Abstract
Traditional Chinese Medicines (TCM) are rapidly gaining attention in the West as sources of new drugs, dietary supplements and functional foods. However, lack of consistent manufacturing practices and quality standards, fear of adulteration, and perceived deficiencies in scientific validation of efficacy and safety impede worldwide acceptance of TCM. In addition, Western pharmaceutical industries and regulatory agencies are partial toward single ingredient drugs based on synthetic molecules, and skeptical of natural product mixtures. This review concentrates on three examples of TCM-derived pharmaceuticals and functional foods that have, despite these usual obstacles, risen to wide acceptance in the West based on their remarkable performance in recent scientific investigations. They are: Sweet wormwood (Artemisia annua), the source of artemisinin, which is the currently preferred single compound anti-malarial drug widely used in combination therapies and recently approved by US FDA; Thunder god vine (Tripterygium wilfordii) which is being developed as a botanical drug for rheumatoid arthritis; and green tea (Camellia sinensis) which is used as a functional beverage and a component of dietary supplements.
Keywords: Artemisia annua, artemisinin, Camellia sinensis, (−)-epigallocatechin-3-gallate (EGCG), functional foods, nutraceuticals, traditional Chinese medicine (TCM), tripdiolide, Tripterygium wilfordii, triptolide
INTRODUCTION
The history of Traditional Chinese Medicines (TCM) dates back more than four thousand years ago to the times of Emperor Yan or Shennong. According to Chinese mythology, Emperor Yan was personally involved in the studies of medicinal plants and died from an herbal poison he had created. He is credited with the development of the first Chinese pharmacopeia and as the father of TCM. The refinement and study of TCM has continued for thousands of years and led to the development an incredibly diverse compendium of modern TCM. According to a recent review, 11,146 plant species representing 2,309 genera and 383 families are used in TCM [1]. In addition, 1,581 species of animals and 80 minerals and substances ranging from precious stones to mineralized fossils are used in preparing TCM. This huge amount of historically accumulated data makes it difficult to document and organize information on the uses and compositions of TCM despite the creation of multiple English and Chinese language databases [2].
Despite rapid globalization and integration of national health care systems, TCM show no signs of losing ground. The World Health Organization reports that sales of herbal traditional medicines in China reached $14 billion in 2005 and may approach $20 billion in 2010 [3]. Moreover, interest in TCM is growing steadily in the U.S. and in Europe, with the associated increase in licensed TCM providers in the U.S. At the same time, the Asian public is increasingly seeking treatments originating in modern Western medicine, often viewing both systems as complimentary to each other. The successes achieved by China in promoting TCM have encouraged scientists worldwide to apply modern experiment-based research methods toward isolating active compounds from TCM, defining their molecular modes of action and characterizing their pharmacodynamic and pharmacokinetic properties. However, these studies have had limited success. Western researchers strive to channel their experiments to a somewhat reductionist 'single chemical entity' paradigm of Western medicine, which is not easily applicable to the multi-dimensional complexities of TCM [4].
Clearly, TCM complexity, variability and underlying philosophical systems present challenges for researchers seeking scientific evidence supporting the use of TCM in modern drug discovery. These challenges primarily lie in the multi-component and multi-species composition of many TCM but can also be attributed to widely reported deficiencies in TCM standardization and quality control. Unscrupulous marketing and questionable manufacturing practices have led to long-standing concerns about adulterations in botanical products in general and TCM in particular, including contamination with undisclosed prescription or over-the-counter drugs [5]. The fundamental belief that that the TCM action is based on interactions between its multiple components makes TCM difficult to standardize and study using available scientific methods. Nevertheless, the realization that multi-component medicines may have advantages over a single component drugs has scientific foundation. The pharmacological advantages of mixtures may in lie in the potentiating action of their multiple bioactive components, in essence making them combination drugs, which are extremely costly and difficult to develop and approve by Western regulatory agencies [6, 7]. These advantages, if scientifically validated and translated into a standardized and properly developed pharmaceutical product, can yield a new generation of efficacious human medicines. Similar reasoning led US Federal Drug and Food Administration (FDA) to publish a botanical drug guidance that, for the first time, outlined a pre-clinical and clinical path for the development of botanical drugs based on multi-component plant extracts rather than on a single active molecule [8].
Regulatory pressures and consumer demand have created a need to develop effective tools to identify and functionally characterize pharmacologically active components of complex mixtures derived from multiple species of plants and animals commonly used in TCM recipes. Recent advances in chromatography, mass spectrometry and nuclear magnetic resonance spectroscopy already allow comprehensive study of TCM derived from a single plant species. As a result, the Western health care establishment is beginning to validate and accept the value of TCM that have been used for centuries. This review provides case studies of three plants used in TCM that are being successfully adapted by the Western pharmaceutical and health and wellness markets thanks to a significant body of evidence derived from rigorous scientific research. These plants are: Sweet wormwood (Artemisia annua), the source of artemisinin, which is the currently preferred single compound anti-malarial drug widely used in combination therapies and recently approved by US FDA; Thunder god vine (Tripterygium wilfordii) which is being developed as a botanical drug for rheumatoid arthritis; and green tea (Camellia sinensis) which is used as functional beverage and a component of dietary supplements.
Growing Western acceptance of the medicinal value of these plants can be at least partially explained by the fact that their pharmacological activity could be traced to a single compound or to a family of related compounds.
CASE STUDY I: ARTEMISININ FROM SWEET WORMWOOD (ARTEMISIA ANNUA L.) - FRONTLINE ANTI-MALARIAL DRUG FROM TCM
Medicinal History and Botany
More than 400 years ago, the western world was introduced to a miracle cure for malaria from Cinchona sp. brought to Europe from Peru [9]. The bark of this plant is the natural source of quinine, which, along with its synthetic derivatives (i.e. chloroquine), has been used as an anti-malarial therapy since its isolation in 1834. However, unknown to the Western world, an arguably more potent anti-malarial plant was known in the East, particularly in China, more than 2000 years earlier. Artemisia annua, known as qinghao, is celebrated in TCM as a treatment for malaria. It is the source of artemisinin (qinghaosu), a compound that has largely replaced quinine and other quinoline anti-malarials as the compound of choice in frontline anti-malarial chemotherapies [10]. The history and life-saving clinical efficacy of A. annua is a testament to the medicinal value of TCM and their successful integration into Western medicine.
The earliest known medicinal record of A. annua (also known as qinghao) is a remedy for hemorrhoids in a text known as “The recipes for 52 kinds of diseases” dated to the West Han Dynasty (168BC) [11]. A. annua is also noted for its use as an anti-inflammatory in shen nong ben cao jing (200 AD), a text thought to represent centuries of traditional medicinal knowledge and may therefore predate that recorded in “The recipes for 52 kinds of diseases” [12]. The anti-febrile properties of A. annua are first mentioned in the zhou hou bei ji fang (“Emergency prescriptions kept up ones sleeve”) written by Ge Hong in 341 AD [13]. Thereafter, one can find many TCM texts and oral traditions stating the anti-malarial value of A. annua, including the ben cao gan mu (Compendium of Materia Medica, 1596) composed by Li Shizen and the wen bing tiao bian (1798) [13–15].
During the 1960’s and 70s when the People’s Republic of China was in the midst of a cultural revolution that separated it from the West, these ancient texts proved invaluable to the isolation and evaluation of artemisinin from A. annua [14]. During this time, the Chinese government sought new chemotherapeutic means of combating malaria, both amongst Chinese citizens and Northern Vietnamese fighting in the Vietnam War [16]. In 1967, “Project 523” was established, which, among other endeavors, sought to systematically evaluate anti-malarial remedies from TCM, and discover novel active compounds (see [17] for a detailed account of the project).
