Abstract
The Neurofibromatosis-2 (NF2) tumor suppressor merlin negatively regulates cell proliferation in numerous cell types. We have previously shown that the NF2 protein (merlin/schwannomin) associates with mixed lineage kinase 3 (MLK3), a mitogen-activated protein kinase (MAPK) kinase kinase that is required for the proliferation of normal and neoplastic cells. In the current study, we show that merlin inhibits MLK3 activity as well as the activation of its downstream effectors, B-Raf, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK). The ability of merlin to regulate MLK3 activity requires a direct association between MLK3 and residues in the C-terminal region of merlin. Merlin integrates Rho GTPase family signaling with MAPK activity by inhibiting the binding between MLK3 and its upstream activator, Cdc42. Furthermore, we demonstrate that MLK3 is required for merlin suppression of cell proliferation and invasion. Collectively, these results establish merlin as a potent inhibitor of MLK3, ERK and JNK activation in cancer, and provide a mechanistic link between deregulated MAPK and Rho GTPase signaling in NF2 growth control.
Keywords: MLK3, NF2, MAPK, B-Raf, Cdc42
Introduction
Mitogen activated protein kinase (MAPK) signaling regulates diverse cellular processes, including proliferation, survival, inflammation, and metabolism in normal cells; and deregulated MAPK activity is a hallmark of numerous human cancers (Gollob et al., 2006; Kyriakis and Avruch, 2001). One key regulator of MAPK activity in response to mitogenic stimuli is mixed lineage kinase 3 (MLK3), a serine/threonine MAPK kinase kinase (MAP3K) (Chadee and Kyriakis, 2004a; Gallo and Johnson, 2002). MLK3 is required for mitogen-activation of B-Raf and ERK/MAPK signaling and MAPK-dependent cell proliferation in both normal cells and neoplastic cells (Chadee and Kyriakis, 2004b). A central role of MLK3 in tumorigenesis is underscored by the observation that stable expression of wild-type MLK3 transforms NIH3T3 cells in a MEK-dependent manner (Hartkamp et al., 1999). In addition, mutations in the kinase domain of MLK3 have recently been identified in gastrointestinal tumors, and are transforming in vitro (Velho et al., 2010).
MLK3 kinase activity is negatively regulated by autoinhibition through an interaction between the N-terminal SH3 domain and proline residue 495; and positively regulated by the Rho GTPases, Rac and Cdc42 (Du et al., 2005; Gallo and Johnson, 2002; Teramoto et al., 1996; Vacratsis and Gallo, 2000). Active Cdc42 binds to the Cdc42/Rac interactive binding (CRIB) domain of MLK3, which leads to MLK3 localization to the plasma membrane, and relief of SH3-mediated autoinhibition (Du et al., 2005; Zhang and Gallo, 2001). Autophosphorylation of Thr277 and Ser281 residues within the activation loop of the kinase domain leads to full activation of MLK3 kinase activity (Leung and Lassam, 2001).
Merlin, the product of the Neurofibromatosis-2 (NF2) gene, is a tumor suppressor protein, which functions as a negative regulator of cell proliferation and motility (Fraenzer et al., 2003; Ikeda et al., 1999; Lutchman and Rouleau, 1995; McClatchey et al., 1997; Sherman et al., 1997; Xiao et al., 2005). Merlin belongs to the family of ezrin, radixin, moesin (ERM) proteins, which link integral membrane proteins to the actin cytoskeleton (Ramesh, 2004; Sun et al., 2002). Loss of function NF2 mutations result in increased cell proliferation and motility in numerous distinct cell types, and culminate in the development of brain tumors (meningiomas) and peripheral nerve tumors (schwannomas) (Reed and Gutmann, 2001).
Since the identification of the NF2 gene in 1993, many investigators have examined the physiological targets of merlin important for its ability to suppress cell growth and motility (Fernandez-Valle et al., 2002; Goutebroze et al., 2000; Jannatipour et al., 2001; Obremski et al., 1998; Scoles et al., 2000). Of the many implicated targets, merlin has been shown to suppress anchorage-independent growth by modulating Rac activity (Kissil et al., 2003; Shaw et al., 2001). However, other reports have shown that merlin growth control involves MAPK signaling (Chadee et al., 2006; Morrison et al., 2007). The relationship between MAPK signaling, Rho GTPase function, and merlin growth suppression is not clear.
We previously reported that merlin associates with MLK3 and inhibits the interaction between MLK3 and B-Raf (Chadee et al., 2006). However, the mechanism by which merlin regulates MLK3 activity has not been defined. Here we demonstrate that merlin inhibits MLK3 kinase activity through a direct association between MLK3 and the C-terminal region of merlin. This association impairs MLK3 activation by blocking the interaction between the Rho GTPase, Cdc42, and MLK3. In addition, we show that merlin knockdown increases endogenous MLK3, B-Raf, ERK and JNK activities, whereas merlin overexpression reduces MLK3, B-Raf, ERK and JNK activities. Furthermore, the increased cell proliferation and invasion, resulting from merlin loss, is ameliorated by MLK3 knockdown. These results establish a requirement for MLK3 in merlin-mediated suppression of B-Raf, ERK and JNK signaling and cell proliferation; and provide a causative mechanistic link between deregulated MAPK and Rho GTPase signaling in NF2 growth control.
