Abstract
Breast milk transmission of human immunodeficiency virus (HIV) remains an important mode of infant HIV acquisition. Interestingly, the majority of infants remain uninfected during prolonged virus exposure via breastfeeding, raising the possibility that immune components in milk prevent mucosal virus transmission. HIV-specific antibody responses are detectable in the milk of HIV-infected women and simian immunodeficiency virus (SIV)-infected monkeys; however, the role of these humoral responses in virus neutralization and local virus quasispecies evolution has not been characterized. In this study, four lactating rhesus monkeys were inoculated with SIVmac251 and monitored for SIV envelope-specific humoral responses and virus evolution in milk and plasma throughout infection. While the kinetics and breadth of the SIV-specific IgG and IgA responses in milk were similar to those in plasma, the magnitude of the milk responses was considerably lower than that of the plasma responses. Furthermore, a neutralizing antibody response against the inoculation virus was not detected in milk samples at 1 year after infection, despite a measurable autologous neutralizing antibody response in plasma samples obtained from three of four monkeys. Interestingly, while IgA is the predominant immunoglobulin in milk, the milk SIV envelope-specific IgA response was lower in magnitude and demonstrated more limited neutralizing capacity against a T-cell line-adapted SIV compared to those of the milk IgG response. Finally, amino acid mutations in the envelope gene product of SIV variants in milk and plasma samples occurred in similar numbers and at similar positions, indicating that the humoral immune pressure in milk does not drive distinct virus evolution in the breast milk compartment.
Breastfeeding is an important component of the maternal-infant immune system, providing the infant with passive maternal immunity and protection against infectious pathogens. In fact, non-breast-fed infants in developing nations experience higher mortality due to respiratory and diarrheal illnesses (45). However, breastfeeding is also a mode of infant human immunodeficiency virus (HIV) acquisition, contributing to a large proportion of infant HIV infections in areas of high HIV prevalence. Therefore, development of feeding strategies that promote HIV-free survival of infants born to HIV-infected mothers in developing nations poses a major public health challenge.
Interestingly, in the absence of antiretroviral prophylaxis, HIV is transmitted via breast milk to only 10% of infants chronically exposed to the virus via breastfeeding (19, 25). This low rate of HIV transmission suggests that antiviral immune factors in milk may protect the majority of infants from mucosal HIV acquisition. HIV envelope-specific antibody responses have been identified in milk, but the magnitude of these responses is similar in women who transmit the virus via breast milk and women whose infants remain uninfected throughout breastfeeding (3, 11, 23). Likewise, the magnitude of simian immunodeficiency virus (SIV) envelope-specific antibody responses in the milk of SIV-infected, lactating rhesus monkeys did not differ in those mothers that did and did not transmit the virus to their suckling infant (1, 42). Proposed mechanisms for HIV-specific breast milk antibody function include virus neutralization and impairment of virus transcytosis through an epithelial cell layer (3, 7, 17). Therefore, the function, rather than the magnitude, of the HIV-specific breast milk antibody response may be the critical feature in protection against infant mucosal transmission. Importantly, passive transfer of broadly neutralizing HIV-specific antibody to neonatal monkeys protected the infants against oral simian-human immunodeficiency virus (SHIV) challenge, indicating that passively transferred humoral immunity can protect infants from virus transmission through breastfeeding (18, 41).
Vertically transmitted HIV variants, including those transmitted via breast milk, have been reported to be resistant to neutralization by systemic maternal antibody responses (9, 38). However, HIV-specific neutralizing antibody responses in breast milk have not been characterized. In fact, the ability of mucosal IgA to neutralize HIV remains an important question in the HIV field. While an HIV-specific mucosal IgA response in the genital tracts of exposed-uninfected individuals has been described, the role of mucosal IgA in protection against mucosal transmission of HIV is unclear and controversial (5, 8–10). Furthermore, the contribution of locally replicating virus at mucosal surfaces to the divergence of the systemic and mucosal antibody responses is unknown. Similarly, the role of mucosal antibody in the shaping of mucosal virus quasispecies evolution is not well characterized. Delineation of the function and role of mucosal antibody responses in defining the pool of transmitted virus will be crucial for the design of immunologic interventions to reduce breast milk transmission of HIV.
SIV infection of lactating rhesus monkeys provides an excellent model to characterize virus-specific immune responses and virus evolution in milk, as the sequence of the virus inoculum, the timing of the infection, and the virus-specific immunodominant responses are well defined in this model. Furthermore, SIV-infected, lactating rhesus monkeys transmit the virus to their suckling infants via breastfeeding (1). We have developed a pharmacologic protocol to induce lactation in nonpregnant rhesus monkeys, facilitating these studies without reliance on breeder monkeys. Moreover, the milk produced by hormone-induced, lactating monkeys has immunoglobulin content and a lymphocyte phenotype similar to that produced by naturally lactating monkeys (35). In this study, we characterized the neutralizing potency of the SIV envelope-specific IgG and IgA responses in milk and their role in shaping the SIV envelope gene evolution of local virus variants.
MATERIALS AND METHODS
Animals and virus
Four female Mamu-A*01+ rhesus monkeys underwent hormone induction of lactation, as previously described (35), and were inoculated intravenously with 2.11 × 105 copies of the previously described stock of SIVmac251 (9). Blood and milk samples were collected two to three times per week until 54 to 76 weeks after infection. Milk samples were separated into cellular, supernatant, and fat fractions by centrifugation, as previously described (35). To measure the SIV virus loads in milk and plasma samples, RNA was isolated from milk supernatant, amplified with SIV gag-specific primers and probes, and compared to amplification of dilutions of a known quantity of gag RNA, as previously described (35). Finally, peripheral blood mononuclear cells (PBMC) were isolated and stained with lymphocyte phenotyping antibodies as previously described (35). The proportion of CD4+ T lymphocytes was multiplied by the automated total lymphocyte count to obtain the CD4+ T-lymphocyte counts.
Quantitation of total and SIV-specific Ig in milk and plasma samples
Plasma and milk supernatant IgM and IgG levels were measured in duplicate using monkey-specific enzyme-linked immunosorbent assay (ELISA) kits (Alpha Diagnostics) and standards per protocol. The plasma and milk supernatant IgA levels were measured by noncommercial ELISA, as previously described (35). Breast milk supernatant was diluted between 1:10 and 1:2,000 for the assays. SIV-specific IgG and IgA were measured by incubation of serial 3-fold dilutions of plasma and milk supernatants in duplicate in a 96-well plate coated with recombinant SIVmac239 gp130 or p27 (ImmunoDiagnostics). After being blocked with phosphate-buffered saline (PBS) with 5% nonfat dried milk and 10% fetal bovine serum, SIV-specific antibody was detected by a horseradish peroxidase (HRP)-conjugated, polyclonal goat anti-monkey IgG (Alpha Diagnostics) or an anti-monkey IgA (Rockland) antibody and by the addition of the ABTS-2 peroxidase substrate system (KPL). The optical density (OD) at 410 nm was measured. The SIV envelope-specific antibody titer was calculated as the inverse of the lowest dilution of plasma or milk supernatant which had an average OD greater than two times the OD of the PBS-negative control. The SIV-specific IgG and IgA titers were normalized by dividing the titer by the average of the total IgG or IgA concentration (mg/ml) measured at three time points during infection for each animal.