Among the top 10 plant species tested in the project was A. annua. In what could have been a misfortune of historic consequences, initial tests using hot water and ether extracts of A. annua did not reveal much of its anti-malarial activity [18]. (In hindsight, this is not surprising as artemisinin is poorly soluble in ether solvent, and is heat labile). However, a reevaluation of TCM texts led Tu Youyou and colleagues to revise their extraction techniques to better reproduce traditional methods. According to Dr. Elizabeth Hsu [14] a simple instruction given by Ge Hong in the zhou hou bei ji fang (Emergency prescriptions kept up ones sleeve) - “qinghao, one bunch, take two sheng (2× 0.2L) of water for soaking it, wring it out, take the juice, ingest in its entirety” - produced, probably through emulsification, bioactive artemisinin extract. In 1971–1972 this extract of A. annua was proven active in vitro as well as in murine, simian and human cases of malaria [16, 17]. In 1973, artemisinin (qinghaosu) was isolated in crystalline form and subsequent tests revealed its chemical properties and structure [15]. The persistence of the Chinese scientists and their faith in TCM proved vital to their success.
A. annua L. is a 70–200 cm tall, much branched herb that can be found growing in a wide variety of environments [19]. It is a member of one of the largest genera (Artemisia, with up to 500 species) of one of the largest plant families, the Asteraceae [20]. It is native to China but has been introduced and grows wild throughout Asia, North American, and Europe [21] and is now broadly cultivated for medicinal purposes [22]. A. annua is so named because it is one of only a dozen or so species of Artemisia to have an annual life cycle [23].
Artemisia spp. are rich in secondary metabolites, which may explain their use as bittering agents, perfumes, culinary spices, and hallucinogens (i.e. A. absinthum, A. dracunculus, A. vulgaris) [20, 22]. Only two other species (A. apiaceae and A. lancea) along with A. annua have been shown to possess artemisinin [24]. Numerous other classes of phytochemicals have also been isolated from A. annua including monoterpenes, sesquiterpenes, triterpenoids, steroids, flavonoids, coumarins, phenolics, and various lipids [25] several of which may be responsible for the overall activity and properties of crude A. annua extracts when compared to that of isolated artemisinin [12].
Artemisinin - The Bioactive
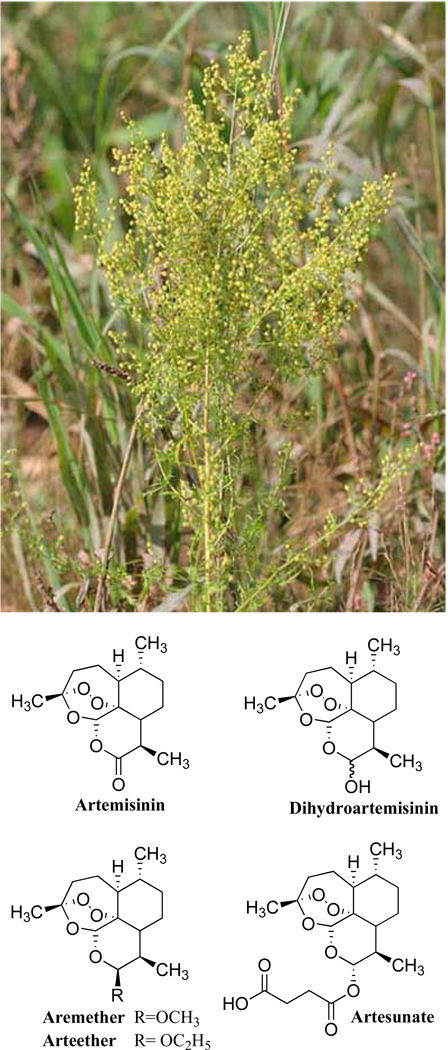
Artemisinin is a sesquiterpene lactone with a unique endoperoxide bridge (Fig. 1). Its biosynthesis in A. annua is restricted to the apices of glandular secretory trichomes on the aerial surface of the plant [26]. Like other sesquiterpenes, artemisinin is synthesized via the mevalonate pathway and includes an amorpha-4,11-diene intermediate, from which related amorphane-type sesquiterpenes in A. annua are also derived [27].
Fig. (1).
Artemisia annua L. and chemical structures of artemisinins.
Artemisinin concentration in A. annua varies from as low as 0.01% to over 2.0% in some selected cultivars [22, 28]. Despite the fact that several de novo methods of artemisinin synthesis have been reported [29] and that microorganisms have been engineered to produce artemisinin precursors [30], A. annua remains the most economical source of artemisinin. Consequently, a great deal of work has been directed toward the cultivation and optimization of high artemisinin-yielding cultivars of A. annua [22, 28].
At present, the clinical importance of artemisinin is over-shadowed by its semi-synthetic derivatives (known as artemisinins), notably artesunate, artemether, and increasingly, dihydroartemisinin (Fig. 1) [31, 32]. The reason for this is mainly related to the poor bioavailability of artemisinin (at 8–10%) when compared to Artemether (54%), and Dihydroartemisinin (85%), Artesunate (82%) [33]. Artemisinin is poorly soluble in both water and oil. However, reduction of the lactone in dihydroartemisinin and artemether has led to increased oil solubility, while the acidic moiety lends water-solubility to artesunate [29]. Furthermore, the metabolism of artemether, artesunate and dihydroartemisinin produces metabolites with comparable activity, artemisinin is metabolized into largely inactive products [33].
Mode of Action
The mode of action of artemisinins is still debated, but is known to be mechanistically different from that of quinine-type molecules, as evidenced by its activity against chloroquine resistant strains of Plasmodium [15]. The endoperoxide bridge is necessary for the activity of artemisinins. Many analogues that share this structural feature are active, while artemisinin derivatives lacking the endoperoxide bridge are inactive [29]. The endoperoxide bridge most likely contributes to the prodrug nature of artemisinins, which are thought to be activated in vivo to give rise to either carbon or oxygen centered radicals that are ultimately responsible for the anti-malarial activity [16].
Several competing theories exist regarding the activation mechanisms and ultimate targets of artemisinins. The parasitized intraerythrocytic environment is rich in Fe2+ (as a result of hemoglobin digestion), which may catalyze the formation of artemisinin radicals [34]. These radicals are thought to selectively react with heme, forming an adduct that kills the parasites either through the inhibition of hemazoin synthesis (the “malaria pigment”) or, more likely, through interaction with a secondary protein target [16, 34, 35].
A contradictory theory holds that because artemisinin localizes outside of the iron rich food vacuole, and the hemazoin lacking ring-stage parasites are also killed by artemisinin, the site of drug action is not the food vacuole. Ecksetein-ludwig et al. propose that artemisinin inhibits parasitic sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [36]. It was noted that artemisinins are structurally similar to other SERCA inhibitors, and Xenopus laevis expressed SERCA analog is specifically and selectively inhibited by artemisinin.
Other mechanisms have been variously proposed to explain the mechanism of action of artemisinins (i.e. inhibition of the electron transport chain by Li et al. [37]), and the numerous well written reviews over the past several years are a testament to the importance of this debate [16, 38, 39].
