Abstract
Purpose
This study was performed to determine whether the serum concentrations of interleukin (IL)-6 family cytokines are elevated in patients with rheumatoid arthritis (RA) and to investigate the relationship between IL-6 family cytokine levels and disease activity in RA patients.
Materials and Methods
We obtained serum samples from 40 patients with RA and 40 age- and sex-matched healthy controls, and we assessed the clinical parameters of disease activity, including the 28-joint disease activity score (DAS28) and C-reactive protein (CRP) levels. Serum samples from five patients with high disease activity (DAS28 > 5.1) were also collected at the eighth week of treatment. Serum concentrations of IL-6, IL-11, and leukemia inhibitory factor (LIF) were measured using an enzyme-linked immunosorbent assay (ELISA).
Results
Serum concentrations of IL-6 family cytokines, including IL-6, IL-11, and LIF, were significantly elevated in patients with RA compared to those of healthy controls. Although there was no significant relationship between IL-6 family cytokine levels and DAS28, the IL-6 levels of patients with RA showed a significant correlation with CRP levels. After eight weeks of medical treatment in patients with high disease activity, a decrease in DAS28 was associated with a significant decrease in the serum concentrations of IL-6 and IL-11.
Conclusion
The serum concentrations of IL-6 family cytokines were significantly elevated in patients with RA, and they decreased with medical treatment. These findings suggest a possible role for IL-6 family cytokines in the pathogenesis of RA.
Keywords: Rheumatoid arthritis, interleukin-6 family cytokines, interleukin-6, interleukin-11, leukemia inhibitory factor
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease that causes inflammation mainly in the synovium and produces destruction and deformity of the joints. The etiology of RA remains unclear, but it is known to be associated with genetic and environmental factors.1
Various proinflammatory cytokines, such as tumor necrotic factor (TNF)-α, interleukin (IL)-1β, interferon (IFN)-γ, and IL-6, are increased in the synovial tissue or synovial fluid of patients with RA.2,3 Increased levels of proinflammatory cytokines lead to the proliferation of synovial tissue, and thereby cause damage in the articular cartilage and bone destruction in the adjacent area.4,5 In particular, IL-6 is a cytokine with various functions. When IL-6 is activated, acute inflammatory responses such as fever or anemia are induced. IL-6 promotes the proliferation of B cells and thus is involved in the production of the rheumatoid factor.6 Recently, RA has been observed to be associated with high levels of IL-6 in the synovial membrane and serum.7,8 This led to the speculation that IL-6 plays a pathogenic role in RA. Recently, an IL-6 receptor antagonist, tocilizumab, was developed and showed clinical efficacy in the treatment of RA.9-11
IL-6 family cytokines consist of IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M, ciliary neurotrophic factor (CNTF), and cardiotrophin-1 (CP-1). Recent studies suggest that some of the IL-6 family cytokines such as IL-6, IL-11, and LIF could be related with RA. IL-6 family cytokines such as IL-11 and LIF share glycoprotein 130 as a receptor for signal transduction, which promotes the secretion of acute reactive proteins.12 Previous studies have shown that IL-6 family cytokines were detected at high concentrations in the synovial membrane and synovial fluid of patients with RA, suggesting a role in the pathogenesis of RA. However, there is a debate on whether serum levels of IL-6 family cytokines are positively correlated with RA disease activity.13-16
In the current study, we sought to examine whether serum levels of IL-6 family cytokines are increased in patients with RA and whether the increased levels are significantly correlated with RA disease activity. We compared the serum concentrations of IL-6 family cytokines in patients with RA and those in normal controls and then investigated the correlation between serum levels of IL-6 family cytokines and the clinical parameters that indicate the disease activity of RA.
MATERIALS AND METHODS
Subjects and clinical assessment
This study was conducted in 40 patients who had visited the Division of Rheumatology at Yonsei University Medical Center between November 2007 and January 2008, and who fulfilled the American College of Rheumatology (ACR) 1987 revised criteria for the classification of RA.17 Forty age- and sex-matched healthy adults without any evidence of chronic inflammatory disease served as the controls. This study was approved by Ethics Committee of our institute, and all study subjects provided their signed informed consent.