SIV Western blotting
SIV Western blotting was performed with milk and plasma samples using an SIV Western blot assay (ZeptoMetrix) per the manufacturer's instructions and the following modifications. Acute breast milk samples (5 to 10 weeks after infection) and all plasma samples were diluted 1:20, and chronic breast milk samples (40 to 54 weeks after infection) were diluted 1:3 in PBS. For IgA detection, an alkaline phosphatase-conjugated, polyclonal goat anti-monkey IgA antibody (Rockland) was used as the secondary antibody in the kit.
Ig isolation from milk and plasma samples
A 1:3 dilution of milk supernatant and 1:10 dilution of plasma samples were run over a NAb protein G spin column (Pierce), according to the manufacturer's instructions. The protein G column elution fractions were then run over a protein L spin column (Pierce). The protein G flowthrough and the protein L elution fraction were both concentrated with Amicon Ultra centrifugal filter units (Millipore) by centrifugation at 1,300 × g and then sterilized through a Durapore Millex 13-mm filter unit (Millipore). The concentrations of the antibody fractions were determined by spectrophotometry (NanoDrop) at A280. An extinction coefficient of 1.36 was used to calculate the antibody concentrations for all fractions (extinction coefficient of monomeric IgG = 1.35 to 1.45; extinction coefficient of secretory component = 1.26) (20, 22) The calculated concentrations of IgG and IgA were validated by total IgA or IgG ELISA, as described above. Total IgG ELISA (Alpha Diagnostic) also confirmed that all non-IgG fractions were composed of less than 1% IgG.
SIV neutralization assays
Stocks of molecularly cloned primary isolate SIVmac251, T-cell line-adapted (TCLA) SIVmac251, and murine leukemia virus (MuLV) envelope-pseudotyped viruses were prepared by transfection in 293T cells and titration in TZM-bl cells, as previously described (26). Neutralization was measured by a reduction in luciferase reporter gene expression after a single round of infection in TZM-bl cells, as previously described (26). Briefly, 200 50% tissue culture infective doses (TCID50) of virus were incubated with 3-fold serial dilutions of plasma or milk supernatant or purified preparations of plasma or milk IgG and IgA in duplicates for 1 h at 37°C in 96-well flat-bottom culture plates. TZM-bl cells were then added (1 × 104 cells/well in a 100-μl volume) in 10% Dulbecco modified Eagle growth medium containing DEAE-Dextran (Sigma) at a final concentration of 11 μg/ml. Assay controls included replicate wells of TZM-bl cells alone (cell control) and TZM-bl cells with virus (virus control). Following 48 h of incubation at 37°C, 150 μl of assay medium was removed from each well, and 100 μl of Bright-Glo luciferase reagent (Promega) was added. The cells were allowed to lyse for 2 min, then 150 μl of the cell lysate was transferred to a 96-well black solid plate, and luminescence was measured. The 50% inhibitory dose (ID50) titer was calculated as the plasma dilution that caused a 50% reduction in the number of relative luminescence units (RLU) compared to the virus control wells after subtraction of the number of cell control RLU. The 50% inhibitory concentration (IC50) titer was calculated as the purified Ig concentration that caused a 50% reduction in the number of RLU.
Single-genome amplification, virus sequencing, and analysis of the SIV envelope gene
Cassettes containing the SIV envelope open reading frame were amplified by single-genome amplification (SGA) and sequenced from simultaneous samples of plasma and milk supernatants collected from 3 chronically SIV-infected, lactating rhesus monkeys between 48 and 74 weeks after SIVmac251 infection, as previously described (36). Sequences were trimmed, translated, and aligned with the sequence of the major inoculation virus species to identify acquired mutations using ClustalW version 2 (2). Amino acid mutations included within 3 minor variants of the inoculation virus population were not included in the analysis.
Statistical analysis
Virus loads, antibody content, and SIV-specific antibody titers were compared by paired, nonparametric t tests (Mann-Whitney U test). Using this test, the lowest obtainable P value in this four-monkey study is 0.12. The numbers of amino acid mutations in plasma and milk virus variants were compared by a nonparametric t test (Wilcoxon's rank sum test).
RESULTS
Breast milk Ig content remains 1 to 2 logs lower than that in plasma samples throughout acute and chronic SIV infection
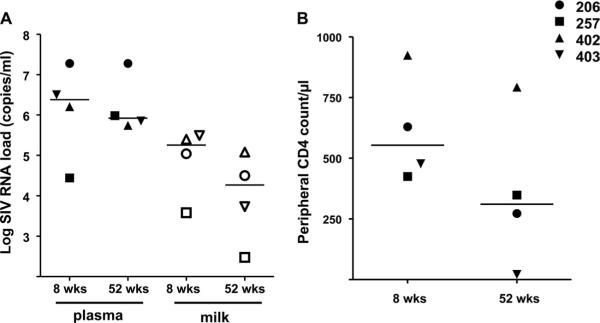
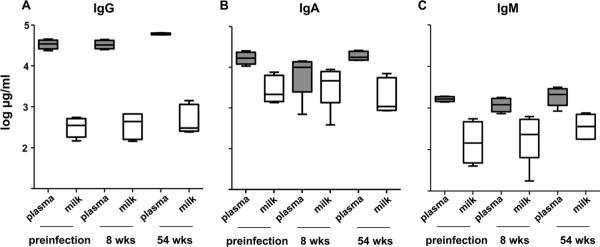
Four Mamu-A*01+, lactating rhesus monkeys inoculated with SIVmac251 had ongoing virus replication in milk samples that remained 1 to 2 logs lower than that in plasma samples throughout acute and chronic infection (Fig. 1A) (all P values = 0.12). IgG content in breast milk samples remained approximately 2 logs lower than that in plasma samples prior to and during acute and chronic infection (all P values = 0.12). Interestingly, the expected increase in plasma IgG (28) was detected during chronic SIV infection, but the milk IgG concentration remained stable (Fig. 2A). IgA content in milk samples was approximately 1 log lower than that in plasma samples prior to infection and during chronic infection (both P values = 0.12) but was not significantly different than that in plasma samples 8 weeks after infection (P = 0.62) (Fig. 2B). Finally, the IgM content in milk samples was approximately 1 log lower than that in plasma samples prior to and during acute and chronic infection (all P values = 0.12) (Fig. 2C). As expected, IgA remained the predominant antibody isotype in milk samples throughout the SIV infection. Importantly, the total antibody concentration in milk samples remained considerably lower than that in plasma samples throughout the infection in all animals.
FIG. 1.
Plasma and milk virus loads and peripheral CD4+ T-lymphocyte counts of SIV-infected, hormone-induced, lactating monkeys. Plasma (closed symbols) and milk (open symbols) virus loads (A) were measured by quantitative reverse transcriptase PCR (RT-PCR). Peripheral blood CD4 counts (B) were measured by flow cytometry and complete blood counting. wks, weeks.
FIG. 2.