Clinical Pharmacology
The artemisinins are the most potent anti-malarial compounds currently known: fever clearance is more than twice as rapid and parasite biomass reduction 1,000 times more efficient when compared with other anti-malarials [12, 31]. Furthermore, artemisinins are among only a few anti-malarial compounds that act on Plasmodium gametocytes, and can drastically reduce parasite transmission [40]. However, artemisinins are metabolized quickly in vivo and, therefore, have a short half-life, on the order of 2–5 hours, compared to multiday half lives of quinoline anti-malarials [41]. Short course administration often results in an incomplete parasite clearance, which may lead to recrudescence (the later redevelopment of malaria from surviving parasites).
In efforts to solve the recrudescence problem and simultaneously discourage the evolution of artemisinin resistant Plasmodium, the WHO has implemented a strict anti-malarial drug regime emphasizing the use of Artemisinin Combination Therapies (ACTs), which combine short acting artemisinins with longer acting mechanistically different anti-malarials [32]. Currently used ACTs include- artesunate/ amodiaquine, artesunate/ mefloquine, artesunate/ sulfadoxine/ pyrimethamine, artemether/ lumefantrine, and dihydroartemisinin/ piperaquine.
Despite preemptive efforts to avoid the development of resistance, several cases of reduced artemisinin sensitivity have been described. According to Cui and Su [16] many proposed cases of resistance are in need of stricter clinical review, however, populations of parasites in Southeast Asia exhibit preliminary signs of resistance. Therefore, caution should be taken in monitoring these areas. It should be noted that southeast Asia represents a hot bed of anti-malarial drug resistance where individual strains resistant to all classes of anti-malarial drugs except artemisinins have been confirmed [42]. Furthermore, A. annua, has been used in this area as an anti-malarial for thousands of years allowing a longer evolutionary window for the development of resistant parasites.
Additional Applications
A. annua has been used as TCM for centuries and, aside from its anti-malarial properties, has been used to induce hair growth, to promote longevity, as a food additive, as an anti-inflammatory, as well as a treatment for numerous external ails including hemorrhoids, lice and boils [13]. The medicinal value of artemisinins beyond the realm of malaria has also been realized [43]. Studies have shown that sesquiterpene lactones from A. annua have activity against phylogenetically unrelated parasites including Trypanosoma spp., which are the causative agents of trypanosomiasis [44], Schistosoma spp., which are the causative agents of schistosomiasis [45], as well as Plasmodium related apicomplexa including Toxoplasma spp. and Babesia spp., which cause toxoplasmosis and babesiosis, respectively. Artemisinins may also be effective for the treatment of cancer, as recently reviewed by Krishna et al. [43].
Conclusion
The development of modern anti-malarials from A. annua is a validation of the lasting value of TCM for the development of modern pharmaceuticals and a proof that plants may still harbor many, yet undiscovered, life-saving drugs. The long and convoluted history of artemisinin demonstrates the difficulties associated with the development of modern pharmaceuticals from ethnobotanical sources and confirms that at least part of this development can be done by nonprofit organizations. The US Food and Drug Administration (FDA) has just written one of the last chapters in the worldwide acceptance of the artemisinin-derived anti-malarial drugs. In April of 2009, the agency approved Novartis-made Coartem® (artemether 20 mg/lumefantrine 120 mg) for the treatment of malaria.
CASE STUDY II: THUNDER GOD VINE (TRIPTERYGIUM WILFORDII HOOK. F.) - TCM IN CLINICAL TRIALS
Medicinal History and Botany
T. wilfordii (Celastraceae), known as Thunder god vine or Lei gong teng, is a climbing shrub with a long history of diverse use in TCM. For many centuries it has been collected in the mountains of Southern China and its roots used in various preparations to “relieve stasis and internal warmth” among many other conditions diagnosed by TCM practitioners. Extensive literature search returns over 1000 publications that address biochemical, pharmacological and other properties of T. wilfordii and its components. Large numbers of Chinese studies suggested therapeutic value of T. wilfordii in a number of autoimmune and inflammatory conditions and numerous attempts have been made to improve its efficacy and safety (for comprehensive reviews see [46, 47]). Despite the fact that various types of T. wilfordii extracts are available in China for treating a number of diseases, few randomized controlled trials had been carried out and little is known about the mechanism of action of T. wilfordii and its bioactive components. In addition, few attempts at standardization or quality control of the extract have been made. The situation changed when scientists working at the University of Texas Southwestern Medical Center and US National Institutes of Health (NIH) began working with anti-inflammatory extracts made from debarked roots of T. wilfordii. Two relatively small, but properly controlled Phase 1 and Phase 2a clinical trials carried out in the US showed a significant benefits of standardized and optimized T. wilfordii extract in patients with rheumatoid arthritis [48, 49]. A recently completed, much larger, randomized and active-controlled, 24 weeks Phase 2b study with 121 patients further confirmed the beneficial effects of T. wilfordii [50]. This study demonstrated that oral administration of T. wilfordii extract was both safe and highly effective in treating patients with active rheumatoid arthritis. T. wilfordii extract showed significantly greater benefits than its comparator, sulfasalazine, and produced rapid improvements in clinical signs and symptoms of rheumatoid arthritis, including joint pain, joint swelling, and measures of overall well-being and inflammatory markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and interleukin-6 (IL-6). T. wilfordii extract was also effective in slowing radiographic joint damage, to levels rarely achieved by oral drugs.
To date, over 380 secondary metabolites have been isolated from T. wilfordii with 350 of these being structurally diverse terpenoids with a wide range of pharmacological activities including anti-inflammatory, immunosuppressive, and anti-neoplastic [46]. Subsequent biochemical analysis and activity guided fractionation of T. wilfordii extracts identified two diterpenoid tri-epoxides as being primarily responsible for the anti-inflammatory and immunosuppressive effects of T. wilfordii preparations - triptolide and tripdiolide (Fig. 2) [51, 52] - allowing the bioactive-based standardization of T. wilfordii extract used in pre-clinical and clinical studies reviewed above. The well accepted assumption that triptolide and tripdiolide are the main active ingredients in the T. wilfordii root extract is based on the fact that both compounds are the most abundant and the most pharmacologically active components present in the extract.
Fig. (2).
Tripterygium wilfordii Hook. F and chemical structures of its main bioactive compounds.
Anti-Inflammatory and Immunosuppressive Effects
Triptolide and tripdiolide (Fig. 2) are among the most powerful and broadly active anti-inflammatory/immunomodulating natural products ever discovered. For example, the successful Phase IIb clinical trial, described above, utilized a daily dose of 180 µg of triptolide/tripdiolide mixture constituting 0.1% of the total extract received by patients. Acting in the nmol concentration range, triptolide effectively inhibits the in vitro production of multiple inflammatory cytokines, such as interleukins (IL) 1, 2, 6 and 8, interferon-gamma (IFN-g), and tumor necrosis factor-a (TNF-a); pro-inflammatory enzymes, such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and metalloproteinases (MMPs); transcription factors, such as , nuclear factor (NF)-κβ, AP-1, NFAT and OCT-1, and the proliferation of T- and B-cells (for comprehensive review see [46]). The efficacy of both compounds was further quantified in a recent study, in which both triptolide and tripdiolide blocked the expression of COX-2, iNOS and IL-1β in mouse macrophages with IC50s between 10 and 50 nmol [51].
