPz/Pz).
Discussion
Although there is growing consensus that the anterior N2 component indexes the mismatch between a presented stimulus and representations held in memory, several issues continue to be debated. Our study sought to examine the extent to which early novelty processing, as measured by the anterior N2, is modulated by the direction of attention. To help assess the relative merits of competing theories, young adult subjects completed two experimental tasks that manipulated attention. Subjects were exposed to the same kinds of visual stimuli under both conditions (standard, target/rare, and perceptually novel), but under the Ignore condition, subjects directed attention to a difficult n-back task in the auditory modality, whereas under the Attend condition subjects focused attention on the visual stimuli in order to respond to designated targets.
As reviewed in the Introduction, we considered the outcomes that would be predicted by several competing hypotheses regarding the extent to which the anterior N2 is influenced by direction of attention. The results of our study are most supportive of the hypothesis that early novelty processing, as indexed by the anterior N2, is not strongly modulated by the direction of attention. First, there was no difference in the amplitude of the anterior N2 to novel visual stimuli between the Attend and Ignore conditions. Second, under the Ignore condition, the size of the anterior N2 component varied considerably across stimulus types, with only perceptually novel stimuli eliciting an enhanced response. Lastly, the anterior N2 to novel visual stimuli did not diminish as a function of task difficulty in the attended auditory channel.3 These results suggest that changing the attentional load did not impact the processing of visual novelty as indexed by the anterior N2 component.
In contrast to the anterior N2, the P3 response to novel visual stimuli did vary across conditions, with novel visual stimuli under the Attend condition eliciting a much larger P3 response than novel visual stimuli under the Ignore condition. This was consistent with our hypothesis and research in the field that the direction of attention strongly modulates the size of the P3 response. It strongly suggests that the N2 and P3 are indexing different aspects of novelty processing.
The results of this study are in keeping with previous findings in our lab (Chong et al., 2008; Daffner et al., 2000). For example, we have reported that unlike the P3 component, the anterior N2 response does not appear to be influenced by task relevance, but rather reflects a relatively automatic process. In the study by Chong and colleagues (2008), subjects completed two experimental tasks in the visual modality that had the same formal task requirements, but divergent instructions that manipulated the significance of novel stimuli. The anterior N2 did not vary across conditions. Similarly, in the current study, the amplitude of the N2 did not differ between two experimental tasks that contained the same kinds of visual stimuli, but in which attention was directed either to visual or auditory events.
Another difference between the current study and the one reported by Chong and colleagues (2008) was the way in which the task was paced. Whereas in the study done by Chong and colleagues subjects controlled how long they viewed the visual stimuli, in the current study, viewing time was predetermined. Nevertheless, in both cases, in contrast to the P3 component, the anterior N2 to novel visual stimuli was not modulated by stimulus significance or direction of attention. Our findings indicate that whether the task is self-paced or experimenter-paced, task-relevance and direction of attention affect the P3 component, but not the anterior N2, in response to novel visual stimuli.
The findings of the current study are not consistent with the argument made in the review article by Folstein and Van Petten (2008) that anterior N2 responses to visual stimuli are dependent upon focal attention to the eliciting stimuli. Our results can be partially reconciled with the observations made by Folstein and Van Petten (2008) by considering differences in the way in which the terms “novel stimuli” and “focal attention” are used. For instance, Folstein and Van Petten (2008) support their claim by citing the sequential matching tasks conducted by Fu et al. (2003) and Wang et al. (2004), who found that mismatches along task-relevant features of a stimulus enhanced the anterior N2 much more than those mismatches along task-irrelevant dimensions. The stimuli used by Fu et al. (2003) were circular square-wave gratings and the stimuli used by Wang et al. (2004) were familiar colored shapes. Thus, in both of these studies, the stimuli used were easily recognizable, and only “novel” in terms of deviation from the immediate context of the experiment.