The total immunoglobulin isotype concentration in breast milk samples is 1 to 2 logs lower than that in plasma samples throughout acute and chronic SIV infection. Total IgG (A), IgA (B), and IgM (C) concentrations were measured by isotype-specific Ig ELISA in plasma samples (shaded box plot) and breast milk samples (unshaded box plot) collected prior to SIV infection and at the indicated time points following SIVmac251 infection. Box plots display values of the median (black line), 25 to 75% interquartile range (box), and range (error bars).
Low-magnitude SIV envelope-specific IgA response in milk samples compared to that in plasma samples during acute and chronic SIV infection
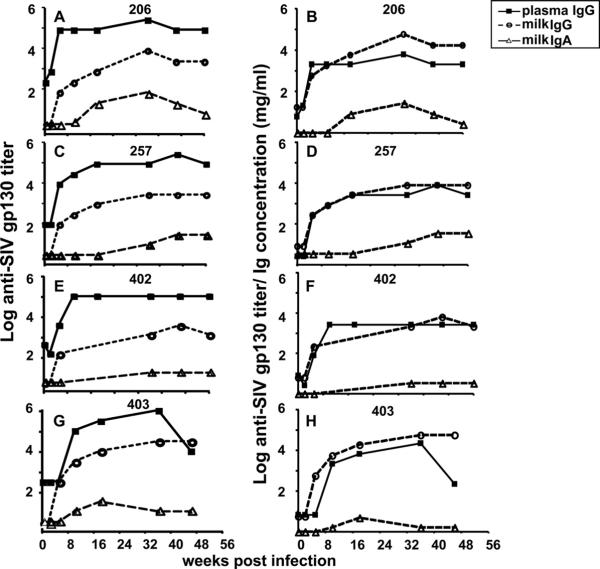
The SIV envelope-specific IgG response in plasma appeared in all monkeys by 5 to 8 weeks after infection and remained relatively constant throughout the SIV infection (Fig. 3A, C, E, and G). However, the SIV envelope-specific plasma IgG response declined in monkey 403 by 48 weeks after infection (Fig. 3G), likely due to significant CD4+ T-lymphocyte loss in this monkey (Fig. 1B). The SIV envelope-specific milk IgG response had kinetics similar to that in plasma samples, with a detectable response at 5 to 8 weeks after infection, but the magnitude of this response in milk samples remained 2 logs lower than that in plasma samples throughout the infection (P = 0.12 for all time points). Interestingly, the milk SIV envelope-specific IgG response in monkey 403 did not display a decrease by 48 weeks after infection similar to that in the plasma SIV envelope-specific IgG response (Fig. 3G and H). When the SIV envelope-specific IgG responses in plasma and milk samples were normalized for the IgG content in each compartment, the plasma and breast milk IgG responses were nearly identical in magnitude (Fig. 3B, D, F, and H).
FIG. 3.
A limited SIV envelope-specific IgA response is detected in breast milk samples obtained from acute and chronically SIV-infected, lactating rhesus monkeys. (A, C, E, and G) SIVmac239 gp130-specific plasma IgG (filled square), milk IgG (open circle), and milk IgA (open triangle) titers were measured by ELISA from samples collected at the indicated time points following SIVmac251 infection. (B, D, F, and H) In addition, SIVmac239 gp130-specific plasma IgG, milk IgG, and milk IgA titers were normalized for Ig isotype-specific concentrations. Monkey identification numbers are indicated above each graph.
In contrast, the SIV envelope-specific IgA response was detectable only in two of four animals during early infection (Fig. 3A and G, monkeys 206 and 403) and remained at low titers throughout acute and chronic infection in all animals (Fig. 3A, C, E, and G). Furthermore, although breast milk has higher total IgA content than IgG content throughout infection, the SIV envelope-specific IgA response remained considerably lower than the SIV envelope-specific IgG response in breast milk samples during acute and chronic infection (Fig. 3B, D, F, and H). Therefore, although IgA is the predominant antibody isotype in milk samples, the SIV envelope-specific IgG response is considerably more robust than the SIV envelope-specific IgA response in breast milk samples throughout infection (P = 0.12 for all time points).
As the SIV envelope IgA response in milk samples was surprisingly minimal compared to the SIV envelope IgG response in milk samples, we assessed the magnitude of the IgA and IgG responses in milk samples directed against another SIV antigen, Gag p27. The anti-Gag p27 IgG response in milk samples was approximately 2 logs lower than that in plasma samples at 12 and 48 weeks after infection (Fig. 4A) and was similar to that in plasma samples after normalization for plasma and milk IgG content (Fig. 4B). In contrast to the low SIV envelope gp130 IgA responses in milk samples, the anti-Gag p27 IgA responses in milk samples were similar in magnitude to the anti-Gag p27 IgG responses in milk samples (Fig. 4C) at both 12 and 48 weeks after infection. Furthermore, the magnitudes of the plasma and milk anti-Gag p27 IgA responses were similar at both of these time points after normalization for total IgA content in each compartment (Fig. 4D). This discrepancy in the magnitudes of the anti-SIV envelope and Gag IgA binding responses in milk suggests poor immunogenicity of the SIV envelope protein in mucosal compartments.
FIG. 4.
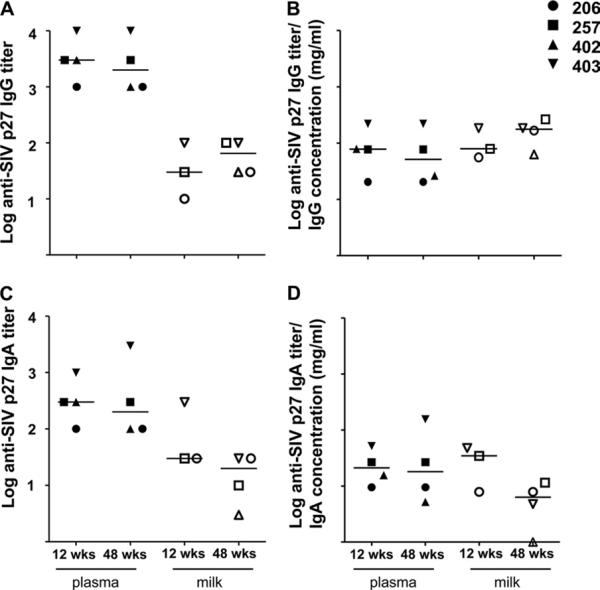
Similar-magnitude SIV Gag-specific IgA and IgG responses in milk samples obtained from acute and chronically SIV-infected, lactating rhesus monkeys. SIVmac239 Gag p27-specific plasma (closed symbols) and milk (open symbols) IgG (A) and IgA (C) titers were measured by ELISA at 12 and 48 weeks after infection. In addition, SIV Gag p27 plasma and milk IgG (B) and IgA (D) titers were normalized for total IgG and IgA content in each compartment, respectively.