Results
Merlin expression and MLK3 activation are inversely correlated in human tumor cell lines
To explore the regulation of MLK3 by merlin in human cancers, we analyzed the levels of merlin and MLK3 protein expression in different tumor-derived cell lines. In all tumor cells analyzed, MLK3 protein levels were similar; however, merlin levels varied (Figures 1a and b). Merlin was detected in NCIH460 lung cancer, HT29 colon cancer, MCF7 breast cancer, but was low or undetectable in HEI193 NF2 schwannoma cell line (expressing a loss of function splice variant of merlin isoform 3) and SKOV3 ovarian cancer cells. In addition, using an activation state, phospho-specific MLK3 antibody (Thr277/Ser281; p-MLK3), high basal levels of p-MLK3 were observed in NCIH460, HEI193, and SKOV3 cells. Interestingly, with the exception of NCIH460 cells, tumor cells with high levels of basal p-MLK3 consistently had low or undetectable levels of merlin (Figures 1a and b). Merlin immunoblots have two distinct bands. The slower migrating form of merlin is phosphorylated on Ser518 and is inactive with respect to growth suppression and the faster migrating (lower) band is the active hypophosphorylated (Ser518) form. Interestingly, in NCIH460 cells, which have high levels of p-MLK3, merlin protein is almost exclusively present in the inactivated form. Thus, similar to the other cell lines analyzed, elevated p-MLK3 levels correlated with reduced levels of functional merlin in NCIH460 cells. It has been previously demonstrated that merlin levels increase with cell confluence (Lee et al., 2006; Morrison et al., 2001). This was evident in SKOV3 cells that had a higher level of merlin and significantly less p-MLK3 in confluent cells (Figure 1b) in comparison to cells harvested at low confluence (Figure 1a). This result suggests that even though merlin levels increased with SKOV3 cell confluence, the inverse relationship between merlin levels and MLK3 kinase activity remained consistent. This finding that tumor cells with low levels of merlin expression have elevated MLK3 activity suggests that merlin might negatively regulate MLK3.
Figure 1.
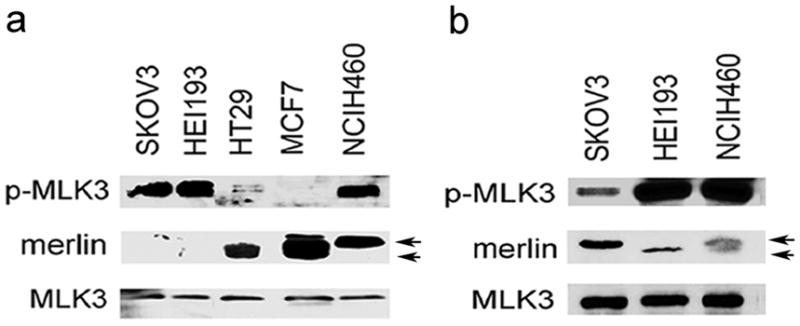
Reduced levels of merlin and increased MLK3 activity in human tumor cell lines. (a and b) Whole cell extracts from tumor cells were prepared at low confluence (panel a) or confluent (panel b) and subjected to Western blotting with p-MLK3 (Thr277/Ser281) antibody which detects active MLK3 enzyme. Cell extracts were also subjected to immunoblotting with MLK3 and merlin antibodies. Arrows indicate Ser518 hyperphosphorylated (upper band) or hypophosphorylated (lower band) forms of merlin.
The C-terminus of merlin interacts with MLK3 and inhibits MLK3 kinase activity
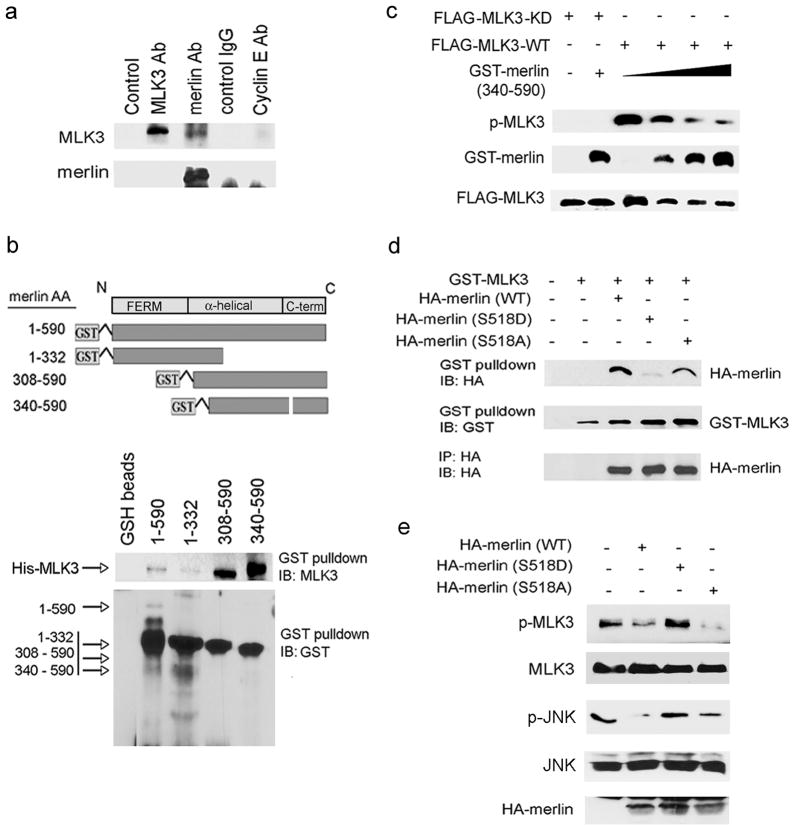
Previously, we observed an association between MLK3 and merlin (residues 1-595) when both proteins were overexpressed in HEK293 cells (Chadee et al., 2006). Here, we demonstrate that endogenous MLK3 and merlin are also associated in situ and co-immunoprecipitate from human TOV21G ovarian tumor cells (Figure 2a). To identify the region of merlin required for the MLK3-merlin interaction, and to determine whether merlin interacts directly with MLK3, in vitro binding assays were performed with GST-merlin deletion mutants (Figure 2b) and His-MLK3 proteins purified from bacteria. Merlin has an N-terminal FERM domain (residues 1-302), a central coiled-coil alpha helical region (residues 303–478) and a unique C-terminal domain (residues 479–595) (Sun et al., 2002). Merlin isoform 2 is generated by alternative splicing and gives rise to a 590 residue protein that differs by 11 residues at the C-terminus (residues 580–590) (Sun et al., 2002). GST-merlin (residues 1–590) or deletion mutants (residues 1–332, residues 308–590 and residues 340–590) (Figure 2b) were incubated with full length His-MLK3 in vitro. GST pulldown assays revealed that GST-merlin (residues 1-590) bound to His-MLK3. MLK3 also binds to merlin isoform 1 (residues 1-595; data not shown). The deletion mutants that contain the alpha helical region and the C-terminus (residues 340–590 and residues 308–590) also bound to His-MLK3; however, the N-terminal deletion mutant (residues 1–332) did not bind (Figure 2b). Together, these results indicate a direct interaction between merlin and MLK3 requiring residues 340–590 in the C-terminal region of merlin. To determine whether the C-terminal region of merlin that bound to MLK3 was sufficient for the inhibition of MLK3 activity, increasing quantities of purified GST-merlin (residues 340–590) were incubated with FLAG-MLK3-wild-type (WT) or a FLAG-MLK3-kinase dead (KD) K144R mutant, and a MLK3 kinase assay was performed. FLAG-MLK3-KD was not detected by the p-MLK3 antibody, which verifies the specificity of the antibody for active MLK3 enzyme. Incubation of FLAG-MLK3-WT with GST-merlin (residues 340–590) dramatically reduced p-MLK3 levels (Figure 2c), suggesting that residues 340–590 in the C-terminus are sufficient for the direct inhibition of MLK3 activity by merlin.