In addition to the strong curative effects of T. wilfordii extract or triptolide in animal rheumatoid arthritis models [53, 54], actives from T. wilfordii were effective in in vivo model systems for a variety of inflammatory and autoimmune diseases. These include: multiple sclerosis [55, 56], chronic colitis [57], lupus nephritis [58], autosomal dominant polycystic kidney disease (ADPKD) [59], prostatitis [60], asthma [61] and in transplant rejection [62]. Several excellent reviews of mainly Chinese literature present the results of the successful clinical use of T. wilfordii extract and its bioactive components for most the above diseases as well as in their in vivo animal models [47, 52]. The beneficial effect of T. wilfordii preparation on human rheumatoid arthritis, systemic lupus erythematosus and psoriasis are particularly well documented in Chinese scientific literature. Unfortunately, few of the reported clinical trials used the appropriate controls and other quality standards accepted in the West.
Despite decades of research and hundreds of manuscripts describing the phenomenology of anti-inflammatory and immunosuppressive effects of triptolide, tripdiolide and related T. wilfordii compounds, their primary cellular targets and modes of molecular action remain largely unknown. Their suppressive effect on the multiple downstream elements of the inflammatory and autoimmune cascade are so dramatic that it is tempting to suggest that, at least triptolide and tripdiolide, interact with several independent cellular targets. Investigations of global pro-inflammatory gene expression in lipopolysaccharide-treated macrophages showed that triptolide caused a >50% inhibition of 47 out of 117 studied genes. Profound inhibition of pro-inflammatory cytokine expression and several transcription factors in addition to NF-κB was observed at triptolide concentrations as low as 10–50 nmol. Thus, it is may be also reasonable to speculate that triptolide interacts with a common component of several transcription factors or transcription coactivators involved in regulating variety of pro-inflammatory and autoimmune genes [63]. Consistent with this hypothesis triptolide inhibited the NF-κB transcriptional activity in human anaplastic thyroid carcinoma cells via blocking the association of p65 subunit with CREB-binding protein (CBP)/p300 [64]. Similarly, in murine neuronal tissue, triptolide inhibited IκBα phosphorylation and NF-κB nuclear translocation by stabilization of the NF-κB/IκBα complex, possibly by enhancing a direct physical interaction between NF-κB and Hsp70 proteins, which were dramatically increased by trip-tolide treatment [55]. HSP70 and related heat shock proteins are known to stabilize various enzymatic and transcription complexes, thus altering their functions [65]. Yet another hypothesis explaining the strength and multiplicity of triptolide effect suggests that it acts as a partial modulator of the glucocorticoid receptor, whereby the modified receptor-triptolide complex cannot activate glucocorticoid-responsive genes while still being able to suppress anti-inflammatory cascades producing a combination of anti-inflammatory and steroid sparing effects [46].
Anti-Cancer Effects
An independent line of research supports the use of triptolide derived from T. wilfordii as a anti-neoplastic agent effective in vitro and in vivo against a great variety of cancers including colorectal cancer [66], oral cancer [67], ovarian cancer [68], breast cancer [69], and a variety of other solid tumors [70]. The anti-tumor activities of triptolide have been attributed to its pronounced apoptotic effects [71] and its recently discovered anti-angiogenesis properties [72].
While the anti-tumor activity of T. wilfordii extracts and triptolide are well documented in vitro and in vivo, the molecular mechanisms of their action, such as primary binding targets and downstream signaling, remain obscure. Several mechanisms for triptolide action have been proposed and documented, some of which are related to its anti-inflammatory activity. They include inhibition of TNF-251658240-mediated induction of c-IAP1 and c-IAP2 [73], inhibition of activation and transcriptional activity of NF251658240B [71, 74] and activation of caspases [75, 76], - possibly via a change in the permeability of the mitochondrial membrane in tumor cells, decreasing its potential and increasing cytochrome C release [77–78]. It was also recently proposed that triptolide may arrest cell growth via inducing Ca2+ release by a PC2-dependent mechanism [59]. In recent years, several triptolide-containing preparations have entered cancer clinical trials in the US.
Spermatocidal Effects
Unexpectedly, the administration of T. wilfordii preparations to male patients enrolled in clinical trials in China produced a pronounced but reversible spermatocidal effect leading to temporary infertility. This observation led to additional studies, which suggested that triptolide and related diterpenoids are the main anti-fertility compounds in T. wilfordii [79]. The discovery of the spermatocidal effect of T. wilfordii preparations and triptolide prompted several research programs directed to the development of human and/or veterinary male contraceptive based on triptolide. It was shown that triptolide did not affect hormonal level in treated animals or cytological and morphological characteristics of their testis, but dramatically reduced epididymal sperm content and mobility [79]. Other compounds present in T. wilfordii extract were recently shown to inhibit T-type Ca2+ currents in mouse spermatogenic cells [80], which may also contribute to the spermatocidal effect of this plant. At present, more emphasis is placed on the development of anti-inflammatory and immunomodulating drugs from T. wilfordii than on male contraceptives. Nevertheless, the spermatocidal effects of this TCM remain one of its most common side effects that may require extensive reproductive toxicology evaluation of all modern drugs derived from this plant. However, rheumatoid arthritis - the main clinical target for T. wilfordii-derived Western drugs, most often affects older women, making spermatocidal side effects of T. wilfordii more acceptable to the general public and regulatory agencies.
Conclusions
T. wilfordii, used for generations in TCM, has not only survived a validation by Western science but has yielded several promising clinical-stage drug candidates for rheumatoid arthritis and cancer. While total synthesis of triptolide and its analogues has been reported, it is rather complex and costly. Today, T. wilfordii extract remains the most cost-effective source of this compound. It remains to be seen whether Western pharmaceutical industries and regulatory agencies will accept a plant extract as a prescription drug, even if its batch-to-batch consistency, safety and efficacy can be assured. The absence of composition of matter patents on triptolide or T. wilfordii may provide another hurdle for its commercialization by the for-profit pharmaceutical company. Nevertheless, the large body of scientific research, preclinical, and clinical studies carried out with T. wilfordii and its components corroborate the value of this ancient TCM in modern medicine.
CASE STUDY III: GREEN TEA (CAMELLIA SINENSIS L. KUNTZE) - MEDICINAL FUNCTIONAL BEVERAGE FROM TCM
The leaves and leaf buds of Camellia sinensis (Fig. 3), a perennial evergreen shrub, are used to produce all types of tea including white tea, green tea, oolong, and black tea, each by using defined processes (including fermentation) resulting in different levels of oxidation. However, green teas alone contain the highest levels of catechins, the primary active compounds, as green tea processing favors retention of these phytochemicals. The centuries-old practice of green tea consumption from the East is now gaining trajectory in the Western world. This species, and in particular the green tea produced from it, is one of the most widely used TCM-derived, food-grade botanicals in the West, with a mounting body of literature researching its medicinal properties.
Fig. (3).
Camellia sinensis (L.) Kuntze and (−)-epigallocatechin-3-gallate (EGCG), the most common bioactive polyphenolic in green tea.
Botany
Camellia sinensis is native to mainland South and Southeast Asia; however, it is cultivated across the world in tropical and subtropical regions. It is an evergreen shrub or small tree that is usually trimmed to below six feet when cultivated for its leaves. It has a strong taproot. The flowers are yellow-white, 2.5–4 cm in diameter, with 7 to 8 petals. Many high quality teas are grown at high elevations, up to 1500 meters (5,000 ft), as the plants grow more slowly and acquires a better flavor - indicative of the influence of environmental stress factors on the production of secondary compounds (polyphenolics) that account for tea’s unique characteristics and value.