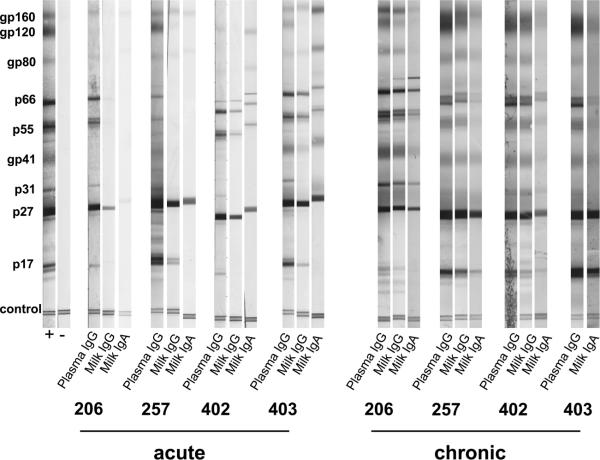
The breadth of the SIV-specific IgG and IgA responses in milk and plasma samples was assessed by SIV Western blotting during acute and chronic infection. A plasma IgG response to eight or nine major SIV proteins was detected by Western blotting by 10 weeks after infection. In breast milk samples, an IgG antibody response was detected at a 1:20 dilution to between four and seven of nine of the major SIV proteins by 10 weeks after infection (Fig. 5). There was no single SIV protein-specific IgG response that was not detected in the breast milk samples obtained from these monkeys. Therefore, the plasma and breast milk IgG responses likely have a similar breadth during early infection, but the lack of detection of responses to all major SIV proteins in breast milk samples by 10 weeks after infection by Western blotting may be to a lower concentration of IgG in milk samples than that in plasma samples (Fig. 2A).
FIG. 5.
The breadth of the SIV-specific humoral response is similar in plasma and breast milk samples. SIV Western blot of plasma and breast milk samples during acute (weeks 5 to 10) and chronic (weeks 40 to 54) infection. All plasma samples are diluted 1:20, whereas acute breast milk samples are diluted 1:20 and chronic breast milk samples are diluted 1:3, due to limited sample availability.
An SIV-specific breast milk IgA antibody response in milk samples against two to seven of the major SIV antigens was detected by Western blotting by five to 10 weeks after infection (Fig. 5). The low number of SIV protein-specific IgA responses detected by Western blotting during acute infection in some monkeys is consistent with the lack of detection of SIV envelope-specific IgA responses in breast milk samples by ELISA early after infection in two of four animals (Fig. 3). However, as the total immunoglobulin level is lower in milk samples than in plasma samples (Fig. 2B), the antibody concentration in milk samples may be too low to detect limited responses against all SIV antigens in this assay.
To further define the breadth of the SIV-specific antibody response in breast milk samples, we repeated the SIV Western blot assay using a less dilute breast milk sample (1:3 dilution) collected during chronic SIV infection. During chronic SIV infection, a plasma IgG response to all major SIV proteins was detectable (Fig. 5). Importantly, breast milk IgG and IgA responses to nearly all major SIV proteins in all animals by approximately 1 year after infection were detected. A breast milk IgG response to all major SIV proteins in three of three monkeys was detected, and a breast milk IgA response to between seven and eight out of nine proteins in four monkeys during chronic infection was detected. There was no single SIV protein-specific response that was not detected in the IgG or the IgA fraction of breast milk samples during chronic infection, indicating similar breadth of the humoral responses in milk and plasma samples during chronic infection.
The inoculation virus-specific neutralizing antibody response was not detected in breast milk during acute or chronic SIV infection
A neutralizing antibody response to the SIVmac251 inoculation virus in plasma in three of four monkeys by 35 weeks after infection was detected and increased in magnitude in the three animals at 1 year after infection (Fig. 6A). This autologous neutralizing antibody response remained undetectable in the plasma obtained from one animal with significant CD4+ T-lymphocyte loss by 1 year after infection (monkey 403) (Fig. 1B). The magnitudes of the neutralizing antibody responses against the autologous challenge virus in plasma samples in our study are consistent with a recent report describing the evolution of this response during SIVmac251 infection (2). In contrast, an inoculation virus-specific neutralizing antibody response was not detected in breast milk samples obtained from any of the four SIV-infected, lactating monkeys through 1 year after infection (Fig. 6B). This finding may indicate that the SIV-specific antibody in breast milk is not effective in neutralizing autologous virus in milk at its intrinsic concentration.
FIG. 6.
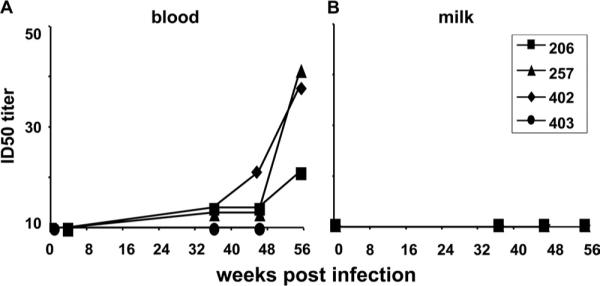
No autologous neutralizing antibody response is detected in breast milk samples during acute or chronic SIV infection. Autologous SIVmac251 envelope pseudovirus neutralization was measured in plasma and milk samples collected at the indicated time points following SIVmac251. ID50 titers in plasma (A) and milk (B) samples are plotted for each animal.
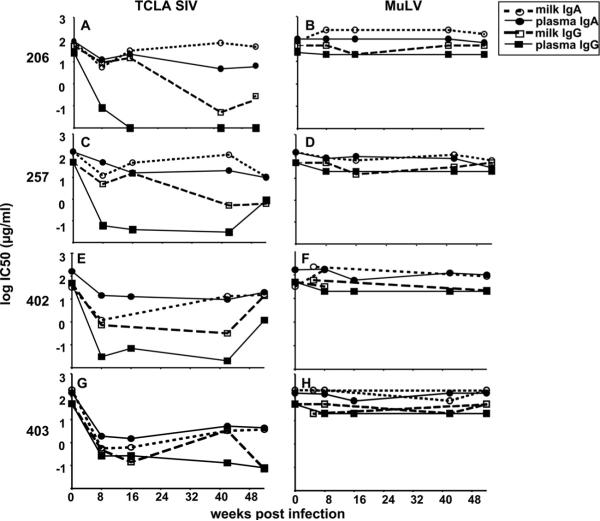
Low neutralizing potency of breast milk IgA compared to plasma or breast milk IgG
In order to further compare the magnitudes of the SIV-specific neutralizing antibody responses in milk and plasma samples, we employed a more easily neutralized, T-cell line-adapted (TCLA) SIVmac251 envelope pseudovirus in the TZM-bl neutralizing antibody assay. While the TCLA SIV-specific neutralizing antibody response in milk samples had kinetics similar to that in plasma samples, the magnitude of the response was approximately 2 logs lower than that in plasma samples obtained from all monkeys (Fig. 7A, C, E, and G). Interestingly, when the TCLA SIV-specific neutralizing antibody responses in plasma and milk samples were normalized for compartment-specific total antibody content, this response remained slightly lower in magnitude in milk samples than in plasma samples in all monkeys (Fig. 7B, D, F, and H) (P = 0.12 for all time points at 16 weeks after infection).
FIG. 7.