At baseline, we calculated the 28-joint disease activity score (DAS28) using the number of joints with tenderness or swelling and the erythrocyte sedimentation rate (ESR),18 and we measured the severity of pain reported by each patient using the pain visual analogue scale (VAS).19 Based on the DAS28, the patients were subdivided into four groups as follows: remission (DAS28 ≤ 2.6), mild (2.6 < DAS28 ≤ 3.2), moderate (3.2 < DAS28 ≤ 5.1) and severe (5.1 < DAS28).20 In patients whose DAS28 exceeded 5.1, a follow-up assessment of DAS28 and blood sampling were conducted to examine whether the serum levels of cytokines were correlated with disease activity.
At the time of clinical assessment for disease activity, blood samples were collected for the measurement of levels of C-reactive protein (CRP) and IL-6 family cytokines.
Measurement of IL-6, IL-11 and LIF levels
Serum samples were collected intravenously and stored in -20℃ after centrifugation (2,500×g for 10 minutes at 4℃) until analysis.
The serum concentration of IL-6 was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems Inc., Minneapolis, MN, USA). One hundred microliters each of serum sample and assay diluent were placed in each well of a 96-well plate coated with a monoclonal mouse IgG against IL-6. This mixture was incubated for two hours at room temperature, and each well was aspirated and washed four times with wash buffer. Subsequently, 200 µL of conjugate solution was placed into each well and the reaction was performed for four hours at room temperature. Again, each well was washed four times with wash buffer. Following this, 200 µL of substrate solution, which was prepared with equal amounts of stabilized hydrogen peroxide (H2O2) and tetramethylbenzidine, was added for a 20-minute reaction under dark conditions. The reaction was quenched by the addition of 50 µL stop solution (2N H2SO4). Within 30 minutes, the optical density was measured at a wavelength of 450 nm using the Spectra Max 340 (Molecular Device Co., Sunnyvale, CA, USA). The serum concentration of IL-6 was determined based on a standard concentration curve. The correlation coefficient (r) of the standard concentration curve was 0.990.
The IL-11 and LIF ELISA kits were purchased from R&D Systems, and serum concentrations of each cytokine were determined using similar methods. The respective correlation coefficients (r) were 0.993 and 0.999 for IL-11 and LIF.
Statistical analysis
Data analyses were performed with SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). All of the descriptive variables were expressed as the mean ± standard deviation. The intergroup analysis of cytokine levels was performed using the Student's t-test and Chi-square test. The correlations among the concentrations of each cytokine, DAS28, pain VAS, and CRP level were tested using Pearson's correlation test. The subgroup analyses of serum cytokine concentrations from the four disease activity subgroups were performed using one-way ANOVA and Tukey's post-hoc analyses. Eight weeks after treatment, the follow-up measurement of cytokine levels was performed using a Paired t-test. For all tests, a p value less than 0.05 was considered statistically significant.
RESULTS
Clinical characteristics of the study subjects
The demographic and clinical data of the subjects are shown in Table 1. The mean age of the 40 patients with RA was 54.7 ± 14.4 years (range: 19-83 years), and the patient group was comprised of 13 males and 27 females. The mean disease duration from symptom onset was 63.6 ± 95.2 months (range: 2-360 months). The mean pain VAS and DAS28 of the RA patients were 4.2 ± 3.3 (range: 0-10) and 3.9 ± 1.3 (range: 1.29-6.50), respectively. Patients were divided into four groups according to DAS28: five in the remission group (DAS28 ≤ 2.6), nine in the mild group (2.6 < DAS28 ≤ 3.2), 19 in the moderate group (3.2 < DAS28 ≤ 5.1), and seven in the severe group (5.1 < DAS28).
Table 1.
Clinical Characteristics of the Study Subjects
RA, rheumatoid arthritis; DAS28, disease activity score 28; VAS, visual analogue scale; NS, not significant.
*The p values were calculated using Student's t-test and the Chi-square test.
The mean age of the healthy controls was 53 ± 12.3 years (range: 28-76 years), and the control group was comprised of 12 males and 28 females.