In contrast to the studies described above, novel stimuli in our experiment were highly unusual/unfamiliar line drawings (e.g., impossible or fragmented objects). Rare stimuli were simple geometric figures (i.e., rectangle with the long side horizontal) that deviated from immediate context (i.e., frequent, repetitive ‘standard’ rectangles with the long side vertical). Rare stimuli were presented as infrequently as novel stimuli. However, under the Ignore condition, the anterior N2 in response to rare stimuli did not differ from that in response to standard stimuli, both of which were much smaller than the anterior N2 in response to novel stimuli. This suggests that infrequency of presentation was not critical to the elicitation of the anterior N2. Rather, the unusual/unfamiliar nature of the novel stimuli appears to be the source of the enhanced anterior N2, even under the Ignore condition.
A similar observation was made in an earlier study from our lab (Daffner et al., 2000), which examined factors that modulate the size of the anterior N2 component under Attend conditions. Infrequency of stimulus presentation and deviance from immediate context by simple geometric figures had limited influence, but deviance from long-term experience by highly unusual stimuli had the most marked impact on N2 amplitude. At the time, we suggested that the amplitude of the visual N2 was strongly linked to unfamiliarity or deviation from representations held in long term storage. Perhaps it is this aspect of novelty that elicits an anterior N2 response even under Ignore conditions. Some evidence suggests that unlike the processing of other stimulus types, there are only limited early selective attention effects for novel stimuli, which Woods (1992) speculated might reflect the fact that neurons responsive to novelty may be located outside of the sensory association cortices mediating selective attention. The existence of a neural mechanism to single out highly novel events, even when they occur outside of the focus of attention, would be consistent with the crucial role that detection of novelty is hypothesized to play in survival and the promotion of cognitive competence (Berlyne, 1960; Daffner et al., 2006; Daffner, Scinto, Weintraub, Guinessey, & Mesulam, 1994; Holdstock et al., 1995; Sokolov, 1963; Woods et al., 1993).
Another important difference between our study and the studies conducted by Fu et al. (2003) and Wang et al. (2004) involves the way in which focal attention was manipulated. In the studies cited, all stimuli were presented within the visual modality and focal attention involved which dimension (e.g., orientation vs. spatial frequency) was to be preferentially processed. In our investigation, focal attention involved which modality (auditory vs. visual) was to be preferentially processed. One could argue that because subjects had their gaze aimed at the middle of the monitor where visual stimuli were presented, it was impossible to ignore the occurrence of visual events. Although this is likely the case, it does not negate the fact that direction of attention varied substantially across the two conditions, but the size of the anterior N2 to novel visual stimuli did not. Moreover, gaze also was aimed at the rare visual stimuli under the Ignore condition. However, this stimulus type did not elicit a robust anterior N2 response. Taken together, these results suggest that the anterior N2 component is particularly sensitive to perceptually novel visual stimuli and is not enhanced by focal visual attention.
Although the amplitude of the anterior N2 did not differ between the Attend and Ignore conditions, its scalp distribution did. It was more centrally distributed under the Ignore condition (Cz max) and more anteriorly distributed under the Attend condition (Fz/FCz max). As reviewed below, there are at least two non-mutually exclusive explanations for this phenomenon. One possibility is that the anterior N2 may be an index of two different aspects of information processing, which are associated with divergent sources of neural activation that lead to different scalp distributions under the Attend and Ignore conditions. We favor an alternative explanation that the anterior N2 indexes only one basic cognitive operation whose scalp distribution varies as a function of overlapping ERP components that are differentially elicited under various conditions.