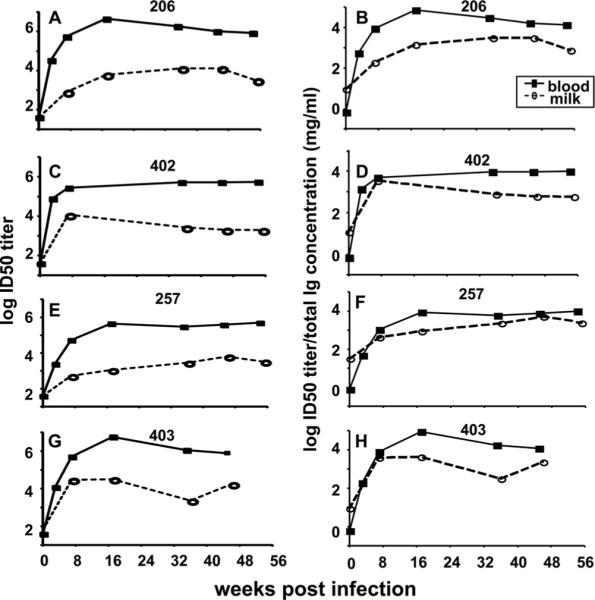
The neutralizing antibody response in breast milk samples against TCLA SIVmac251 envelope pseudovirus remains lower than that in plasma samples after normalization for total Ig content. TCLA SIVmac251 envelope-specific neutralization was measured in plasma and milk samples collected at the indicated time points following infection. (A, C, E, and G) ID50 titers in plasma (filled squares) and milk (open circles) samples are shown for each monkey; (B, D, F, and H) ID50 titers in plasma and milk samples normalized for total Ig content are shown in each graph.
Next, we compared the SIV-neutralizing potency of breast milk IgG and IgA to plasma IgG and IgA. We isolated IgG and IgA fractions from plasma and milk samples obtained from the four animals at various time points during acute and chronic SIV infection. We then measured the neutralizing potency of each Ig fraction against the TCLA SIVmac251 envelope pseudovirus and a murine leukemia virus (MuLV) envelope pseudovirus (negative control). Using dilutions of the quantitated IgA and IgG fractions, the IC50 titer for each pseudovirus was calculated.
The most potent TCLA SIV-specific neutralizing response was detected in the plasma IgG fraction, with a median IC50 titer of 0.06 μg/ml (range, 0.03 to 0.26 μg/ml) by 8 weeks after infection (Fig. 8A, C, E, and G; Table 1). A potent TCLA SIV-specific neutralizing response was also detected in the breast milk IgG fraction. TCLA SIV-neutralizing activity was detected in the breast milk IgG fraction at 8 weeks after infection (median IC50, 4.9 μg/ml; range, 0.48 to 8.8 μg/ml) but increased in potency during chronic infection (week 42 after infection) (median IC50, 0.32 μg/ml; range, 0.05 to 3.4 μg/ml). Importantly, minimal or no nonspecific neutralizing activity was detected against the MuLV envelope pseudovirus (Fig. 8B, D, F, and H; Table 1).
FIG. 8.
Low-potency neutralizing activity of breast milk IgA against TCLA SIVmac251 envelope pseudovirus compared to those of plasma and breast milk IgG. IgG and IgA were purified from plasma and milk samples collected at the indicated time points after SIVmac251 infection and tested for TCLA SIVmac251 pseudovirus neutralizing activity (A, C, E, and G) and for neutralizing activity against a negative control MuLV envelope pseudovirus (B, D, F, and H). IC50 titers of plasma IgG (closed squares), plasma IgA (closed circles), breast milk IgG (open squares), and breast milk IgA (open circles) during acute and chronic infection are shown for each monkey. A decreasing IC50 titer indicates increasing neutralizing potency.
TABLE 1.
IC50 titers of plasma and milk IgG and IgA fractions from SIV-infected, lactating rhesus monkeys
| IC50 titer (μg/ml) in monkeya: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fraction | Week | 206 |
257 |
402 |
403 |
||||
| TCLA SIV | MuLV | TCLA SIV | MuLV | TCLA SIV | MuLV | TCLA SIV | MuLV | ||
| Plasma IgG | 0 | >25 | >25 | >50 | >50 | >50 | >50 | >50 | >50 |
| 8 | 0.08 | >20 | 0.06 | >20 | 0.03 | >20 | 0.26 | >20 | |
| 16 | <0.01 | >20 | 0.04 | >20 | 0.07 | >20 | 0.27 | >20 | |
| 42 | <0.01 | >20 | 0.03 | >20 | 0.02 | >20 | 0.13 | >20 | |
| 52 | <0.01 | >20 | 0.9 | >20 | 1.2 | >20 | 0.08 | >20 | |
| Milk IgG | 0 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 8 | 8.8 | >50 | 4.9 | >50 | 0.73 | >32 | 0.48 | >50 | |
| 16 | 14.6 | >20 | >15 | >15 | NA | NA | 3.14 | >20 | |
| 42 | 0.05 | >50 | 0.6 | >32 | 0.32 | >22 | 3.4 | >50 | |
| 52 | 0.19 | >50 | 0.6 | >50 | 14 | >60 | 0.07 | >20 | |
| Plasma IgA | 0 | 84.5 | >100 | >150 | >150 | >165 | >165 | >150 | >150 |
| 8 | 12.3 | >100 | 48.5 | >80 | 14.5 | >176 | 1.5 | >137 | |
| 16 | 21.5 | >100 | 16.1 | >100 | 12.9 | >60 | 1.5 | >70 | |
| 42 | 4.8 | >100 | 20.8 | >80 | 9.6 | >120 | 5.2 | >150 | |
| 52 | 5.8 | 68.7 | 9.7 | >30 | 20 | >100 | 4.4 | >150 | |
| Milk IgA | 0 | >80 | >80 | >150 | >150 | >32 | >32 | >200 | >200 |
| 8 | 5.5 | 250 | 11.9 | >85 | 1.2 | >165 | 0.62 | >200 | |
| 16 | 30.7 | 245 | 47.6 | >65 | NA | NA | 0.62 | >70 | |
| 42 | 70.8 | >250 | 109.5 | >120 | 13.4 | >87 | 3.7 | >200 | |
| 52 | 47.5 | >160 | 10.4 | >65 | 15.3 | >220 | 3.7 | >200 | |
Titers were obtained using a TZM-bl neutralization assay with TCLA SIVmac251 and MuLV (negative-control) envelope pseudoviruses. NA, sample not available.
The activities of the milk and plasma IgA neutralizing responses were considerably less potent than those of the milk and plasma IgG neutralizing responses throughout acute and chronic infection (Fig. 8B, D, F, and H; Table 1). However, the TCLA SIV-neutralizing potency of breast milk IgA was similar in magnitude and kinetics to that of plasma IgA throughout infection. Interestingly, the neutralizing potency of the milk IgA peaked at 8 weeks after infection (median IC50, 5.5 μg/ml; range, 0.56 to 11.9 μg/ml) and remained static or decreased at later time points, requiring a similar or larger amount of IgA to neutralize the TCLA SIV pseudovirus at week 42 (median IC50, 70.8 μg/ml; range, 3.3 to 109.5 μg/ml) than at week 8 after infection. This pattern is in contrast to the plasma and breast milk IgG responses, which continued to increase in potency at 42 weeks after infection. Importantly, approximately 2- to 3-log more milk IgA (median IC50, 70.8 μg/ml; range, 3.3 to 109.5 μg/ml) than milk IgG (median IC50, 0.32 μg/ml; range, 0.05 to 3.4 μg/ml) was required to neutralize the TCLA SIV pseudovirus at 42 weeks after infection in three of four monkeys, confirming that the SIV-specific milk IgA response is considerably less potent than the SIV-specific milk IgG response. The reduced neutralizing potency of the milk IgA response compared to that of the milk IgG response is similar to the reduced neutralizing potency of the plasma IgA response compared to that of the plasma IgG response. Therefore, this mucosal SIV-specific IgA response does not seem to be of higher functional quality than the plasma SIV-specific IgA response.