Figure 2.
The C-terminus of merlin interacts with MLK3 and inhibits MLK3 kinase activity. (a) Endogenous merlin was immunoprecipitated from TOV21G cell lysates and associated endogenous MLK3 was detected with MLK3 antibody. Control immunoprecipitations were performed with rabbit IgG and Cyclin E antibodies. (b) Diagram of merlin GST-fusion proteins (upper panel). Bacterial purified His-MLK3 was incubated with GST-merlin deletion mutants in an in vitro binding assay (lower panel). GST-pulldowns were performed and associated His-MLK3 and GST-merlin proteins were detected with the indicated antibodies. (c) MLK3 kinase assays were performed with immunopurified FLAG-MLK3 protein expressed in HEK293 cells and C-terminal GST-merlin mutant residues 340–590 (0.35–1.25 μg), expressed and purified from bacteria. GST-merlin and FLAG-MLK3 protein expression was verified with the indicated antibodies. (d) GST-MLK3 and HA-merlin (wild-type, S518D and S518A) were overexpressed in HEK293 cells. GST pulldowns were performed and HA-merlin and GST-MLK3 proteins in the pulldowns were detected by Western blotting with the indicated antibodies. (e) HA-merlin (wild-type, S518D and S518A) were overexpressed in SKOV3 cells and cell extracts were immunoblotted with the indicated antibodies. IB, immunoblot; IP, immunoprecipitate.
Phosphorylation of merlin on Ser518 inhibits merlin intramolecular interactions and inactivates merlin-dependent growth suppressive activity (Rong et al., 2004). Thus, we sought to determine whether phosphorylation of merlin impaired its interaction with MLK3. The interaction between MLK3 and merlin was evaluated using wild-type merlin and mutant merlin proteins with either a Ser to Ala substitution at residue 518 (S518A) that cannot be phosphorylated, or a Ser to Asp substitution at residue 518 (S518D) that is phosphomimetic. Wild-type and S518A merlin, but not the S518D mutant, co-immunoprecipitated with MLK3 in HEK293 cells (Figure 2d). Furthermore, exogenous wild-type and S518A merlin, but not the S518D mutant substantially reduced p-MLK3 and p-JNK levels in SKOV3 cells. These results indicate that phosphorylation of merlin on Ser518 impairs the interaction between merlin and MLK3 and the inhibition of MLK3 activity by merlin.
Merlin inhibits MLK3, B-Raf, ERK and JNK activation
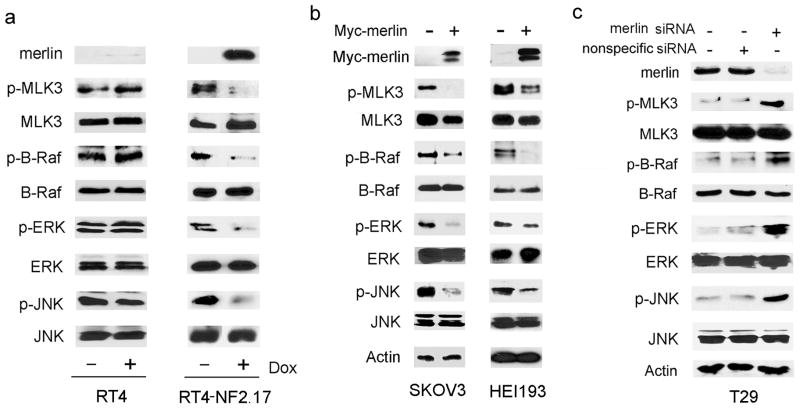
To determine if merlin negatively regulates endogenous MLK3 kinase activity in schwann cells, we induced merlin expression in RT4-NF2.17 rat schwannoma cells that have doxycylcine-inducible expression of wild-type merlin and a low level of endogenous merlin. Induction of merlin expression in these cells significantly reduced the basal levels of p-MLK3 and phosphorylated JNK (p-JNK) with no effect on total MLK3 or total JNK protein levels. Furthermore, induction of merlin expression reduced the basal levels of B-Raf phosphorylation (activation) on residues Thr599/Ser601 (p-B-Raf), and phosphorylated ERK (p-ERK) with no effect on total B-Raf or total ERK protein levels (Figure 3a, right panel). Treatment of control RT4 cells that do not express an inducible merlin with doxycycline had no effect on total and phosphorylated MLK3, B-Raf, ERK and JNK levels (Figure 3a, left panel). These data suggest that merlin is a potent inhibitor of endogenous MLK3, B-Raf, ERK and JNK kinase activities in schwann cells. Next, we wished to test the effect of expression of functional merlin in SKOV3 and HEI193 cells that have reduced or inactive merlin and elevated levels of p-MLK3. Consistent with the results obtained for RT4-NF2.17 cells, overexpression of merlin in SKOV3 and HEI193 cells significantly reduced the levels of p-MLK3, p-B-RAF, p-ERK and p-JNK (Figure 3b).
Figure 3.
