Abstract
Tumor necrosis factor receptor (TNFR) p55-knockout (KO) mice are susceptible profoundly to Salmonella infection. One day after peritoneal inoculation, TNFR-KO mice harbor 1,000-fold more bacteria in liver and spleen than wild-type mice despite the formation of well organized granulomas. Macrophages from TNFR-KO mice produce abundant quantities of reactive oxygen and nitrogen species in response to Salmonella but nevertheless exhibit poor bactericidal activity. Treatment with IFN-γ enhances killing by wild-type macrophages but does not restore the killing defect of TNFR-KO cells. Bactericidal activity of macrophages can be abrogated by a deletion in the gene encoding TNFα but not by saturating concentrations of TNF-soluble receptor, suggesting that intracellular TNFα can regulate killing of Salmonella by macrophages. Peritoneal macrophages from TNFR-KO mice fail to localize NADPH oxidase-containing vesicles to Salmonella-containing vacuoles. A TNFR-KO mutation substantially restores virulence to an attenuated mutant bacterial strain lacking the type III secretory system encoded by Salmonella pathogenicity island 2 (SPI2), suggesting that TNFα and SPI2 have opposing actions on a common pathway of vesicular trafficking. TNFα–TNFRp55 signaling plays a critical role in the immediate innate immune response to an intracellular pathogen by optimizing the delivery of toxic reactive oxygen species to the phagosome.
Tumor necrosis factor (TNF) α participates in a broad spectrum of biological activities including resistance to parasites, fungi, and intracellular bacteria (1–8). The multitude of signaling pathways and transcription factors triggered after the binding of soluble and membrane TNFα to their cognate p55 and p75 TNF receptors underlie the molecular basis for the multifaceted TNF response (9). By triggering chemokine production and modulating the expression of vascular cell adhesion molecule-1, intercellular cell-adhesion molecule-1, and E-selectin, TNFα orchestrates the recruitment of proinflammatory leukocytes to sites of infection (1). TNFα participates more directly in resistance to microbial pathogens by controlling the expression of antimicrobial effector molecules. TNFα synergizes with secondary signals as diverse as β2-integrins and IFN-γ for the production of superoxide and NO (3, 4, 8, 10–12), precursors of a battery of reactive oxygen and nitrogen species capable of inhibiting or killing a broad range of viral, bacterial, fungal, and parasitic microorganisms.
Salmonella is a facultative intracellular pathogen of mononuclear phagocytes associated with a variety of clinical syndromes ranging from self-limiting diarrhea to life-threatening septicemia. Experimental evidence strongly points to the importance of TNFα in resistance to salmonellosis. Administration of neutralizing anti-TNFα antibodies or genetic abrogation of TNFα signaling in TNF receptor (TNFR) p55-knockout (KO) mice increase the susceptibility of both naïve and vaccinated mice to Salmonella (2, 5, 6). However, the mechanisms by which this cytokine confers resistance against Salmonella infections have not been elucidated completely. Because reactive oxygen and nitrogen species generated by the NADPH phagocyte oxidase and inducible NO synthase contribute to resistance to salmonellosis and Salmonella killing by macrophages (13–17), we used TNFRp55-KO mice to determine whether TNFα controls oxygen-dependent anti-Salmonella actions of phagocytes.
Materials and Methods
Murine Salmonellosis Model.
Viability of C57BL/6 Nramp1 G169D (itys) wild-type (WT) mice or congenic Nramp1 G169D (itys) TNFRp55-KO mice (The Jackson Laboratory) was recorded daily after i.p. challenge with approximately 103 colony-forming units of virulent 12023 (synonymous with ATCC 14028s) or attenuated isogenic sseB mutant Salmonella typhimurium (18). Mice were killed by CO2 inhalation, and their livers were removed aseptically and homogenized in sterile PBS (16). Viable counts were determined after overnight culture on Luria-Bertani agar plates.
Histopathology.
Hepatic tissue from Salmonella-infected WT or TNFR-KO mice was obtained after 3 days of infection, fixed in 10% formalin, stained in hematoxylin/eosin, and examined by light microscopy for the presence of microabscesses and granulomata.