A comparison of cytokine levels at baseline
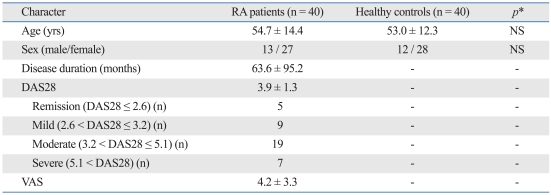
The serum concentration of IL-6 was 41.76 ± 20.28 pg/mL (range: 18.0-109.1 pg/mL) in the patient group and 6.56 ± 5.33 pg/mL (range: 0-16.55 pg/mL) in the control group. The serum concentration of IL-11 was 378.32 ± 230.31 pg/mL (range: 45-900 pg/mL) in the patient group and 102.43 ± 101.72 pg/mL (range: 0-375 pg/mL) in the control group. The serum concentration of LIF was 57.44 ± 33.82 pg/mL (range: 1.3-120.3 pg/mL) in the patient group and 10.38 ± 7.46 pg/mL (range: 0-25.8 pg/mL) in the control group. Serum concentrations for all three IL-6 family cytokines were significantly elevated in the patient group compared with those of the control group (p < 0.001) (Fig. 1).
Fig. 1.
A comparison of serum concentrations of IL-6, IL-11, and LIF between the two groups. Serum concentrations of IL-6, IL-11, and LIF were significantly higher in the patient group than in the control group. IL, interleukin; LIF, leukemia inhibitory factor.
Relationship of baseline cytokine levels to disease activity
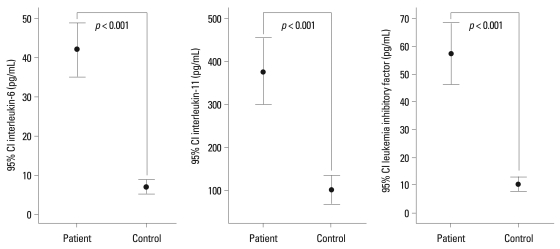
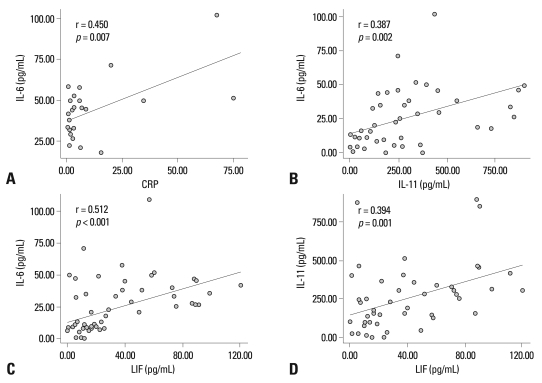
At baseline, serum concentrations of IL-6 showed a significantly positive correlation with CRP levels (r = 0.45, p = 0.007) (Fig. 2A), but not with DAS28 (r = 0.28, p = 0.102), or pain VAS (r = 0.04, p = > 0.807). Serum concentrations of IL-11 and LIF were not significantly correlated with CRP levels, DAS28, or pain VAS (Table 2). The IL-6 family cytokines showed significant correlations with each other at baseline (Fig. 2B, C and D).
Fig. 2.
The correlations among serum concentrations of IL-6, IL-11, LIF, and CRP level. (A) Serum concentrations of IL-6 showed a significant positive correlation with CRP levels (r = 0.45, p = 0.007) in RA patients. (B) Serum concentrations of IL-6 showed significant correlations with serum concentrations of IL-11. (C) Serum concentrations of IL-6 showed significant correlations with serum concentrations of LIF. (D) Serum concentrations of IL-11 showed significant correlations with serum concentrations of LIF. IL, interleukin; LIF, leukemia inhibitory factor; CRP, C-reactive protein; RA, rheumatoid arthritis.
Table 2.
The Correlations among IL-6 Family Cytokines and RA Disease Activity
RA, rheumatoid arthritis; DAS28, disease activity score 28; VAS, visual analogue scale; CRP, C-reactive protein; IL, interleukin; LIF, leukemia inhibitory factor.
*Pearson's correlation analysis was performed.