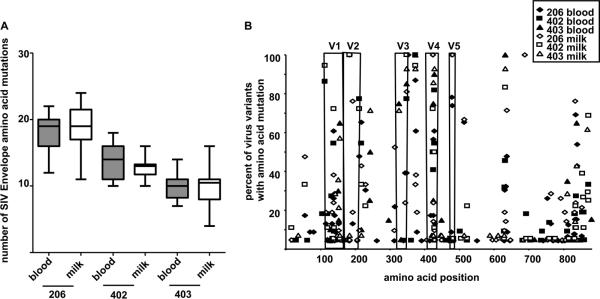
Similar SIV envelope evolution of breast milk and plasma virus variants during chronic SIV infection
HIV and SIV are known to rapidly and continually escape neutralizing antibody responses by mutation of the virus envelope gene. Recent studies have indicated that virus variants in breast milk are not phylogenetically compartmentalized from those in plasma but may be partially produced by local infected cells (15, 36). Therefore, the magnitude of the humoral immune pressure in an anatomic compartment can be assessed by determining the amino acid mutation rate of the SIV envelope in the compartment-specific virus population. We performed single genome amplification and virus sequencing to determine the number of amino acid mutations in the envelopes of plasma and milk SIV variants collected between 48 and 74 weeks after infection compared to those of the inoculation virus quasispecies. The median number of amino acid mutations in the SIV envelope were similar in plasma and milk virus variants during chronic infection in three monkeys, compared by paired, nonparametric t tests (Fig. 9A) (P = 0.5; P = 0.19; P = 0.67). Moreover, a specific pattern of SIV envelope amino acid mutation was not identified in milk virus variants compared to plasma virus variants (Fig. 9B). The amino acid mutations clustered in the variable loops and gp41 in both plasma and milk viruses in a similar pattern to what has been previously described in SIV-vaccinated and unvaccinated SIV-infected monkeys (2). Therefore, the evolution of the SIV envelope is parallel in blood and milk virus populations, suggesting that the humoral immune pressure in milk is not distinct from that in plasma.
FIG. 9.
Similar SIV envelope evolution of virus variants in plasma and breast milk samples during chronic SIV infection. (A) The median number of acquired mutations in the virus envelope of plasma (filled box) and breast milk (unfilled box) virus variants are similar in all three monkeys. The results from paired, nonparametric t testing of the median number of plasma and milk virus envelope mutations are as follows: monkey 206 at week 74, 23 blood virus variants and 21 milk virus variants (P = 0.5); monkey 402 at week 67, 22 blood virus variants and 18 milk virus variants (P = 0.19); and monkey 403 at week 48, 20 blood virus variants and 15 milk virus variants (P = 0.67). (B) The frequency of acquired amino acid mutations in the SIV envelope gene product is similar in plasma (closed symbol) and breast milk (open symbol) virus variants from chronic infection in three monkeys. The frequency of an amino acid mutation is graphed as a function of amino acid position within the envelope. Envelope variable regions 1 to 5 (V1 to −5) are indicated. Amino acid positions with no mutations in all virus variants were not plotted.
DISCUSSION
Breast milk is an important means of maternal antibody transfer to the developing infant and provides passive immune protection against a variety of neonatal pathogens. However, the role of breast milk antibody in the protection of infants from viral pathogens transmitted via breast milk is unclear. The magnitude of HIV-specific antibody responses is not associated with HIV transmission via breast milk (3, 11, 23). However, neutralization-resistant viruses have been reported to initiate infant HIV infections (9, 38), suggesting that functional maternal antibody responses are critical in protecting the infant from virus acquisition. In this study, we describe the limited potency of SIV-specific antibody responses in milk samples and find no divergence in the envelope sequence evolution of breast milk and systemic virus populations, suggesting that the mucosal antibody response in milk has a limited role in milk immunodeficiency virus neutralization.
The predominant antibody isotype in human breast milk is secretory IgA, whereas IgG comprises only 1 to 5% of breast milk Ig (6, 29). The IgA in milk is either produced by local plasma cells in the mammary gland or actively transported by epithelial cells via the polymeric Ig receptor (32). In contrast, IgG traffics mainly from the serum to the mucosal surface of the mammary gland by transudation across the mucosal epithelium (16). While the principal antibody isotype in milk is locally produced secretory IgA, and hormone-induced lactating monkeys have a slightly higher secretory IgA content than naturally lactating monkeys (35), we found that the SIV-specific humoral response in milk obtained from rhesus monkeys is predominantly the IgG isotype. Furthermore, the limited virus-specific mucosal IgA responses occurred in a setting of less dramatic pathogenicity and CD4+ T-lymphocyte loss than previously described in these Mamu-A*01+ animals (33). In contrast, SIV Gag p27-specific IgA responses were similar in magnitude to that of SIV Gag p27-specific IgG responses in milk samples, suggesting a specific impairment of the SIV envelope-specific IgA responses in breast milk.
Our finding of a limited mucosal SIV envelope-specific IgA response in breast milk is in concordance with several other studies of HIV-specific humoral responses in breast milk (3, 42) and in other mucosal compartments, such as the genital tract and saliva (4, 12, 14, 27, 31, 39, 43, 46, 47). This low-magnitude virus-specific mucosal IgA response in breast milk is distinct from those described for other mucosal pathogens. Antibody responses specific for respiratory syncytial virus (13) and rotavirus (37) in breast milk are predominantly the IgA isotype. The specific impairment of HIV/SIV envelope-specific IgA responses in milk and other mucosal areas may be associated with poor HIV/SIV envelope immunogenicity, hypothesized to be the result of few envelope glycoprotein spikes on the surface of the virion or heavy glycosylation of the antigen (34, 44, 48). Moreover, HIV-induced humoral immune dys-regulation and significant CD4+ T-lymphocyte loss in the gastrointestinal tract may impair the lymphocyte responses of the gut-mammary axis during infection.
We were not able to detect an inoculation virus-specific neutralizing antibody response in breast milk samples obtained from SIV-infected lactating rhesus monkeys by 1 year after infection, indicating that breast milk antibody may not be effective at neutralizing autologous virus at its in vivo concentration. As the concentrations of antibodies are significantly lower in breast milk than in plasma, we assessed the contribution of plasma and milk IgG and IgA to the neutralization of a TCLA SIV pseudovirus using purified immunoglobulin to normalize the concentrations of antibodies. These data demonstrated that breast milk SIV-specific IgG and IgA are considerably lower in potency than that of plasma IgG. The low neutralizing potency of the locally produced or actively transported IgA response may be explained by impairment of mucosal lymphocyte responses, as previously discussed. However, the breast milk IgG response also had lower neutralizing potency than that of plasma IgG, despite evidence that IgG in human milk is comprised mainly of IgG that traffics to the milk by transudation from the plasma. Identification of a difference in the function of milk and plasma IgG at the same concentration of antibody supports selective transfer of plasma IgG into breast milk. In fact, selective transfer of IgG1 from plasma into milk mediated by Fc receptors on mucosal epithelial cells has been described in cattle (21, 24) and has been hypothesized in humans (30). Therefore, the regulation of the transport of IgG isotypes from blood into human milk remains to be fully characterized.