Merlin inhibits MLK3, B-Raf, ERK and JNK activities. (a) RT4 (control, left panel) and RT4-NF2.17 (inducible merlin, right panel) cells were treated with 1.0 μg/ml doxycycline for 24 h and cell extracts were analyzed by Western blotting. (b) SKOV3 and HEI193 cells were transfected with Myc-merlin and cell extracts were immunoblotted with the indicated antibodies. (c) T29 cells were transfected with nonspecific or merlin siRNA oligos and cell extracts were analyzed by Western blotting with the indicated antibodies.
Merlin expression in SKOV3 ovarian tumor cells inhibited MLK3, B-Raf, ERK and JNK activities. We wished to test if loss of merlin in immortalized ovarian epithelial cells that have functional merlin, would elevate MLK3, B-Raf, ERK and JNK activities. Merlin was depleted from T29 immortalized ovarian epithelial cells by siRNA-mediated knockdown and p-MLK3, p-B-Raf, p-ERK and p-JNK levels were analyzed (Figure 3c). In T29 cells treated with merlin siRNA, p-MLK3, p-B-Raf, p-ERK and p-JNK levels were increased compared to control cells treated with nonspecific siRNA, supporting our findings above that merlin negatively regulates MLK3, B-Raf, ERK and JNK activation.
Merlin inhibits binding of Cdc42 to MLK3
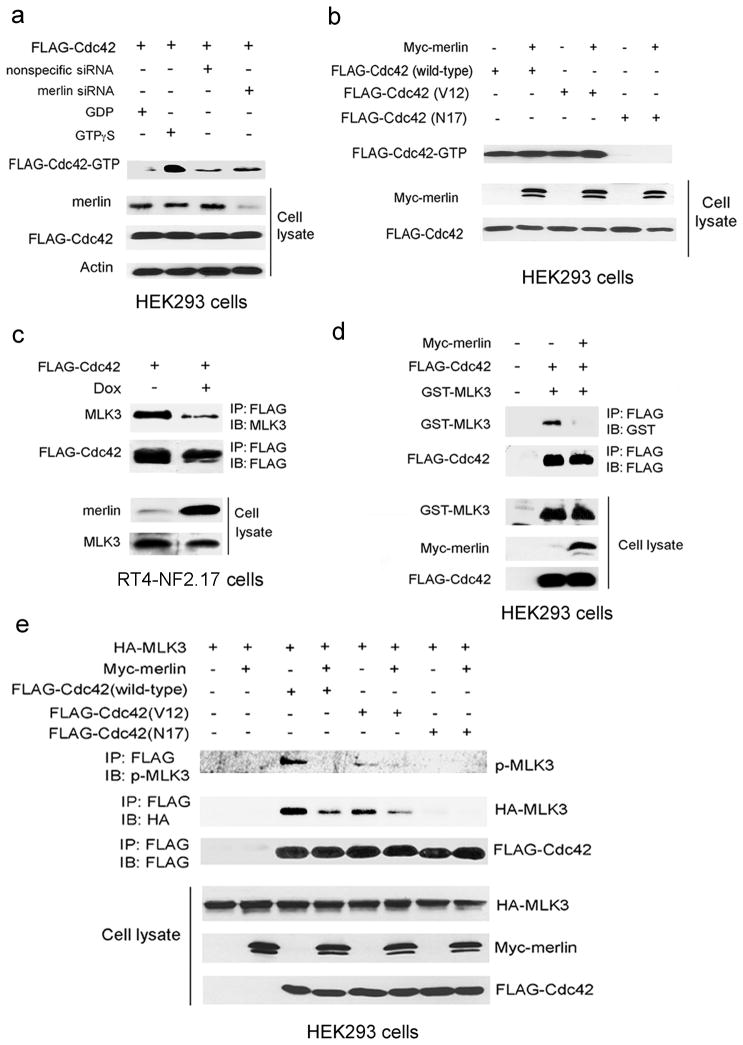
Cdc42 is a key upstream regulator of MLK3 activity, such that binding of active Cdc42 to the MLK3 CRIB domain relieves MLK3 autoinhibition and allows MLK3 autophosphorylation and activation (Du et al., 2005). The effect of merlin on Cdc42 activity is unknown. To determine whether merlin inhibits MLK3 indirectly by inhibiting Cdc42 activity, FLAG-Cdc42 activity was analyzed in HEK293 cells treated with merlin siRNA (Figure 4a). Active FLAG-Cdc42 was isolated from cell extracts with Pak-1 binding domain (PDB) agarose beads. Cdc42 bound to PBD beads in control cell lysates pre-incubated with GTPγ S but not GDP, indicating that the PBD beads bound only to active FLAG-Cdc42-GTP (Figure 4a). The amount of FLAG-Cdc42-GTP isolated from cells transfected with nonspecific siRNA or with merlin siRNA was similar, indicating that merlin expression does not affect Cdc42 activity (Figure 4a). A similar assay was performed to determine the effect of overexpression of Myc-merlin on FLAG-Cdc42 activity (Figure 4b). Active FLAG-Cdc42-GTP was detected bound to PBD beads in cells expressing FLAG-Cdc42-WT or dominant active FLAG-Cdc42-V12 (Figure 4b). As a negative control, cells were also transfected with dominant negative FLAG-Cdc42-N17, which does not bind to the PBD. Overexpression of Myc-merlin did not alter the levels of GTP bound FLAG-Cdc42-WT or constitutively active FLAG-Cdc42-V12 (Figure 4b). Taken together these data suggest that merlin suppression of MLK3 activity does not occur through inhibition of Cdc42 activity. An alternative possibility is that merlin blocks MLK3 activation by hindering the interaction between MLK3 and active Cdc42. To test this possibility, merlin expression was induced in RT4-NF2.17 cells and the effect on Cdc42-MLK3 binding was analyzed. Induction of merlin expression caused a dramatic reduction in the interaction between endogenous MLK3 and FLAG-Cdc42 in RT4-NF2.17 cells (Figure 4c). A similar reduction in Cdc42-MLK3 binding was observed when GST-MLK3 and FLAG-Cdc42 were co-expressed with Myc-merlin in HEK293 cells (Figure 4d). Myc-merlin expression led to a substantial decrease in the amount of total and phosphorylated HA-MLK3 immunoprecipitated with wild-type FLAG-Cdc42 in HEK293 cells (Figure 4e). The amount of MLK3 in FLAG-Cdc42-V12 immunoprecipitates was less than in wild-type FLAG-Cdc42 immunoprecipitates, and may be a result of Cdc42-V12-mediated relocalization of MLK3 to the plasma membrane (Du et al., 2005). Nevertheless, merlin expression also significantly reduced the amount of total and phosphorylated MLK3 immunoprecipitated with FLAG-Cdc42-V12. Collectively, these findings indicate that merlin inhibits Cdc42-mediated activation of MLK3 by blocking the Cdc42-MLK3 interaction.