Bioactive Phytochemical Constituents: Epigallocatechin-3-Gallate (EGCG)
Green tea is particularly rich in the catechin class of polyphenolic flavonoids. The eight major types of catechins include catechin, epicatechin, catechin gallate, epicatechin gallate (ECG), gallo-catechin, gallocatechin gallate, (−)-epigallocatechin-3-gallate (EGCG), and epigallocatechin [81, 82]. Most bioactivity is linked to the ‘epi’ catechins rather than the catechins, and the most abundant (up to 65% of total catechin content in green tea) and most pharmacologically-active of the epicatechins in green tea is EGCG [83, 84] (Fig. 3).
Medicinal Uses
The health benefits of green tea catechins, in particular the major polyphenolic constituent in green tea, EGCG, are extensively documented in traditional medicine, in epidemiological studies, and through both in vitro and in vivo screening and clinical trials [82, 85]. Tea foliage and extracts have been used in traditional Chinese medicine and other traditional systems to treat asthma (functioning as a bronchodilator), angina pectoris, peripheral vascular disease, and coronary artery disease. Epidemiology has highlighted benefits of green tea consumption for cardiovascular diseases (CVD), several cancers, and neurological disorders. In vitro assays and testing in animal models have corroborated these traditional uses, linking the consumption of green tea with a wide variety of biological interventions including prevention and/or treatment of cancer, Parkinson’s and Alzheimer’s’ diseases, hypercholesterolemia, and atherosclerosis (for a comprehensive review see [86]). Recently, attention both in the scientific community and amongst the general public has escalated in the wake of research and subsequent publicity regarding treatment with green tea extracts of metabolic syndrome, including weight loss and prevention of CVD. Green tea consumption as a beverage has risen worldwide, and green tea extract has become a featured ingredient in nutritional and multivitamin supplement [82].
Cancer chemoprevention
Green tea extracts and its primary catechins have been used for a variety of different cancers [87]. The in vivo and in vitro anti-cancer activity of tea polyphenols has been extensively reported in tumor xenografts, carcinogen-induced tumors in digestive organs, mammary glands, hepatocarcinomas, lung cancers, skin tumors, leukemia, tumor promotion and metastasis [81]. Oral consumption of green tea was reported to inhibit chemical carcinogen- or ultraviolet radiation-induced skin tumorigenesis in animal models. Consumption of green tea has been shown to block or reduce the frequency of cigarette smoke-induced mutations in humans [88]. In another study, tea polyphenolics (EGCG) inhibited testosterone-mediated induction of ornithine decarboxylase (ODC) in vitro and in vivo [89]. The polyphenolic constituents in green tea were reported to induce the mitochondrial pathway of apoptosis and, therefore, can be used as potential chemopreventive agents against skin cancer [84, 90]. In a recent study, tea polyphenolics were reported to inhibit COX-2 expression and block activation of nuclear NF-βB and Akt in diethylnitrosoamine-induced lung cancer in mice [90]. The molecular mechanisms of the cancer chemopreventive effects of tea polyphenols are most likely related to antioxidative activities, modulation of xenobiotic metabolite enzymes, inhibition of tumor promotion, and modulation of mitotic signal transduction [91]. Generally, the mechanisms of antimutagenesis and anticarcinogenesis of green tea polyphenols suggest that the inhibition of tumors may be due to both extracellular and intracellular mechanisms, including the modulation of metabolism, blocking or suppressing of DNA replication and repair mechanisms, inhibition of invasion and metastasis, and induction of novel mechanisms [81].
Antibacterial
Tea extracts, due to their antibacterial properties, are used in preservation of processed organic food and the treatment of persistent bacterial infections. Green tea drinking is long recognized as antagonistic to dental caries. The antimutagenic activity of tea extracts containing ECG and EGCG against a range of mutagens was established in microbial systems (Salmonella typhimurium and Escherichia coli), mammalian cell systems and in vivo animal studies. Green tea inhibition of multidrug resistant Staphylococcus aureaus infections as well as HIV-1 infection are most significant recent discoveries [92].
UVB protection
Topical application of EGCG on human skin, prior to irradiance exposure, significantly decreased ultraviolet B (UVB)-induced erythema and prostaglandin metabolites, and blocked leukocytes infiltration [84], suggesting that EGCG may provide protection from UVB-induced ROS-associated photoaging, dermatoses, and photo-carcinogenesis [93].
Exercise metabolism, diabetes, and age-related declines
The benefits of green tea catechins to counteract age-related reductions in motor performance, and to increase overall endurance, have been extensively demonstrated in both animal and human trials. Green teas have been used to improve mental alertness, in weight control, and to lower cholesterol levels [93]. Anti-diabetic benefits (amelioration of insulin resistance and improved glucose transporter content in animal models) have been also demonstrated [94, 95]. Murase et al. [96] showed that improved skeletal muscle mitochondrial function in catechin-fed mice, in combination with a habitual exercise regimen, was responsible for suppressing age-related declines in physical performance and improving energy metabolism. In a series of recent human clinical trials, EGCG, often in combination with quercetin, has proven to be an effective modulator in human stress performance trials. A two week supplementation treatment was able to counter an inflammatory response and augment granulocyte oxidative burst activity after three days of heavy exertion by trained cyclists [97]. In another counter-balanced cross-over design, healthy male volunteers realized significantly improved insulin sensitivity and glucose tolerance as well as increased fat oxidation during moderate-intensity exercise following ingestion of green tea catechins [98].
CVD prevention
Perhaps the most compelling recent evidence for medicinal efficacy of green tea concerns its role in the prevention of cardiovascular diseases (CVD) in parallel to the evidence in favor of high polyphenol dark chocolates [99]. Green tea catechins have been shown to effectively counter a range of CVD risk factors such as LDL oxidation, diabetes incidence, excess body weight, platelet ‘stickiness’, and low HDL levels. Hypocholesterolemic efficacy of green tea and green tea extracts and the ability to prevent LDL oxidation and atherosclerosis has been well documented [100, 101]. Green tea extract possesses well-documented anti-inflammatory activity [102, 103], which relates to the efficacy of green tea catechins against inflammation- related cardiovascular disease. EGCG from green tea attenuates blood pressure increases in a stroke rodent model [104]. Recently a large prospective cohort trial with over 40,000 Japanese subjects demonstrated inverse association between green tea consumption and CVD [105].
Mode of Action
The wide span of biological effects of green tea (anti-cancer, anti-obesity, anti-diabetes, cardioprotective, etc.) suggests broad-spectrum mechanisms of action. Its antioxidant properties are well documented [106] and corroborate the efficacy against many human diseases that involve reactive oxygen species such as cancer, neurodegeneration, and CVD. Green tea constituents effect cellular and molecular targets in signal transduction pathways, however, it is unclear whether these effects are downstream events of the modulation of pro-oxidant/antioxidant balance, or rather direct actions of catechins on molecular targets [82]. In addition, the mechanisms of action appear to be dependent on cell-type and dosage of the catechins in a green tea preparation. Given the breadth of medicinal indications for green tea, it is no surprise that the efficacy of tea extracts have been documented in a diverse range of bioassays including antioxidant assays, antibacterial assays, several assays targeting chemopreventive properties of tea constituents (e.g. human DNA topoisomerase II catalytic assay, and HepG2 cell cytotoxicity assay), and anti-inflammatory screens. One of the most robust, reproducible assays for bioefficacy of green tea extracts uses RT-PCR for anti-inflammatory gene expression through the induction of pro-inflammatory genes in stimulated macrophages [103, 107, 108]. This routine screening has been adopted by industry, but the endpoints are not specific to the cardiovascular protective properties of tea. More directed detection of cardioprotective capacity by tea catechins could be approached by using an eNOS (endothelial nitric oxide synthase) bioassay. Endothelial-dependent NO is produced by this enzyme (eNOS), which is important to cardiovascular homeostasis [108]. Recent evidence suggests that the effects of catechins (those containing a gallocatechin moiety or a galloyl residue) are mediated in part by activation AMPK in different tissues, and that reactive oxygen species are involved in catechin activation of the LKB1/AMPK pathway [109].