Finally, in our evaluation of the SIV envelope evolution of plasma and breast milk virus populations during chronic infection, we found no difference in the number or location of acquired amino acid mutations in plasma and breast milk virus variants. No compartment-specific SIV envelope evolution was identified in milk samples, in spite of evidence that virus in breast milk replicates locally (36). However, this finding further supports the lack of a potent, functional SIV-specific antibody response in breast milk that would be expected to drive compartment-specific neutralization escape. The SIV envelope of milk virus is therefore likely to be under humoral immune pressure similar to that in plasma.
Taken together, the findings of a low-potency SIV-specific neutralizing antibody response in milk samples and the lack of breast milk compartment-specific SIV envelope evolution indicate that the humoral immune response in milk is not likely to neutralize virus in the infant gastrointestinal tract. Alternative breast milk antibody virus-specific functions, such as inhibition of virus transcytosis or Fc receptor-mediated blocking of virus and target cell interaction, may play a role in protection of the infant from virus transmission via breastfeeding. Importantly, the limited mucosal HIV/SIV-specific humoral response detected in milk samples obtained from infected individuals does not preclude the possibility that induction of an HIV-specific humoral response in breast milk through a maternal vaccination could protect breastfeeding infants from virus acquisition. In fact, a vaccine-elicited HIV-specific binding antibody response may have protected vaccinated subjects from virus acquisition in a recent phase III HIV vaccine trial (40). The design of a maternal vaccine to prevent breast milk transmission of HIV will be aided by identification of virus-specific humoral immune responses in milk that can prevent the transmission of HIV in the infant gastrointestinal tract.
ACKNOWLEDGMENTS
This work was supported by the Center for HIV/AIDS Vaccine Immunology (grant R01AI067854; to S.R.P. and N.L.L.), NIH grant K08AI087992 (to S.R.P.), the Children's Hospital Boston Faculty Development Award (to S.R.P.), the Pediatric Infectious Disease Society/St. Jude Children's Hospital Basic Science Research Award (to S.R.P.), and the Bill and Melinda Gates Collaboration for AIDS Vaccine Discovery Vaccine Immune Monitoring Consortium grant 38619 (to M.S.S.).
Footnotes
Published ahead of print on 2 June 2010.
No conflict of interest exists among us.
REFERENCES
- 1.Amedee AM, Lacour N, Ratterree M. Mother-to-infant transmission of SIV via breast-feeding in rhesus macaques. J. Med. Primatol. 2003;32:187–193. doi: 10.1034/j.1600-0684.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 2.Basavapathruni A, Yeh WW, Coffey RT, Whitney JB, Hraber PT, Giri A, Korber BT, Rao SS, Nabel GJ, Mascola JR, Seaman MS, Letvin NL. Envelope vaccination shapes viral envelope evolution following simian immunodeficiency virus infection in rhesus monkeys. J. Virol. 2010;84:953–963. doi: 10.1128/JVI.01679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becquart P, Hocini H, Levy M, Sepou A, Kazatchkine MD, Belec L. Secretory anti-human immunodeficiency virus (HIV) antibodies in colostrum and breast milk are not a major determinant of the protection of early postnatal transmission of HIV. J. Infect. Dis. 2000;181:532–539. doi: 10.1086/315255. [DOI] [PubMed] [Google Scholar]
- 4.Belec L, Dupre T, Prazuck T, Tevi-Benissan C, Kanga JM, Pathey O, Lu XS, Pillot J. Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J. Infect. Dis. 1995;172:691–697. doi: 10.1093/infdis/172.3.691. [DOI] [PubMed] [Google Scholar]
- 5.Belec L, Ghys PD, Hocini H, Nkengasong JN, Tranchot-Diallo J, Diallo MO, Ettiegne-Traore V, Maurice C, Becquart P, Matta M, Si-Mohamed A, Chomont N, Coulibaly IM, Wiktor SZ, Kazatchkine MD. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 2001;184:1412–1422. doi: 10.1086/324375. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. The secretory immune system of lactating human mammary glands compared with other exocrine organs. Ann. N. Y. Acad. Sci. 1983;409:353–382. doi: 10.1111/j.1749-6632.1983.tb26883.x. [DOI] [PubMed] [Google Scholar]
- 7.Chomont N, Hocini H, Gody JC, Bouhlal H, Becquart P, Krief-Bouillet C, Kazatchkine M, Belec L. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology. 2008;370:246–254. doi: 10.1016/j.virol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Devito C, Hinkula J, Kaul R, Kimani J, Kiama P, Lopalco L, Barass C, Piconi S, Trabattoni D, Bwayo JJ, Plummer F, Clerici M, Broliden K. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J. Virol. 2006;80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorrell L, Hessell AJ, Wang M, Whittle H, Sabally S, Rowland-Jones S, Burton DR, Parren PW. Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. AIDS. 2000;14:1117–1122. doi: 10.1097/00002030-200006160-00008. [DOI] [PubMed] [Google Scholar]
- 11.Duprat C, Mohammed Z, Datta P, Stackiw W, Ndinya-Achola JO, Kreiss JK, Holmes KK, Plummer FA, Embree JE. Human immunodeficiency virus type 1 IgA antibody in breast milk and serum. Pediatr. Infect. Dis. J. 1994;13:603–608. doi: 10.1097/00006454-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Fiore JR, Laddago V, Lepera A, La Grasta L, Di Stefano M, Saracino A, Lopalco P, Pastore G, Angarano G. Limited secretory-IgA response in cervicovaginal secretions from HIV-1 infected, but not high risk seronegative women: lack of correlation to genital viral shedding. New Microbiol. 2000;23:85–92. [PubMed] [Google Scholar]
- 13.Fishaut M, Murphy D, Neifert M, McIntosh K, Ogra PL. Bronchomammary axis in the immune response to respiratory syncytial virus. J. Pediatr. 1981;99:186–191. doi: 10.1016/s0022-3476(81)80447-7. [DOI] [PubMed] [Google Scholar]
- 14.Haimovici F, Mayer KH, Anderson DJ. Quantitation of HIV-1-specific IgG, IgA, and IgM antibodies in human genital tract secretions. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;15:185–191. doi: 10.1097/00042560-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Heath L, Conway S, Jones L, Semrau K, Nakamura K, Walter J, Decker WD, Hong J, Chen T, Heil M, Sinkala M, Kankasa C, Thea DM, Kuhn L, Mullins JI, Aldrovandi GM. Restriction of HIV-1 genotypes in breast milk does not account for the population transmission genetic bottleneck that occurs following transmission. PLoS One. 2010;5:e10213. doi: 10.1371/journal.pone.0010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochwald GM, Thorbecke GJ. Occurrence of myeloma-like gamma-globulin in C.S.F. of a four-month old infant with hydrocephalus. Pediatrics. 1964;33:435–440. [PubMed] [Google Scholar]