Figure 4.
Merlin regulates MLK3 activity by inhibiting the Cdc42-MLK3 interaction. (a) HEK293 cells were transfected with FLAG-Cdc42 and nonspecific or merlin siRNA. GTP-bound FLAG-Cdc42 was isolated from cell lysates with Pak-1 binding domain (PBD) beads and detected with FLAG antibody. For control samples, cell lysates were incubated with GTP γS or GDP prior to the addition of PBD beads. Protein expressions were verified by Western blotting with the indicated antibodies. (b) HEK293 cells were transfected with FLAG-Cdc42 (wild-type, V12 or N17) with or without Myc-merlin. GTP-bound FLAG-Cdc42 was isolated from cells lysates with PBD beads and detected with FLAG antibody. Western blotting of cell extracts was performed with FLAG and Myc antibodies to verify protein expression. (c) FLAG-Cdc42 was overexpressed in RT4-NF2.17 cells. Cells were untreated or treated with 1.0μg/ml doxcycline for 24 h to induce merlin expression. FLAG-Cdc42 immunoprecipitates were analyzed by Western blotting with the indicated antibodies. (d) Myc-merlin, FLAG-Cdc42, and GST-MLK3 were overexpressed in HEK293 cells. FLAG-Cdc42 immunoprecipitates and cell lysates were analyzed by Western blotting with the indicated antibodies. (e) Myc-merlin, FLAG-Cdc42 (wild-type, V12, or N17), and HA-MLK3 were overexpressed in HEK293 cells. FLAG-Cdc42 immunoprecipitates and cell extracts were analyzed by Western blotting with the indicated antibodies. IB, immunoblot; IP, immunoprecipitate.
MLK3 is required for merlin-mediated growth suppression
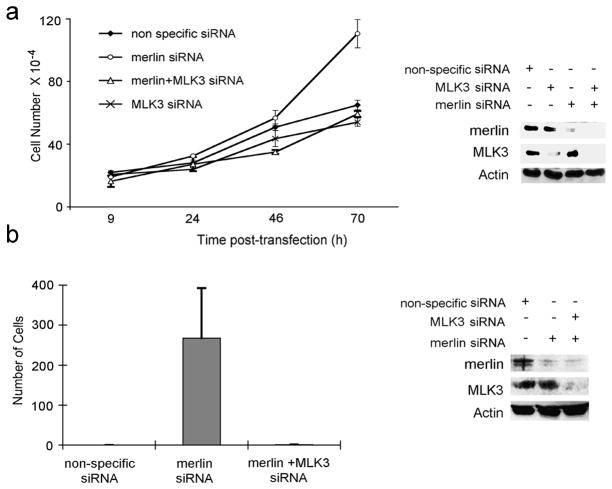
Whereas MLK3 knockdown inhibits normal and tumor cell proliferation, merlin loss leads to increased proliferation (Chadee and Kyriakis, 2004b; Morrison et al., 2007; Okada et al., 2005; Xiao et al., 2005). To determine if merlin suppression of cell proliferation requires MLK3 in normal ovarian cells, cell proliferation was measured in T29 cells that were treated with merlin, MLK3, or MLK3 and merlin siRNA. Cells with merlin knockdown had increased rates of proliferation, whereas cells with MLK3 knockdown had reduced cell proliferation rates compared to cells transfected with nonspecific siRNA (Figure 5a). MLK3 suppression in merlin knockdown cells reversed the increased cell growth phenotype observed in merlin knockdown cells, suggesting that MLK3 is required for merlin-dependent growth suppression in ovarian epithelial cells.
Figure 5.
MLK3 is required for merlin suppression of cell proliferation and invasiveness. (a) T29 cells were transfected with nonspecific, human merlin, human MLK3, or human merlin plus human MLK3 siRNA. Cell proliferation was determined by counting cells at various time intervals after transfection. Experiments were performed in triplicate and repeated three times. Cell extracts were prepared from cells at each time interval and analyzed by Western blotting with the indicated antibodies. (b) SW10 cells were transfected with nonspecific siRNA oligo, merlin siRNA, or both MLK3 and merlin siRNAand cell invasiveness was assessed using a modified Boyden chamber. Cell extracts were analyzed by Western blotting with the indicated antibodies.
Next we wished to determine if MLK3 is required, in normal schwann cells, for merlin-mediated suppression of cellular invasion. Merlin and MLK3 were depleted in normal SW10 murine schwann cells using siRNA, and cellular invasiveness was analyzed by invasion assays using an artificial basement membrane (Figure 5b). Consistent with previous reports, depletion of merlin led to a significant increase in cell invasion (Figure 5b) (Poulikakos et al., 2006), which was reversed when both merlin and MLK3 were depleted. These results indicate that MLK3 is also required for merlin-dependent suppression of invasion.
Discussion
In this report, we demonstrated an inverse relationship between merlin expression and MLK3 activity in schwannoma, ovarian and lung tumor cell lines, and showed that merlin regulates MLK3 activity. Moreover, we demonstrate that merlin growth suppression requires MLK3 activation. Merlin inhibits MLK3 activation by directly interfering with MLK3 binding to Cdc42, a major MLK3 activator; thus providing a critical link between previous reports describing merlin suppression of Rac1 or MAPK activity (Chadee et al., 2006; Morrison et al., 2007).