CONCLUSIONS
Green tea, of course, is not only a TCM - it is an extremely popular beverage, which is consumed at any time of the day. Precisely because tea is so versatile and accepted in business and social settings, it is easy to consume multiple cups within a single day, which can be a well-tolerated and efficacious way to deliver the active EGCG to the user. Although the polyphenolic catechins seem to have poor oral bioavailability [82], and users vary widely in the preferred strength of the brewed beverage, continuous exposure to even low dosages over the long time may provide health benefits. While perhaps most routine tea drinkers are oblivious to the medicinal value of the beverage, others have embraced tea as a functional food, and green teas as a functional ingredient now frequently supplement popular snack foods and other beverage formulations. The multifaceted biomedical properties and resultant publicity associated with green tea consumption, its relatively low cost and its accessibility as a functional beverage rather than a natural product-derived pharmaceutical, have all certainly contributed to the surge in green tea consumption in the West in recent years. Because the bioavailability of the most active catechins in human systems appears to be limited [82], and the methods for brewing tea over a wide range of strengths varies with personal preference, it has not yet been possible to develop robust recommendations for daily intake. Because health-associated benefits of tea and EGCG are so well known to consumers around the world, the adoption of tea catechins as functional foods, nutritional supplements, or templates for drug analog design has been particularly well received by the general public, and demand continues to arise in pace with expanding research on this ancient TCM.
ACKNOWLEDGEMENTS
Partially supported by the Medicines for Malaria Ventures, NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome, grant # 1-P50 AT002776-01, NIH Center for Dietary Supplement Research NIH, 2 P50 AT000477-06, and Phytomedics Inc.
ABBREVIATIONS
- ACTs
Artemisinin Combination Therapies
- CVD
Cardiovascular Disease
- eNOS
Endothelial nitric oxide synthase
- ECG
Epicatechin gallate
- EGCG
(−)-epigallocatechin-3-gallate
- ROS
Reactive oxygen species
- TCM
Traditional Chinese Medicines
- UVB
Ultraviolet B
- US FDA
United States Food and Drug Administration
REFERENCES
- 1.Li W-F, Jiang J-G, Chen J. Chinese medicine and its modernization demands. Arch Med Res. 2008;39(2):246–251. doi: 10.1016/j.arcmed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Wu Z, Zhou X, Zhou Z, Fan W. Knowledge discovery in traditional Chinese medicine: State of the art and perspectives. Artif Intell Med. 2006;38(3):219–236. doi: 10.1016/j.artmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Traditional Medicine. World Health Organization; [cited 2009 1-Dec-2009];2008 Available from: http://www.who.int/mediacentre/factsheets/fs134/en/
- 4.Normile D. Asian medicine. The new face of traditional Chinese medicine. Science. 2003;299(5604):188–190. doi: 10.1126/science.299.5604.188. [DOI] [PubMed] [Google Scholar]
- 5.Marcus DM, Grollman AP. Botanical medicines--the need for new regulations. N Engl J Med. 2002;347(25):2073–2076. doi: 10.1056/NEJMsb022858. [DOI] [PubMed] [Google Scholar]
- 6.Raskin I, Ripoll C. Can an apple a day keep the doctor away? Curr Pharm Des. 2004;10(27):3419–3429. doi: 10.2174/1381612043383070. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat Chem Biol. 2007;3(7):360–366. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human S. Food and Drug A, Center for Drug Evaluation and Research C. Guidance for Industry: Botanical Drug Products. 2004
- 9.Rocco F. Quinine: malaria and the quest for a cure that changed the world. New York: HarperCollins; 2004. [Google Scholar]
- 10.World Health Organization. Aregawi M, World Health O, editors. World malaria report 2008. Geneva: World Health Organization; 2008
- 11.Li Y, Wu YL. An over four millennium story behind qinghaosu (artemisinin)--a fantastic antimalarial drug from a traditional chinese herb. Curr Med Chem. 2003;10(21):2197–2130. doi: 10.2174/0929867033456710. [DOI] [PubMed] [Google Scholar]
- 12.Willcox M, Bodeker G, Bourdy G, et al. Artemisia annua as a Traditional Herbal Antimalarial. In: Willcox M, Bodeker G, Rasoanaivo P, editors. Traditional medicinal plants and malaria. Boca Raton: CRC Press; 2004. [Google Scholar]
- 13.Hsu E. The history of qing hao in the Chinese materia medica. Trans R Soc Trop Med Hyg. 2006;100(6):505–508. doi: 10.1016/j.trstmh.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Hsu E. Reflections on the 'discovery' of the antimalarial qinghao. Br J Clin Pharmacol. 2006;61(6):666–670. doi: 10.1111/j.1365-2125.2006.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.QARCG. Antimalaria studies on Qinghaosu. Chin Med J (Engl) 1979;92(12):811–816. [PubMed] [Google Scholar]
- 16.Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7(8):999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J. A Detailed Chronological Record of Project 523 and the Discovery and Development of Qinghaosu (Artemisinin): Yang Cheng Evening News Publishing Company. 2005 [Google Scholar]
- 18.Tu YY. The Development of new antimalarial drugs: Qinghaosu and Dihydro-Qinqhaosu. Chin Med J (Engl) 1999;112(111):976–977. [PubMed] [Google Scholar]
- 19.Wu ZY, Raven PH, Hong DY. Flora of China. Bejing St. Louis: Science Press Missouri Botanical Garden Press; 2009. [Google Scholar]
- 20.Watson L, Bates P, Evans T, Unwin M, Estes J. Molecular phylogeny of Subtribe Artemisiinae (Asteraceae), including Artemisia and its allied and segregate genera %U http://www.biomedcentral.com/1471-2148/2/17. BMC Evol Biol. 2002;2(1):17. doi: 10.1186/1471-2148-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GBIF. Biodiversity occurrence data provided by: Catalogue of Life: 2007 Annual Checklist: The Integrated Taxonomic Information System and The International Plant Names Index: IPNI. [cited 2009 1-Dec-2009];2009 Available from: www.gbif.net.