Consistent with the first explanation, Nittono et al. (2007) raise the possibility that the anterior N2 may represent two components that index somewhat different processes, one with a frontal, and the other a central scalp distribution. They suggest that the frontal N2 component may be particularly sensitive to stimulus unfamiliarity, whereas the central N2 component may be especially sensitive to stimulus complexity. In the context of our experiment, this would mean that under the Ignore condition, the N2 elicited by novel stimuli would be a marker of their complexity, whereas under the Attend condition, the N2 elicited by novel stimuli would be a marker of their unfamiliarity. If this were true, it would suggest that under the Ignore condition, the brain automatically registers the complexity of the novel stimuli, which elicits a central N2, but does not mark its degree of unfamiliarity (i.e., lack of a ready match to stored representations), an operation that may be more dependent upon the allocation of focal attention.
The alternative explanation, which we consider more likely, is that the differential scalp distribution of the anterior N2 between conditions reflects the impact of an overlapping P3 component under the Attend condition. Greater central-posterior positivity (from the P3 component) would reduce the size of the N2 response over those regions of the scalp, giving it a more frontally distributed appearance. Consistent with this view is the observation that the P3 response to novel visual stimuli was more posteriorly distributed under the Attend condition than under the Ignore condition. Additionally, the difference in N2 scalp distribution between conditions did not vary as a function of stimulus type (no interaction between condition, site, and stimulus type). Thus, the difference in scalp distribution under the Attend relative to the Ignore condition was not limited to the processing of novelty, but more likely reflected a non-specific effect of directing attention to the visual task. It has been well established that the P3 component is much larger under Attend than Ignore conditions (Chong et al., 2008; Knight & Scabini, 1998), which was observed in the current study.
Also of note, under the Ignore condition, the N2 to novel stimuli is followed by a small positive deflection (P3a) and subsequent negativity, the latter of which is not seen in the Attend condition. We suspect that the subsequent negativity may be absent under the Attend condition due to the overlapping P3b in the same temporal window. The functional significance of this subsequent negativity remains to be determined. We have suggested previously (Daffner et al., 1998) that it may be a remnant in young adults of the anterior Nc wave observed in younger children, which Courchesne (1978) suggested is elicited by potentially ‘attention-getting or interesting events,’ a description which aptly characterizes the novel stimuli in our paradigm. Alternatively, it may reflect what has been labeled the picture N300, which has been understood to indicate the degree of effort necessary to integrate nonverbal figural material into higher level conceptual representations (Daffner et al., 2000; Holcomb & McPherson, 1994; McPherson & Holcomb, 1999). The picture N300 is especially robust in response to visual stimuli (e.g. non-objects) that deviate from long-term prior experience, much like the unfamiliar novel stimuli used in the current study.
One concern about the current study’s findings of no difference in the amplitude of the anterior N2 component under the Attend and Ignore conditions is that they lead to support of a null hypothesis. There are several reasons why we do not believe that our results simply reflect a Type-II statistical error, and that if the study were better powered it would bring about the rejection of the null hypothesis in favor of its alternative that attention enhances the anterior N2. Of note, there was not even a suggestion of a weak trend toward a significant difference between conditions in the size of the anterior N2 response to novel stimuli, with the effect of condition having an F value of less than 1. There was an interaction between condition and electrode site that was due to a trend at Cz toward a difference between conditions. However, the effect was actually contrary to the hypothesis that the N2 is enhanced by attention, as the size of the N2 was smaller under the Attend than the Ignore condition.
Future investigations should address several other limitations in the current study. Although subjects were presented the same types of visual stimuli under both conditions, only the Ignore condition included exposure to auditory stimuli. We reduced the likelihood that the electrophysiological response to auditory events would have an impact on pertinent ERPs in response to the visual stimuli by varying the jitter between the presentation of auditory and visual stimuli (Woldorff, 1993). Although the influence of overlapping electrophysiologic activity remains a possibility, there is no reason to believe that it would affect one stimulus type more than the others. Also noteworthy is the fact that under the Attend condition all non-novel, rare stimuli were targets, which confounded ‘rareness’ with ‘targetness’. Future studies should include rare visual stimuli that are neither targets nor novels. The inclusion of this stimulus type would allow one to address whether attended rare stimuli, like those in the sequential matching task conducted by Wang and colleagues (2004), would elicit a larger anterior N2 than ignored rare stimuli. We would predict that perceptually novel stimuli would still generate a much larger anterior N2 response than attended simple rare stimuli. Moreover, if the scalp distribution of the anterior N2 did not differ between perceptually novel and simple rare stimuli, it would argue against the notion (Nittono et al., 2007) that an N2 component with a more central distribution is a marker of stimulus complexity. Although outstanding issues remain, the current study confirms the brain’s exquisite sensitivity to novelty, with robust early processing that occurs even in the absence of focal attention.