MLK3 is required for cell proliferation in normal and neoplastic cells, and a number of studies have demonstrated that exogenous expression of members of the MLK family is sufficient to promote neoplastic transformation (Cho et al., 2004; Hartkamp et al., 1999; Velho et al., 2010). However, deregulated MLK3 kinase activity in tumor-derived cells has not been previously reported. Here we report that SKOV3, NCIH460 and HEI193 tumor cells have elevated basal MLK3 kinase activity, suggesting that MLK3 kinase activity is deregulated in these cells. Interestingly, these cells also have reduced levels of functional merlin. Our results demonstrate that merlin and MLK3 are associated in situ and the C-terminal residues 340–590 of merlin directly interact with MLK3. This region of merlin is also sufficient for the direct inhibition of MLK3 kinase activity in vitro. Furthermore, an inactive mutant of merlin (S518D) exhibited markedly reduced binding to MLK3 and could not inhibit MLK3 activity in comparison to wild-type merlin, indicating that only active merlin can bind to MLK3 and inhibit MLK3 activity. Merlin is activated upon dephosphorylation of Ser518 by the myosin phosphatase MYPT-1-PP1đ (Jin et al., 2006). MYPT1 is inhibited by the oncoprotein CPI-17, and altered CPI-17 protein levels in tumor cells leads to deregulation of MYPT-1-PP1đ activity and increased levels of phosphorylated, inactive merlin (Jin et al., 2006). Possibly, inactivation of merlin through this mechanism could also elevate the level of active MLK3 in cells. We propose that elevated basal MLK3 activity in tumor cells could be due to a loss of negative regulation of MLK3 by merlin.
MLK3 interacts with B-Raf and facilitates B-Raf activation; and merlin expression disrupts the MLK3-B-Raf interaction (Chadee et al., 2006). Our current results indicate that silencing merlin elevates B-Raf and ERK activities in normal ovarian epithelial cells and overexpression of merlin blocks B-Raf and ERK activities in ovarian tumor cells. Possibly, loss of functional merlin promotes MLK3-B-Raf complex formation, thereby facilitating ERK activation in SKOV3 ovarian tumor cells. In addition, we observed that silencing merlin in normal T29 ovarian epithelial cells significantly elevated MLK3 and JNK kinase activities. MLK3 directly phosphorylates and activates the MAP2K, MKK4/SEK1, which in turn phosphorylates and activates JNK (Rana et al., 1996). Thus, deregulation of MLK3 activity, resulting from loss of functional merlin, may cause persistent JNK signaling which can also promote cellular transformation and tumorigenesis (Khatlani et al., 2007; Rennefahrt et al., 2004; Rennefahrt et al., 2002). Consistent with this hypothesis, we observed that merlin suppression of cell growth and invasiveness is dependent on MLK3.
It has been previously reported that merlin inhibits both Rac1 and MAPK signaling through different mechanisms (Chadee et al., 2006; Morrison et al., 2007). MLK3 is a critical mediator in Cdc42 activation of MAPK signaling. (Du et al., 2005; Teramoto et al., 1996; Vacratsis and Gallo, 2000). Our results demonstrate that merlin inhibits MLK3, B-Raf, ERK and JNK activation; and blocks Cdc42 binding to MLK3, providing a novel mechanistic link by which merlin can suppress both Rho-GTPase and MAPK signaling. We propose a model where merlin directly binds to and inhibits MLK3 activation by inhibiting the Cdc42-MLK3 interaction. In schwannoma and ovarian tumor cells, loss of functional merlin elevates MLK3, ERK and JNK signaling by allowing the formation of Cdc42-MLK3 and B-Raf-MLK3 signaling complexes.
Collectively, our data implicate merlin as a critical regulator of MLK3 function. Loss of functional merlin and enhanced MLK3-dependent signaling could be early events in cellular transformation and tumorigenesis in schwann, ovarian and other cell types. Inhibiting MLK3 is effective in slowing the growth of neoplastic cells and targeting MLK3 could be beneficial to the treatment of human tumors that lack functional merlin.
Materials and methods
Cell lines
Human embryonic kidney (HEK293), colon cancer (HT29), ovarian cancer (SKOV3 and TOV21G), lung cancer (NCIH460), breast cancer (MCF7), and mouse normal schwann (SW10) cells were obtained from the American Type Culture Collection, Manassas, VA, USA. RT4 is a rat schwannoma cell line. The RT4-NF2.17 cell line was derived from RT4 cells, and have been engineered to express a tetracycline-inducible NF2 gene (Gutmann et al., 2001). HEI193 are patient-derived NF2 schwannoma cells (Hung et al., 2002). T29 are immortalized human ovarian epithelial cells (Liu et al., 2004). RT4-NF2.17, NCIH460, HEI193, SKOV3, SW10, and HT29 and MCF7 cells were cultured in DMEM (Mediatech, Herndon, VA, USA) supplemented with 10% FBS (fetal bovine serum) (Hyclone, Logan, UT, USA). T29 and TOV21G cells were cultured in medium 199 (Mediatech, Inc.), with 10% MCDB 105 (Sigma-Aldrich, St. Louis, MO, USA) and 10% FBS. All tissue culture media were supplemented with 25μg/ml streptomycin and 25 I.U. penicillin (Mediatech). Cells were cultured in a humidified atmosphere with 5% CO2 at 37° C.
Plasmids and siRNA transfections
The following vectors: pCMV5-FLAG-MLK3 (wild-type and K144R), pCMV5-Myc-merlin, pCDNA3-HA-merlin (wild-type, S518D and S518A), pCMV5-FLAG-Cdc42 (wild-type,V12 and N17), pCMV-HA-MLK3 and pEBG-GST-MLK3 were used in this study. Transient transfections were performed as previously described (Chadee et al., 2006). Human and mouse merlin, and MLK3 siRNA oligonucleotide sequences have been previously described (Chadee and Kyriakis, 2004b; Morrison et al., 2007; Okada et al., 2005). SiRNA with non-targeting sequence (Dharmacon, Lafayette, CO, USA) was used as a control and all oligofections were performed as previously described (Chadee and Kyriakis, 2004b).