- 22.Ferreira JFS, Simon JE, Janick J. Artemisia annua: Botany, Horticulture, Pharmacology. New York: John Wiley and Sons; 1997. [Google Scholar]
- 23.Riggins C. Molecular Phylogenetic and Biogeographis Study of the Genus Artemisia (Asteraceae), with an Emphasis on the Section Absinthium. Urbana: University of Illinois at Urbana-Champagne; 2008. [Google Scholar]
- 24.Tan RX, Zheng WF, Tang HQ. Biologically active substances from the genus Artemisia. Planta Med. 1998;64(4):295–302. doi: 10.1055/s-2006-957438. [DOI] [PubMed] [Google Scholar]
- 25.Bhakuni RS, Jain DC, Sharma RP. Phytochemistry of Artemisia annua and the Development of Artemisinin-Derived Antimalarial Agents. In: Wright CW, editor. Artemisia. London: Taylor and Francis; 2002. [Google Scholar]
- 26.Olsson ME, Olofsson LM, Lindahl AL, Lundgren A, Brodelius M, Brodelius PE. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular secretory trichomes of Artemisia annual. Phytochemistry. 2009;70(9):1123–1128. doi: 10.1016/j.phytochem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Covello PS, Teoh KH, Polichuk DR, Reed DW, Nowak G. Functional genomics and the biosynthesis of artemisinin. Phytochemistry. 2007;68(14):1864–1871. doi: 10.1016/j.phytochem.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Laughlin JC, Heazlewood GN, Beattie BM. Cultivation of Artemisia annua L. In: Wright CW, editor. Artemisia. New York: Taylor and Francis; 2002. [Google Scholar]
- 29.Kumar V, Mahajan A, Chibale K. Synthetic medicinal chemistry of selected antimalarial natural products. Bioorg Med Chem. 2009;17(6):2236–2275. doi: 10.1016/j.bmc.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Q, Qiu F, Yuan L. Production of artemisinin by genetically-modified microbes. Biotechnol Lett. 2008;30(4):581–592. doi: 10.1007/s10529-007-9596-y. [DOI] [PubMed] [Google Scholar]
- 31.Aweeka FT, German PL. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47(2):91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2006
- 33.Balint GA. Artemisinin and its derivatives: An important new class of antimalarial agents. Pharmacol Ther. 2001;90(2–3):261–265. doi: 10.1016/s0163-7258(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 34.Meshnick SR. Artemisinin: Mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 35.Kannan R, Kumar K, Sahal D, Kukreti S, Chauhan VS. Reaction of artemisinin with haemoglobin: Implications for antimalarial activity. Biochem J. 2005;385(Pt 2):409–418. doi: 10.1042/BJ20041170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424(6951):957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Mo W, Shen D, et al. Yeast model uncovers dual roles of mitochondria in action of artemisinin. PLoS Genet. 2005;1(3):e36. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36(14):1427–1441. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Olliaro PL, Haynes RK, Meunier B, Yuthavong Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001;17(3):122–126. doi: 10.1016/s1471-4922(00)01838-9. [DOI] [PubMed] [Google Scholar]
- 40.Butcher GA. Antimalarial drugs and the mosquito transmission of Plasmodium. Int J Parasitol. 1997;27(9):975–987. doi: 10.1016/s0020-7519(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 41.Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg. 1997;56(1):17–23. doi: 10.4269/ajtmh.1997.56.17. [DOI] [PubMed] [Google Scholar]
- 42.White NJ. Antimalarial drug resistance. J Clin Investig. 2004;113(8):1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishna S, Bustamante L, Haynes RK, Staines HM. Artemisinins: Their growing importance in medicine. Trends Pharmacol Sci. 2008;29(10):520–527. doi: 10.1016/j.tips.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51(5):1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Curr Opin Investig Drugs. 2007;8(2):105–116. [PubMed] [Google Scholar]
- 46.Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68(6):732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao X, Lipsky PE. The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin North Am. 2000;26(1):29–50. doi: 10.1016/s0889-857x(05)70118-6. viii. [DOI] [PubMed] [Google Scholar]
- 48.Tao X, Cush JJ, Garret M, Lipsky PE. A phase I study of ethyl acetate extract of the chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28(10):2160–2167. [PubMed] [Google Scholar]
- 49.Tao X, Younger J, Fan FZ, Wang B, Lipsky PE. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: A double-blind, placebo-controlled study. Arthritis Rheum. 2002;46(7):1735–1743. doi: 10.1002/art.10411. [DOI] [PubMed] [Google Scholar]
- 50.Goldbach-Mansky R, Wilson M, Fleischmann R, et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med. 2009;151(4):229–240. doi: 10.7326/0003-4819-151-4-200908180-00005. W49-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma J, Dey M, Yang H, et al. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry. 2007;68(8):1172–1178. doi: 10.1016/j.phytochem.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook F. Drugs RD. 2003;4(1):1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 53.Lin N, Liu C, Xiao C, et al. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol. 2007;73(1):136–146. doi: 10.1016/j.bcp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 54.Lipsky PE, Tao XL. A potential new treatment for rheumatoid arthritis: Thunder god vine. Semin Arthritis Rheum. 1997;26(5):713–723. doi: 10.1016/s0049-0172(97)80040-6. [DOI] [PubMed] [Google Scholar]
- 55.Kizelsztein P, Komarnytsky S, Raskin I. Oral administration of triptolide ameliorates the clinical signs of experimental autoimmune encephalomyelitis (EAE) by induction of HSP70 and stabilization of NF-kappaB/IkappaBalpha transcriptional complex. J Neuroimmunol. 2009;217(1–2):28–37. doi: 10.1016/j.jneuroim.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Mei Y, Feng D, Xu L. Triptolide modulates T-cell inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86(11):2441–2449. doi: 10.1002/jnr.21683. [DOI] [PubMed] [Google Scholar]
- 57.Wei X, Gong J, Zhu J, et al. The suppressive effect of triptolide on chronic colitis and TNF-alpha/TNFR2 signal pathway in interleukin-10 deficient mice. Clin Immunol. 2008;129(2):211–218. doi: 10.1016/j.clim.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Tao X, Fan F, Hoffmann V, et al. Effective therapy for nephritis in (NZB x NZW)F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum. 2008;58(6):1774–1783. doi: 10.1002/art.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leuenroth SJ, Okuhara D, Shotwell JD, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci USA. 2007;104(11):4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Zhang W, Ouyang J. Effects of Tripterygium wilfordii Polyglycosidium on the Expression of ICAM-1 and TNF-alpha in the rat model for nonbacterial chronic. Prostatitis Zhongguo Nankexue Zazhi. 2007;21(4):24–28. [Google Scholar]
- 61.Morota T, Saitoh K, Maruno M, Yang C-X, Qin W-Z, Yang B-H. leukotriene D4 antagonists in Tripterygium wilfordii Hook F (TWHF) and Cyclosporin A in rat liver transplantation. Nat Med. 1995;49(4):468–471. [Google Scholar]
- 62.He X, Verran D, Hu C, et al. Synergistic effect of Tripterygium wilfordii Hook.F (TWHF) and cyclosporin A in rat liver transplantation. Transplant Proc. 2000;32(7):2054. doi: 10.1016/s0041-1345(00)01555-4. [DOI] [PubMed] [Google Scholar]
- 63.Matta R, Wang X, Ge H, Ray W, Nelin LD, Liu Y. Triptolide induces anti-inflammatory cellular responses. Am J Trans Res. 2009;1(3):267–282. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu W, Ou Y, Li Y, et al. A small-molecule triptolide suppresses angiogenesis and invasion of human anaplastic thyroid carcinoma cells via down-regulation of the nuclear factor-kappa B pathway. Mol Pharmacol. 2009;75(4):812–819. doi: 10.1124/mol.108.052605. [DOI] [PubMed] [Google Scholar]