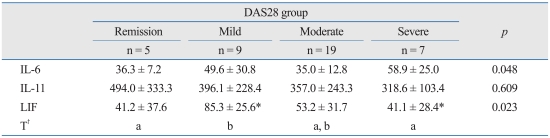
In subgroup analysis, serum concentrations of IL-6 were 36.3 ± 7.2 pg/mL in the remission group, 49.6 ± 30.8 pg/mL in the mild group, 35.0 ± 12.8 pg/mL in the moderate group, and 58.9 ± 25.0 pg/mL in the severe group. The differences among the four groups were statistically significant (p = 0.048), but this was not confirmed by post-hoc analysis. Serum concentrations of IL-11 were 494.0 ± 333.3 pg/mL, 396.1 ± 228.4 pg/mL, 357.0 ± 243.3 pg/mL, and 318.6 ± 103.4 pg/mL in the corresponding group order from mildest to most severe. Serum concentrations of IL-11 were not significantly different among the four groups (p = 0.609). Serum concentrations of LIF were 41.2 ± 37.6 pg/mL, 85.3 ± 25.6 pg/mL, 53.2 ± 31.7 pg/mL, and 41.1 ± 28.4 pg/mL, in the remission, mild, moderate, and severe groups, respectively. There was a significant difference in the serum concentrations of LIF between the mild and severe group (p = 0.023), with serum concentrations of LIF significantly higher in the mild group (Table 3).
Table 3.
A Comparison of the Serum Concentrations of IL-6 Family Cytokines among the Groups Divided Based on DAS28
DAS28, disease activity score 28; IL, interleukin; LIF, leukemia inhibitory factor.
Remission group, DAS28 ≤ 2.6; mild disease activity group, 2.6 < DAS28 ≤ 3.2; moderate disease activity group, 3.2 < DAS28 ≤ 5.1; severe disease activity group, 5.1 < DAS28.
*Tukey's post-hoc analysis was used.
†The same letters indicate non-significant difference between groups based on Tukey's multiple comparison test.
Changes in concentrations of IL-6 family cytokines after treatment
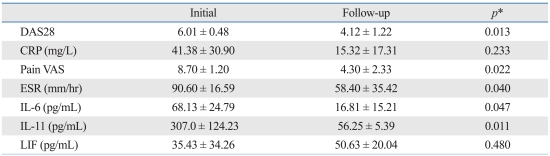
To examine whether the serum concentrations of IL-6 family cytokines depend on the clinical course in patients whose DAS28 exceeded 5.1, the serum concentrations of IL-6 family cytokines were measured again after eight weeks of treatment with disease-modifying anti-rheumatic drugs (DMARDs). Five out of seven patients with high initial DAS were tested again and two patients were lost during the follow-up period. Five patients who had high disease activity showed decreased DAS28 of less than 5.1 after the eight-week treatment. The mean DAS28 was 6.01 ± 0.48 at baseline, and 4.12 ± 1.22 at the eight-week follow-up. The decrease in DAS28 was statistically significant based on the results of a Paired t-test (p = 0.013). Serum concentrations of IL-6 (p = 0.047) and IL-11 (p = 0.011) were also significantly decreased after the eight-week treatment period, though serum concentrations of LIF did not significantly differ from baseline after treatment (p = 0.480) (Table 4).
Table 4.
The Changes in RA Disease Activity and the Concentrations of Cytokines of Five Patients with SevereDisease Activity at Baseline and after Eight-Week Treatment
RA, rheumatoid arthritis; DAS28, disease activity score 28; CRP, C-reactive protein; VAS, visual analogue scale; ESR, erythrocyte sedimentation rate; IL, interleukin; LIF, leukemia inhibitory factor.
*Paired sample t-test was used.