Immunoblotting and immunoprecipitation
Immunoblotting, cell lysis and immunoprecipitations were performed as described previously (Chadee and Kyriakis, 2004b). Antibodies used for immunoblotting and immunoprecipitations were Cyclin E (M-20), rabbit IgG, GST (Z–5), Myc (9E10), B-Raf (F-3), MLK3(C-20), ERK (C-14), JNK (C-17), β-Actin (C-4), p-B-Raf (Thr598/Ser601), HA (Y-11) and merlin (A-19) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Activation-state p-ERK (Thr202/Tyr204), p-JNK (Thr183/Tyr185) and p-MLK3 (Thr277/Ser281) antibodies were from Cell Signaling Technology (Beverly, MA, USA) and FLAG antibody was from Stratagene (La Jolla, CA, USA).
GST fusion protein preparation and binding assay
pGEX2T-merlin deletion mutant constructs residues 1-590, residues 1–322, residues 308–590, and residues 340–590 were from Dr. V. Ramesh (Gonzalez-Agosti et al., 1999). Merlin GST fusion deletion mutants were purified from bacteria on glutathione-sepharose beads and eluted from the beads with glutathione (Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA). His-MLK3 protein was purified from bacteria on nickel agarose beads and eluted from the beads with imidazole (Pierce, Thermo Fisher Scientific Inc.). GST-merlin fusion proteins were incubated with His-MLK3 for 3h. GST beads were collected and protein complexes bound to GST-sepharose were analyzed by immunoblotting with GST and MLK3 antibodies.
Cell proliferation assays
Cell proliferation assays were performed as previously described (Chadee and Kyriakis, 2004b). Briefly, cells were seeded at 1x105 cells per 6-cm dish. The next day cells were transfected with the indicated siRNA, or vehicle (Lipofectamine 2000, Invitrogen, Carlsbad, CA, USA). At 9, 24, 46 and 70 h post transfection, cells were counted with a haemocytometer. In addition, parallel cell extracts were prepared and subjected to western blot analysis with antibodies as indicated.
Invasion assays
Cell invasion assays were performed with 100μl of 1 mg/ml BD matrigel matrix (BD Bioscience, San Jose, CA, USA) in 24-well transwells with 8.0μm pore size and 24 mm diameter polycarbonate membrane (Corning, Acton, MA, USA). Cells were washed in DMEM containing 0.5% FBS. Ten thousand cells were seeded onto the upper chamber. Matrigel and cells remaining in the upper chamber were removed after 16 h incubation. The cells on the underside surface of the membrane were fixed in Diff Quick Stain Kit (Fisher Scientific, Pittsburgh, PA, USA) and the number of cells per field of view was counted. All experiments were run in triplicate and repeated at least three times.
Assay of MLK3 kinase activity
Preparation of cell lysates and immunoprecipitation of FLAG-MLK3 for kinase assays was performed as previously described (Chadee et al., 2002). Immunoprecipitated FLAG-MLK3-WT or FLAG-MLK3-KD or was incubated with 0.35, 0.88 or 1.25μg GST-merlin (residues 340–590), 100 μM ATP and 10 mM MgCl2 for 30 min at 30°C. FLAG-MLK3-KD was incubated with 0.88μg GST-merlin (residues 340–590). The reaction was stopped with the addition of 6X SDS sample buffer containing EDTA. Samples were boiled and subjected to 15% SDS PAGE and immunoblotting with phospho-MLK3 antibody (Thr277/Ser281) to detect active MLK3.
Assay of Cdc42 activity
Cell lysates were collected and Cdc42 activity was determined using the Cdc42 activity assay kit (Upstate Biotechnologies, Lake Placid, NY, USA) according to the manufacturer’s instructions.
Acknowledgments
We acknowledge Dr. J. Kyriakis for the FLAG-Cdc42 constructs and Dr. D. Lim for the HEI193 cells. This work was supported by a National Institutes of Health grant 1 R15 CA132006-01 and an American Cancer Society (Ohio Division) grant (to D.N.C.)
Grant support: This work was supported by a National Institutes of Health grant 1 R15 CA132006-01 and an American Cancer Society (Ohio Division) grant (to D.N.C.)
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Chadee DN, Kyriakis JM. A novel role for mixed lineage kinase 3 (MLK3) in B-Raf activation and cell proliferation. Cell Cycle. 2004a;3:1227–1229. doi: 10.4161/cc.3.10.1187. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004b;6:770–776. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Xu D, Hung G, Andalibi A, Lim DJ, Luo Z, et al. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci U S A. 2006;103:4463–4468. doi: 10.1073/pnas.0510651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee DN, Yuasa T, Kyriakis JM. Direct activation of mitogen-activated protein kinase kinase kinase MEKK1 by the Ste20p homologue GCK and the adapter protein TRAF2. Mol Cell Biol. 2002;22:737–749. doi: 10.1128/MCB.22.3.737-749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YY, Bode AM, Mizuno H, Choi BY, Choi HS, Dong Z. A novel role for mixed-lineage kinase-like mitogen-activated protein triple kinase alpha in neoplastic cell transformation and tumor development. Cancer Res. 2004;64:3855–3864. doi: 10.1158/0008-5472.CAN-04-0201. [DOI] [PubMed] [Google Scholar]
- Du Y, Bock BC, Schachter KA, Chao M, Gallo KA. Cdc42 induces activation loop phosphorylation and membrane targeting of mixed lineage kinase 3. J Biol Chem. 2005;280:42984–42993. doi: 10.1074/jbc.M502671200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E, et al. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet. 2002;31:354–362. doi: 10.1038/ng930. [DOI] [PubMed] [Google Scholar]
- Fraenzer JT, Pan H, Minimo L, Jr, Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol. 2006;33:392–406. doi: 10.1053/j.seminoncol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Agosti C, Wiederhold T, Herndon ME, Gusella J, Ramesh V. Interdomain interaction of merlin isoforms and its influence on intermolecular binding to NHE-RF. J Biol Chem. 1999;274:34438–34442. doi: 10.1074/jbc.274.48.34438. [DOI] [PubMed] [Google Scholar]