- 65.Wegele H, Muller L, Buchner J. Hsp70 and Hsp90--a relay team for protein folding. Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Jin H, Xu R, Mei Q, Fan D. Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp Mol Med. 2009;41(10):717–727. doi: 10.3858/emm.2009.41.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen YW, Lin GJ, Chia WT, Lin CK, Chuang YP, Sytwu HK. Triptolide exerts anti-tumor effect on oral cancer and KB cells in vitro and in vivo. Oral Oncol. 2009;45(7):562–568. doi: 10.1016/j.oraloncology.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Westfall SD, Nilsson EE, Skinner MK. Role of triptolide as an adjunct chemotherapy for ovarian cancer. Chemotherapy. 2008;54(1):67–76. doi: 10.1159/000112419. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Jiang Z, Xiao J, et al. Effects of triptolide from Tripterygium wilfordii on ER[alpha] and p53 expression in two human breast cancer cell lines. Phytomedicine. 2009;16(11):1006–1013. doi: 10.1016/j.phymed.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Yang S, Chen J, Guo Z, et al. Triptolide inhibits the growth and metastasis of solid tumors. Mol Cancer Ther. 2003;2(1):65–72. [PubMed] [Google Scholar]
- 71.Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappaB activation. Exp Mol Med. 2002;34(6):462–468. doi: 10.1038/emm.2002.64. [DOI] [PubMed] [Google Scholar]
- 72.He MF, Huang YH, Wu LW, Ge W, Shaw PC, But PP. Triptolide functions as a potent angiogenesis inhibitor. Int J Cancer. 2010;126(1):266–278. doi: 10.1002/ijc.24694. [DOI] [PubMed] [Google Scholar]
- 73.Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. PG490 (triptolide) cooperates with tumor necrosis factor-alpha to induce apoptosis in tumor cells. J Biol Chem. 1999;274(19):13451–13455. doi: 10.1074/jbc.274.19.13451. [DOI] [PubMed] [Google Scholar]
- 74.Qiu D, Zhao G, Aoki Y, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274(19):13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 75.Choi YJ, Kim TG, Kim YH, et al. Immunosuppressant PG490 (triptolide) induces apoptosis through the activation of caspase-3 and down-regulation of XIAP in U937 cells. Biochem Pharmacol. 2003;66(2):273–280. doi: 10.1016/s0006-2952(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 76.Liu Q, Chen T, Chen H, et al. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004;319(3):980–986. doi: 10.1016/j.bbrc.2004.04.201. [DOI] [PubMed] [Google Scholar]
- 77.Carter BZ, Mak DH, Schober WD, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108(2):630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su Y, Yang S, Xiao Z, Wang W, Okunieff P, Zhang L. Triptolide alters mitochondrial functions. Adv Exp Med Biol. 2007;599:139–146. doi: 10.1007/978-0-387-71764-7_19. [DOI] [PubMed] [Google Scholar]
- 79.Matlin SA, Belenguer A, Stacey VE, et al. Male antifertility compounds from Tripterygium wilfordii Hook F. Contraception. 1993;47(4):387–400. doi: 10.1016/0010-7824(93)90036-7. [DOI] [PubMed] [Google Scholar]
- 80.Bai J-P, Shi Y-L. Inhibition of Ca2+ channels in mouse spermatogenic cells by male antifertility compounds from Tripterygium wilfordii Hook F. Contraception. 2002;65(6):441–445. doi: 10.1016/s0010-7824(02)00312-8. [DOI] [PubMed] [Google Scholar]
- 81.Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436(1):69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 82.Zaveri NT. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection (Review) Int J Oncol. 2001;18(6):1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 84.Katiyar SK, Matsui MS, Elmets CA, Mukhtar H. Polyphenolic antioxidant (−)-epigallocatechin-3-gallate from green tea reduces UVB-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem Photobiol. 1999;69(2):148–153. [PubMed] [Google Scholar]
- 85.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134(12 Suppl):3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 86.Cabrera C, Artacho R, Gimenex R. Beneficial Effects of Green Tea - A Review. J Am College Nutrition. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 87.Boehm K, Borrelli F, Ernst E, et al. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD005004.pub2. CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee IP, Kim YH, Kang MH, Roberts C, Shim JS, Roh JK. Chemopreventive effect of green tea (Camellia sinensis) against cigarette smoke-induced mutations (SCE) in humans. J Cell Biochem Suppl. 1997;27:68–75. [PubMed] [Google Scholar]
- 89.Gupta S, Ahmad N, Mohan RR, Husain MM, Mukhtar H. Prostate cancer chemoprevention by green tea: In vitro and in vivo inhibition of testosterone-mediated induction of ornithine decarboxylase. Cancer Res. 1999;59(9):2115–2120. [PubMed] [Google Scholar]
- 90.Roy P, Nigam N, Singh M, et al. Tea polyphenols inhibit cyclooxygenase-2 expression and block activation of nuclear factor-kappa B and Akt in diethylnitrosoamine induced lung tumors in Swiss mice. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9274-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Lin JK, Liang YC. Cancer chemoprevention by tea polyphenols. Proceedings of the National Science Council, Republic of China Part B. 2000;24(1):1–13. [PubMed] [Google Scholar]
- 92.Stapleton PD, Shah S, Anderson JC, Hara Y, Hamilton-Miller JM, Taylor PW. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents. 2004;23(5):462–467. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 93.Sharangi A. Medicinal and therapeutic potentialities of tea (Camillia sinensis L.) A review. Food Res Int. 2009;42:529–535. [Google Scholar]
- 94.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52(3):643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 95.Wu LY, Juan CC, Hwang LS, Hsu YP, Ho PH, Ho LT. Green tea supplementation ameliorates insulin resistance and increases glucose transporter IV content in a fructose-fed rat model. Eur J Nutr. 2004;43(2):116–124. doi: 10.1007/s00394-004-0450-x. [DOI] [PubMed] [Google Scholar]
- 96.Murase T, Haramizu S, Ota N, Hase T. Tea catechin ingestion combined with habitual exercise suppresses the aging-associated decline in physical performance in senescence-accelerated mice. Am J Physiol - Regul Integr Comp Physiol. 2008;295(1):R281–R289. doi: 10.1152/ajpregu.00880.2007. [DOI] [PubMed] [Google Scholar]
- 97.Nieman DC, Henson DA, Maxwell KR, et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc. 2009;41(7):1467–1475. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 98.Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87(3):778–784. doi: 10.1093/ajcn/87.3.778. [DOI] [PubMed] [Google Scholar]
- 99.Grassi D, Desideri G, Necozione S, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 100.Yang TT, Koo MW. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 2000;66(5):411–423. doi: 10.1016/s0024-3205(99)00607-4. [DOI] [PubMed] [Google Scholar]
- 101.Yang TT, Koo MW. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis. 2000;148(1):67–73. doi: 10.1016/s0021-9150(99)00239-7. [DOI] [PubMed] [Google Scholar]
- 102.Adcocks C, Collin P, Buttle DJ. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type II collagen degradation in vitro. J Nutr. 2002;132:341–346. doi: 10.1093/jn/132.3.341. [DOI] [PubMed] [Google Scholar]
- 103.Wang BS, Yub HM, Changa LW, Yena WJ, Duha PD. Protective effects of pu-erh tea on LDL oxidation and nitric oxide generation in macrophage cells. LWT - Food Sci Technol. 2008;41(6):1122–1132. [Google Scholar]
- 104.Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- 105.Kuriyama S, Shimazu T, Ohmori K, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. J Am Med Assoc. 2006;296(10):1255–1265. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 106.Yang CS, Kim S, Yang GY, et al. Inhibition of carcinogenesis by tea: Bioavailability of tea polyphenols and mechanisms of actions. Proc Soc Exp Biol Med. 1999;220(4):213–217. doi: 10.1046/j.1525-1373.1999.d01-36.x. [DOI] [PubMed] [Google Scholar]
- 107.Hou D, Masuzaki S, Hashimoto F, et al. Green tea proanthocyanidins inhibit cyclooxygenase-2 expression in LPS-activated mouse macrophages: Molecular mechanisms and structure-activity relationship. Arch Biochem Biophys. 2007;460:67–74. doi: 10.1016/j.abb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 108.Lorenz M, Wessler S, Follmann E, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem. 2004;279(7):6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 109.Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]