DISCUSSION
The current study showed that serum concentrations of IL-6, IL-11, and LIF were significantly elevated in patients with RA compared to those in healthy controls. As seen in previous reports, this finding supports the hypothesis that IL-6 family cytokines are involved in the pathogenesis of RA.13-16
IL-6 is a cytokine that causes an acute inflammatory response, and it is well-documented that IL-6 plays a crucial role in the pathogenesis of various inflammatory diseases including RA.6,7 IL-11, one of the IL-6 family cytokines, is found in high concentrations in the synovial membrane, synovial fluid, and serum of patients with RA, and it is also thought to be involved in the pathogenesis of RA.21 According to studies where the immunological role of IL-11 in RA was examined, IL-11 was shown to mediate an anti-inflammatory response by inhibiting the activity of macrophages and thereby diminishing the production of proinflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ.22 However, according to Phase I and II clinical trials where recombinant IL-11 was administered to patients with RA, IL-11 did not affect the clinical course.23 There is still a controversy as to the role of IL-11 in the pathogenesis of RA.
It has also been reported that LIF is found in high concentrations in cartilage and synovial fluid of patients with RA.16 IL-1 and TNF-α mediate the secretion of LIF through chondrocytes, and LIF contributes to cartilaginous destruction by inducing the absorption of proteoglycan in patients with chronic arthritis.24,25
In addition to these previous observations, the significant increase in the IL-6 family cytokines observed in the current study indicates that these cytokines might play a role in inducing inflammatory responses or mediating anti-inflammatory responses in the pathogenesis of RA.
However, the only positive correlation was found between serum concentrations of IL-6 and CRP levels at baseline. We could not find any other significant relationships between the cytokine levels and the clinical parameters that reflect RA disease activity at baseline. This may be explained by the difference of involvement of the IL-6 family cytokines in the local and systemic reactions; in the synovium and serum. A previous study has shown that the concentrations of IL-6 family cytokines can be measured in both the serum and synovium of patients with RA, but these cytokines are mainly involved in the local reaction.14 In the systemic circulation, this may be the reason that increased serum concentrations of cytokines do not significantly correlate with disease activity.
In addition, the current study was limited; it only included a small number of patients and the types of drugs taken by the patients were not assessed. These factors might reduce statistical significance. DMARDs can reduce the concentrations of IL-6 family cytokines;26 therefore, further study is warranted to accurately compare the correlation between disease activity and the concentrations of cytokines, with consideration for the effects of the drugs in a larger patient population.
However, the most interesting finding in the current study was that serum concentrations of IL-6 family cytokines decreased significantly after treatment in patients with high disease activity. We investigated the changes in cytokine levels associated with changes in disease activity before and after treatment for patients with high disease activity. We further investigated whether IL-6 family cytokines levels can change and found that the concentrations of IL-6 and IL-11 significantly decreased as DAS28 decreased (Table 4). Although cross-sectional analysis did not document a significant correlation between disease activity and cytokine concentration, the change in the concentration of cytokines correlated well with the clinical course in patients with high disease activity. This finding suggests that the levels of these cytokines could reflect the disease activity.
IL-6 family cytokines have pleiotropic activities in inflammatory response.13 IL-6 might have dual roles in inflammation; pro-inflammatiory27,28 or anti-inflammatiory,29 and it is thought to be partly determined by concentration of soluble IL-6 receptors.30 IL-11 also might have pro-inflammatory31 and anti-inflammatory32 activity, even though the mechanism is obscure. In this study, IL-6 and IL-11 levels decreased by treatment, but the level of LIF did not. This result might be another example of dual effects of IL-6 family cytokines with unique characteristics to LIF which would require further investigation with a large number of subjects.