Macrophage-Killing Assays.
Peritoneal exudate cells from WT C57BL/6 Nramp1 G169D (itys), TNFR-KO Nramp1 G169D (itys), and TNFα-KO Nramp1 G169D (itys) mice (The Jackson Laboratory) were harvested 4 days after i.p. inoculation of 1 mg/ml sodium periodate as described (19). Peritoneal exudate cells were resuspended in RPMI medium 1640 supplemented with 10% (vol/vol) heat-inactivated FCS (Gemini Biological Products, Calabasas, CA)/1 mM sodium pyruvate/10 mM Hepes/2 mM L-glutamine (all from Sigma–Aldrich). The macrophages were selected by adherence to a 96-well plate and cultured for 48 h at 37°C in a 5% CO2 incubator. In selected experiments, macrophages were incubated with 20 units per ml of murine IFN-γ (Life Technologies, Grand Island, NY) 24 h before infection. Some groups of macrophages were pretreated with soluble TNFRp55 (Amgen Biologicals). Biological activity of the TNF-soluble receptor was confirmed by its ability to inhibit TNFα-induced macrophage IL-6 production by specific ELISA (Endogen, Cambridge, MA). TNFα was obtained from PeproTech (Rocky Hill, NJ). Adherent macrophages were challenged with S. typhimurium opsonized with 10% normal mouse serum at a multiplicity of infection of 10, allowed to internalize the bacteria for 15 min, and washed with prewarmed medium containing 6 μg/ml gentamicin. At designated time points after challenge, macrophages were lysed with 0.5% sodium deoxycholate to allow enumeration of surviving bacteria on Luria-Bertani agar plates.
Quantification of Reactive Oxygen and Nitrogen Species.
Reactive oxygen species produced by Salmonella-infected macrophages were measured by using 12.5 μM lucigenin (bis-N-methylacridinium) (Sigma–Aldrich) with a Lumistar chemiluminometer (BMG Lab Technologies, Durham, NC) (13). Production of reactive nitrogen species by Salmonella-infected macrophages was determined spectrophotometrically at 550 nm by using the Griess reagent (13).
Immunofluorescence Microscopy.
Green fluorescent protein (GFP)-expressing 12023 or sseB mutant S. typhimurium were used for fluorescence microscopy (13, 20). Macrophages adhered onto coverslips were challenged with constitutively GFP-expressing S. typhimurium strains. After 90 min of infection, the coverslips were fixed with 2% paraformaldehyde in PBS and washed with 0.1% Tween 20 and 1% normal goat serum. Nonspecific labeling was blocked with a 10% normal goat serum solution. The cells were stained with 5 μg/ml of rabbit anti-p22phox polyclonal antibody (gift of M. Dinauer, Indiana University, Indianapolis, IN), followed by a rhodamine-conjugated goat anti-rabbit polyclonal antibody (Jackson ImmunoResearch). After washing, the coverslips were examined with a Leica DMR XA microscope equipped with a CCD camera controlled by the SLIDEBOOK image-processing software (Intelligent Imaging Innovations, Denver, CO).
Transmission Electron Microscopy.
Ultrastructural studies were performed with macrophages from WT and TNFR-KO mice challenged for 1 h with 12023 or sseB mutant S. typhimurium as described (20). Cerium chloride was added to detect products of the respiratory burst (20).
Results
TNFR-KO Mice Rapidly Succumb to Infection With Virulent or Attenuated Salmonella Pathogenicity Island 2 (SPI2)-Deficient Salmonella.