Acknowledgments
This research was funded in part by NIA grant 1R01 AGO17935 and by generous support from D. Wimberly and S. Muss. The authors thank Katie Gartner for her excellent administrative assistance.
Footnotes
Participants completed a neuropsychological test battery that included assessments of estimated IQ (AMNART (Ryan & Paolo, 1992)), global cognitive status (MMSE (Folstein, Folstein, & McHugh, 1975)), frontal-executive functioning (Digit Span, WAIS-III (Wechsler, 1997a)), Controlled Oral Fluency (COWAT (Ivnik, Malec, Smith, Tangalos, & Petersen, 1996)), semantic access (Category Fluency (animals) (Spreen & Strauss, 1998)), verbal and visual memory (Logical Memory II, WMS-III (Wechsler, 1997b)), Visual Retention Test (Youngjohn, Larrabee, & Crook, 1993), and language (Boston Naming Test (Tombaugh & Hubley, 1997)). Neuropsychological test scores were standardized using age-matched norms. On average, the sample had an AMNART estimated IQ of 119.2 (5.5). On tests of neuropsychological performance, the group had an overall composite percentile score of 59.3 (15.1) (based on the mean percentile score relative to age-matched norms for the Digit Span WAIS-III, Controlled Oral Fluency (COWAT), Category Fluency (animals), Logical Memory II WMS-III, Visual Retention Test, and Boston Naming Test).
When the anterior N2 elicited by standard and target/rare stimuli is discussed, it refers to the amplitude of the waveforms in the N2 latency range observed in response to these stimuli.
Although unlikely, it is possible that subjects performed better on the 2-back task than on the 3-back task as a result of a practice effect, and not task difficulty, as the 3-back task always preceded the 2-back task.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. 4th ed. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Barcelo F, Escera C, Corral MJ, Perianez JA. Task switching and novelty processing activate a common neural network for cognitive control. Journal of Cognitive Neuroscience. 2006;18:1734–1748. doi: 10.1162/jocn.2006.18.10.1734. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Perianez JA, Knight RT. Think differently: a brain orienting response to task novelty. NeuroReport. 2002;13:1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Berlyne D. Conflict, Arousal and Curiosity. New York, NY: McGraw-Hill; 1960. [Google Scholar]
- Chao LL, Nielsen-Bohlman L, Knight RT. Auditory event-related potentials dissociate early and late memory processes. Electroencephalography and Clinical Neurophysiology. 1995;96:157–168. doi: 10.1016/0168-5597(94)00256-e. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb PJ, Daffner KR. To ignore or explore: top-down modulation of novelty processing. J.Cogn Neurosci. 2008;20:120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Calvo V, Faust R, Scinto LFM, Holcomb PJ. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000;37:737–747. [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Cohen LG, Kennedy BP, West WC, et al. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. NeuroReport. 1998;9:787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, et al. Increased responsiveness to novelty is associated with successful cognitive aging. Journal of Cognitive Neuroscience. 2006;18:1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LFM, Weintraub S, Guinessey J, Mesulam MM. The impact of aging on curiosity as measured by exploratory eye movements. Archives of Neurology. 1994;51:368–376. doi: 10.1001/archneur.1994.00540160062009. [DOI] [PubMed] [Google Scholar]
- Dale AM. Dissertation Abstracts International 55-07B. 1994. Source localization and spatial discriminant analysis of event-related potentials: linear approaches (brain cortical surface) p. 2559. [Google Scholar]