In summary, we found the serum concentrations of IL-6 family cytokines were significantly increased in patients with RA compared with those of normal controls. The cytokine levels were not significantly correlated with RA disease activity in a cross-sectional analysis at baseline. But serum levels of IL-6 correlated with CRP levels, and follow-up measurements of cytokine levels in patients with high disease activity showed that the levels of IL-6 and IL-11 decreased as symptoms improved. These findings suggest that IL-6 family cytokines might be involved in the pathogenesis of RA and that levels of IL-6 family cytokines might reflect the activity of the disease.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (2009-0064825).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH. Rheumatology. In: MacGregor AJ, Silman AJ, editors. Classification and epidemiology. 4th ed. Spain: Mosby; 2008. pp. 755–762. [Google Scholar]
- 2.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 3.Brennan F, Beech J. Update on cytokines in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:296–301. doi: 10.1097/BOR.0b013e32805e87f1. [DOI] [PubMed] [Google Scholar]
- 4.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 5.Huber LC, Distler O, Tarner I, Gay RE, Gay S, Pap T. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology. 2006;45:669–675. doi: 10.1093/rheumatology/kel065. [DOI] [PubMed] [Google Scholar]
- 6.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 7.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen LS, Christensen IJ, Lottenburger T, Svendsen MN, Nielsen HJ, Nielsen L, et al. Pre-analytical and biological variability in circulating interleukin 6 in healthy subjects and patients with rheumatoid arthritis. Biomarkers. 2008;13:59–78. doi: 10.1080/13547500701615017. [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 10.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 11.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 13.Wong PK, Campbell IK, Egan PJ, Ernst M, Wicks IP. The role of the interleukin-6 family of cytokines in inflammatory arthritis and bone turnover. Arthritis Rheum. 2003;48:1177–1189. doi: 10.1002/art.10943. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–1105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 15.Robak T, Gladalska A, Stepień H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm. 1998;7:347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waring PM, Carroll GJ, Kandiah DA, Buirski G, Metcalf D. Increased levels of leukemia inhibitory factor in synovial fluid from patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1993;36:911–915. doi: 10.1002/art.1780360707. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Aletaha D. Activity assessments in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20:306–313. doi: 10.1097/BOR.0b013e3282fbd382. [DOI] [PubMed] [Google Scholar]
- 20.Zatarain E, Strand V. Monitoring disease activity of rheumatoid arthritis in clinical practice: contributions from clinical trials. Nat Clin Pract Rheumatol. 2006;2:611–618. doi: 10.1038/ncprheum0246. [DOI] [PubMed] [Google Scholar]
- 21.Hermann JA, Hall MA, Maini RN, Feldmann M, Brennan FM. Important immunoregulatory role of interleukin-11 in the inflammatory process in rheumatoid arthritis. Arthritis Rheum. 1998;41:1388–1397. doi: 10.1002/1529-0131(199808)41:8<1388::AID-ART7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley M, Butler DM, Marinova-Mutafchieva L, Feldmann M. An anti-inflammatory role for interleukin-11 in established murine collagen-induced arthritis. Immunology. 1998;95:31–37. doi: 10.1046/j.1365-2567.1998.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreland L, Gugliotti R, King K, Chase W, Weisman M, Greco T, et al. Results of a phase-I/II randomized, masked, placebo-controlled trial of recombinant human interleukin-11 (rhIL-11) in the treatment of subjects with active rheumatoid arthritis. Arthritis Res. 2001;3:247–252. doi: 10.1186/ar309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll GJ, Bell MC. Leukaemia inhibitory factor stimulates proteoglycan resorption in porcine articular cartilage. Rheumatol Int. 1993;13:5–8. doi: 10.1007/BF00290327. [DOI] [PubMed] [Google Scholar]
- 25.Campbell IK, Waring P, Novak U, Hamilton JA. Production of leukemia inhibitory factor by human articular chondrocytes and cartilage in response to interleukin-1 and tumor necrosis factor alpha. Arthritis Rheum. 1993;36:790–794. doi: 10.1002/art.1780360608. [DOI] [PubMed] [Google Scholar]
- 26.Straub RH, Müller-Ladner U, Lichtinger T, Schölmerich J, Menninger H, Lang B. Decrease of interleukin 6 during the first 12 months is a prognostic marker for clinical outcome during 36 months treatment with disease-modifying anti-rheumatic drugs. Br J Rheumatol. 1997;36:1298–1303. doi: 10.1093/rheumatology/36.12.1298. [DOI] [PubMed] [Google Scholar]
- 27.Kopf M, Baumann H, Freer G, Feudenberg M, Lamers M, Kishimoto T, et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 28.Sasai M, Saeki Y, Ohshima S, Nishioka K, Mima T, Tanaka T, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–1643. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronstein BN. Interleukin-6--a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 31.Yin TG, Schendel P, Yang YC. Enhancement of in vitro and in vivo antigen-specific antibody responses by interleukin 11. J Exp Med. 1992;175:211–216. doi: 10.1084/jem.175.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]