- Goutebroze L, Brault E, Muchardt C, Camonis J, Thomas G. Cloning and characterization of SCHIP-1, a novel protein interacting specifically with spliced isoforms and naturally occurring mutant NF2 proteins. Mol Cell Biol. 2000;20:1699–1712. doi: 10.1128/mcb.20.5.1699-1712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Haipek CA, Burke SP, Sun CX, Scoles DR, Pulst SM. The NF2 interactor, hepatocyte growth factor-regulated tyrosine kinase substrate (HRS), associates with merlin in the “open” conformation and suppresses cell growth and motility. Hum Mol Genet. 2001;10:825–834. doi: 10.1093/hmg/10.8.825. [DOI] [PubMed] [Google Scholar]
- Hartkamp J, Troppmair J, Rapp UR. The JNK/SAPK activator mixed lineage kinase 3 (MLK3) transforms NIH 3T3 cells in a MEK-dependent fashion. Cancer Res. 1999;59:2195–2202. [PubMed] [Google Scholar]
- Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS, et al. Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2. Int J Oncol. 2002;20:475–482. [PubMed] [Google Scholar]
- Ikeda K, Saeki Y, Gonzalez-Agosti C, Ramesh V, Chiocca EA. Inhibition of NF2-negative and NF2-positive primary human meningioma cell proliferation by overexpression of merlin due to vector-mediated gene transfer. J Neurosurg. 1999;91:85–92. doi: 10.3171/jns.1999.91.1.0085. [DOI] [PubMed] [Google Scholar]
- Jannatipour M, Dion P, Khan S, Jindal H, Fan X, Laganiere J, et al. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J Biol Chem. 2001;276:33093–33100. doi: 10.1074/jbc.M105792200. [DOI] [PubMed] [Google Scholar]
- Jin H, Sperka T, Herrlich P, Morrison H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature. 2006;442:576–579. doi: 10.1038/nature04856. [DOI] [PubMed] [Google Scholar]
- Khatlani TS, Wislez M, Sun M, Srinivas H, Iwanaga K, Ma L, et al. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene. 2007;26:2658–2666. doi: 10.1038/sj.onc.1210050. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lee JY, Moon HJ, Lee WK, Chun HJ, Han CW, Jeon YW, et al. Merlin facilitates ubiquitination and degradation of transactivation-responsive RNA-binding protein. Oncogene. 2006;25:1143–1152. doi: 10.1038/sj.onc.1209150. [DOI] [PubMed] [Google Scholar]
- Leung IW, Lassam N. The kinase activation loop is the key to mixed lineage kinase-3 activation via both autophosphorylation and hematopoietic progenitor kinase 1 phosphorylation. J Biol Chem. 2001;276:1961–1967. doi: 10.1074/jbc.M004092200. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- Lutchman M, Rouleau GA. The neurofibromatosis type 2 gene product, schwannomin, suppresses growth of NIH 3T3 cells. Cancer Res. 1995;55:2270–2274. [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Ramesh V, Gusella JF, Jacks T. The Nf2 tumor suppressor gene product is essential for extraembryonic development immediately prior to gastrulation. Genes Dev. 1997;11:1253–1265. doi: 10.1101/gad.11.10.1253. [DOI] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–527. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- Obremski VJ, Hall AM, Fernandez-Valle C. Merlin, the neurofibromatosis type 2 gene product, and beta1 integrin associate in isolated and differentiating Schwann cells. J Neurobiol. 1998;37:487–501. [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- Ramesh V. Merlin and the ERM proteins in Schwann cells, neurons and growth cones. Nat Rev Neurosci. 2004;5:462–470. doi: 10.1038/nrn1407. [DOI] [PubMed] [Google Scholar]
- Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, et al. The mixed lineage kinase SPRK phosphorylates and activates the stress–activated protein kinase activator, SEK-1. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- Reed N, Gutmann DH. Tumorigenesis in neurofibromatosis: new insights and potential therapies. Trends Mol Med. 2001;7:157–162. doi: 10.1016/s1471-4914(01)01955-4. [DOI] [PubMed] [Google Scholar]
- Rennefahrt U, Illert B, Greiner A, Rapp UR, Troppmair J. Tumor induction by activated JNK occurs through deregulation of cellular growth. Cancer Lett. 2004;215:113–124. doi: 10.1016/j.canlet.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Rennefahrt UE, Illert B, Kerkhoff E, Troppmair J, Rapp UR. Constitutive JNK activation in NIH 3T3 fibroblasts induces a partially transformed phenotype. J Biol Chem. 2002;277:29510–29518. doi: 10.1074/jbc.M203010200. [DOI] [PubMed] [Google Scholar]
- Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- Scoles DR, Huynh DP, Chen MS, Burke SP, Gutmann DH, Pulst SM. The neurofibromatosis 2 tumor suppressor protein interacts with hepatocyte growth factor-regulated tyrosine kinase substrate. Hum Mol Genet. 2000;9:1567–1574. doi: 10.1093/hmg/9.11.1567. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–2509. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.44.27225. [DOI] [PubMed] [Google Scholar]
- Vacratsis PO, Gallo KA. Zipper-mediated oligomerization of the mixed lineage kinase SPRK/MLK-3 is not required for its activation by the GTPase cdc 42 but Is necessary for its activation of the JNK pathway. Monomeric SPRK L410P does not catalyze the activating phosphorylation of Thr258 of murine MITOGEN-ACTIVATED protein kinase kinase 4. J Biol Chem. 2000;275:27893–27900. doi: 10.1074/jbc.M002858200. [DOI] [PubMed] [Google Scholar]
- Velho S, Oliveira C, Paredes J, Sousa S, Leite M, Matos P, et al. Mixed lineage kinase 3 gene mutations in mismatch repair deficient gastrointestinal tumours. Hum Mol Genet. 2010;19:697–706. doi: 10.1093/hmg/ddp536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gallo KA. Autoinhibition of mixed lineage kinase 3 through its Src homology 3 domain. J Biol Chem. 2001;276:45598–45603. doi: 10.1074/jbc.M107176200. [DOI] [PubMed] [Google Scholar]