- Donchin E. Surprise!…surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: the variation in event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10:590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Detecting novelty and significance. Journal of Cognitive Neuroscience. 2010;22:404–411. doi: 10.1162/jocn.2009.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C, Rose SA. Novelty and conflict in the categorization of complex stimuli. Psychophysiology. 2008;45:467–479. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ford JM. Does P300 reflect template match/mismatch? In: Otto DA, editor. Multidisciplinary Perspectives in Event-Related Brain Potential Research. Washington, DC: U.S. Government Printing Office; 1978. pp. 181–183. [Google Scholar]
- Fu S, Fan S, Chen L. Event-related potentials reveal involuntary processing of orientation changes in the visual modality. Psychophysiology. 2003;40:770–775. doi: 10.1111/1469-8986.00077. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annual Review of Psychology. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, McPherson WB. Event-related brain potentials reflect semantic priming in an object decision task. Brain Cogn. 1994;24:259–276. doi: 10.1006/brcg.1994.1014. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Rugg MD. The effect of attention on the P300 deflection elicited by novel sounds. Journal of Psychophysiology. 1995;9:18–31. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Johnson R. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints: a PET investigation. Brain. 1994;117:1055–1071. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: a comparison of lexical, object and reality decisions. Journal of Verbal Learning and Verbal Behavior. 1984;23:39–66. [Google Scholar]
- McPherson WB, Holcomb PJ. An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology. 1999;36:53–65. doi: 10.1017/s0048577299971196. [DOI] [PubMed] [Google Scholar]
- Naatanen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Nittono H, Shibuya Y, Hori T. Anterior N2 predicts subsequent viewing time and interest rating for novel drawings. Psychophysiology. 2007;44:687–696. doi: 10.1111/j.1469-8986.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, McGinnnis S, Tarbi E, Sun X, Holcomb PJ, et al. Age-related changes in early novelty processing as measured by ERPs. Biological Psychology. 2009;82:33–44. doi: 10.1016/j.biopsycho.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6:53–62. [Google Scholar]
- Sokolov EN. Higher nervous functions: the orienting reflex. Annual Review of Physiology. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd ed. vols. 599. New York: Oxford University Press; 1998. [Google Scholar]
- Squires KC, Hillyard SA, Lindsay PH. Vertex potentials evoked during auditory signal detection: relation to decision criteria. Perceptual Psychophysiology. 1973;14:25–31. [Google Scholar]
- Tombaugh TN, Hubley AM. The 60-item Boston Naming test: norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19:922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui L, Wang H, Tian S, Zhang X. The sequential processing of visual feature conjunction mismatches in the human brain. Psychophysiology. 2004;41:21–29. doi: 10.1111/j.1469-8986.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. WAIS-III. Administration and Scoring Manual. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. WMS-III. Administration and Scoring Manual. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Woods DL, Alho K, Algazi A. Intermodal selective attention. I. Effects on event-related potentials to lateralized auditory and visual stimuli. Electroencephalography and Clinical Neurophysiology. 1992;82:341–355. doi: 10.1016/0013-4694(92)90004-2. [DOI] [PubMed] [Google Scholar]
- Woods DL, Knight RT, Scabini D. Anatomical substrates of auditory selective attention: behavioral and electrophysiological effects of posterior association cortex lesions. Cognitive Brain Research. 1993;1:227–240. doi: 10.1016/0926-6410(93)90007-r. [DOI] [PubMed] [Google Scholar]
- Youngjohn JR, Larrabee GJ, Crook TH. New adult- and education-correction norms for the Benton Visual Retention Test. The Clinical Neuropsychologist. 1993;7:155–160. doi: 10.1080/13854049308401517. [DOI] [PubMed] [Google Scholar]