- 17.Hocini H, Becquart P, Bouhlal H, Adle-Biassette H, Kazatchkine MD, Belec L. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin. Diagn. Lab. Immunol. 2000;7:515–518. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann-Lehmann R, Rasmussen RA, Vlasak J, Smith BA, Baba TW, Liska V, Montefiori DC, McClure HM, Anderson DC, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Katinger H, Stiegler G, Posner MR, Cavacini LA, Chou TC, Ruprecht RM. Passive immunization against oral AIDS virus transmission: an approach to prevent mother-to-infant HIV-1 transmission? J. Med. Primatol. 2001;30:190–196. doi: 10.1034/j.1600-0684.2001.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 19.John GC, Richardson BA, Nduati RW, Mbori-Ngacha D, Kreiss JK. Timing of breast milk HIV-1 transmission: a meta-analysis. East Afr. Med. J. 2001;78:75–79. doi: 10.4314/eamj.v78i2.9092. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone A, Thorpe R. Immunochemistry in practice. Blackwell Scientific Publications; Oxford, England: 1982. [Google Scholar]
- 21.Kemler R, Mossmann H, Strohmaier U, Kickhofen B, Hammer DK. In vitro studies on the selective binding of IgG from different species to tissue sections of the bovine mammary gland. Eur. J. Immunol. 1975;5:603–608. doi: 10.1002/eji.1830050905. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K. Studies on human secretory IgA comparative studies of the IgA-bound secretory piece and the free secretory piece protein. Immunochemistry. 1971;8:785–800. doi: 10.1016/0019-2791(71)90446-0. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn L, Trabattoni D, Kankasa C, Sinkala M, Lissoni F, Ghosh M, Aldrovandi G, Thea D, Clerici M. HIV-specific secretory IgA in breast milk of HIV-positive mothers is not associated with protection against HIV transmission among breast-fed infants. J. Pediatr. 2006;149:611–616. doi: 10.1016/j.jpeds.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leary HL, Jr., Larson BL, Nelson DR. Immunohistochemical localization of IgG1 and IgG2 in prepartum and lactating bovine mammary tissue. Vet. Immunol. Immunopathol. 1982;3:509–514. doi: 10.1016/0165-2427(82)90016-2. [DOI] [PubMed] [Google Scholar]
- 25.Leroy V, Newell ML, Dabis F, Peckham C, Van de Perre P, Bulterys M, Kind C, Simonds RJ, Wiktor S, Msellati P. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu FX. Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin. Immunol. 2000;97:59–68. doi: 10.1006/clim.2000.4910. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Maza O, Crabb E, Mitsuyasu RT, Fahey JL, Giorgi JV. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 1987;138:3720–3724. [PubMed] [Google Scholar]
- 29.McClelland DB, Warwick RR, Shearman DJ. IgA concentration. Am. J. Dig. Dis. 1973;18:347–348. doi: 10.1007/BF01070997. [DOI] [PubMed] [Google Scholar]
- 30.Mehta PD, Mehta SP, Isaacs CE. Distribution of IgG subclasses in human colostrum and milk. Immunol. Lett. 1989;22:235–238. doi: 10.1016/0165-2478(89)90197-1. [DOI] [PubMed] [Google Scholar]
- 31.Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, Sabbaj S, Mulligan MJ, Goepfert PA. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res. Hum. Retroviruses. 2004;20:972–988. doi: 10.1089/aid.2004.20.972. [DOI] [PubMed] [Google Scholar]
- 32.Mostov KE, Blobel G. Biosynthesis, processing, and function of secretory component. Methods Enzymol. 1983;98:458–466. doi: 10.1016/0076-6879(83)98173-9. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P, Rudersdorf RR, Liebl ME, Krebs K, Vasquez J, Dodds E, Loffredo J, Martin S, McDermott AB, Allen TM, Wang C, Doxiadis GG, Montefiori DC, Hughes A, Burton DR, Allison DB, Wolinsky SM, Bontrop R, Picker LJ, Watkins DI. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J. Virol. 2003;77:9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pantophlet R, Burton DR. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 2006;24:739–769. doi: 10.1146/annurev.immunol.24.021605.090557. [DOI] [PubMed] [Google Scholar]
- 35.Permar SR, Kang HH, Carville A, Mansfield KG, Gelman RS, Rao SS, Whitney JB, Letvin NL. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J. Immunol. 2008;181:3643–3650. doi: 10.4049/jimmunol.181.5.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Permar SR, Kang HH, Wilks AB, Mach LV, Carville A, Mansfield KG, Learn GH, Hahn BH, Letvin NL. Local replication of simian immunodeficiency virus in the breast milk compartment of chronically-infected, lactating rhesus monkeys. Retrovirology. 2010;7:7. doi: 10.1186/1742-4690-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman MM, Yamauchi M, Hanada N, Nishikawa K, Morishima T. Local production of rotavirus specific IgA in breast tissue and transfer to neonates. Arch. Dis. Child. 1987;62:401–405. doi: 10.1136/adc.62.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rainwater SM, Wu X, Nduati R, Nedellec R, Mosier D, John-Stewart G, Mbori-Ngacha D, Overbaugh J. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr. HIV Res. 2007;5:189–197. doi: 10.2174/157016207780076986. [DOI] [PubMed] [Google Scholar]
- 39.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, Blondeau C. Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res. Hum. Retroviruses. 1999;15:1365–1376. doi: 10.1089/088922299310070. [DOI] [PubMed] [Google Scholar]
- 40.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 41.Ruprecht RM, Ferrantelli F, Kitabwalla M, Xu W, McClure HM. Antibody protection: passive immunization of neonates against oral AIDS virus challenge. Vaccine. 2003;21:3370–3373. doi: 10.1016/s0264-410x(03)00335-9. [DOI] [PubMed] [Google Scholar]
- 42.Rychert J, Amedee AM. The antibody response to SIV in lactating rhesus macaques. J. Acquir. Immune Defic. Syndr. 2005;38:135–141. doi: 10.1097/01.qai.0000148947.03416.b5. [DOI] [PubMed] [Google Scholar]
- 43.Schafer F, Kewenig S, Stolte N, Stahl-Hennig C, Stallmach A, Kaup FJ, Zeitz M, Schneider T. Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut. 2002;50:608–614. doi: 10.1136/gut.50.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 45.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–455. [PubMed] [Google Scholar]
- 46.Williams SB, Flanigan TP, Cu-Uvin S, Mayer K, Williams P, Ettore CA, Artenstein AW, Duerr A, VanCott TC. Human immunodeficiency virus (HIV)-specific antibody in cervicovaginal lavage specimens obtained from women infected with HIV type 1. Clin. Infect. Dis. 2002;35:611–617. doi: 10.1086/342201. [DOI] [PubMed] [Google Scholar]
- 47.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, VanCott TC, Trabattoni D, Sannella E, Mestecky J. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res. Hum. Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 48.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]