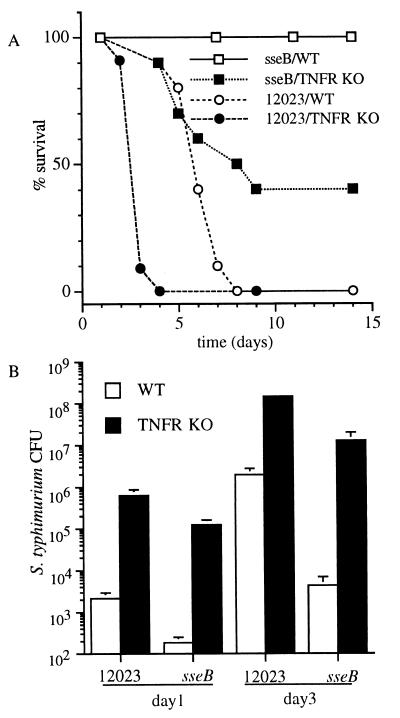
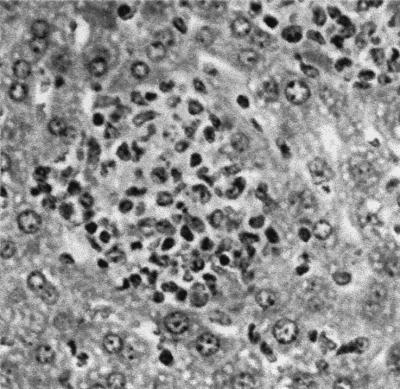
sseB mutant S. typhimurium, deficient in the SPI2-encoded type III secretory system (18, 21), is attenuated for virulence in WT mice (Fig. 1A; ref. 20). The virulence of the sseB mutant is restored substantially in congenic TNFR-KO mice (Fig. 1A), and these immunodeficient mice are even more susceptible to infection with the virulent S. typhimurium strain 12023 (Fig. 1A). The increased lethality of Salmonella infection in TNFR-KO mice coincides with a 102- to 103-fold increased bacterial burden in liver during the initial 1–3 days of infection (Fig. 1B). Similar differences were observed in splenic bacterial burden (data not shown). The dramatic differences in the number of 12023 and sseB mutant S. typhimurium recovered from WT mice are less pronounced in TNFR-KO mice. Infection of TNFR-KO mice with sseB mutant S. typhimurium is associated with the early formation of hepatic granulomas rich in neutrophils and mononuclear cells (Fig. 2). In contrast, the immunodeficient mice develop necrotic lesions in hepatic parenchyma after 3 days of infection with virulent S. typhimurium strain 12023 (data not shown).
Figure 1.
TNFR-KO mice are hypersusceptible to early phases of Salmonella infection. (A) Survival of WT and congenic TNFR-KO mice was recorded over time after infection with roughly 103 colony-forming units of virulent 12023 or attenuated sseB mutant S. typhimurium. (B) The bacterial burden present in livers of WT and TNFR-KO mice was measured after 1 and 3 days of Salmonella infection. The data represent the mean ± SEM of six to 14 mice from three independent experiments.
Figure 2.
Salmonella-infected TNFR-KO mice develop early granulomas. Hematoxylin/eosin-stained sections of hepatic tissue of TNFR-KO mice were obtained after 3 days of infection. The picture is representative of four independent experiments.
Macrophages from TNFR-KO Mice Are Impaired in Their Ability to Kill Virulent or SPI2-Deficient Salmonella.
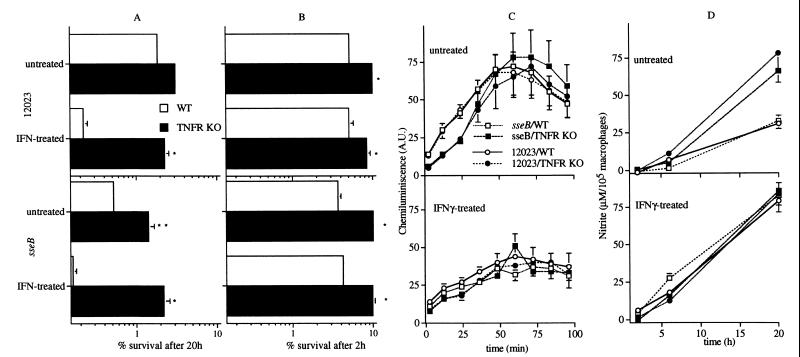
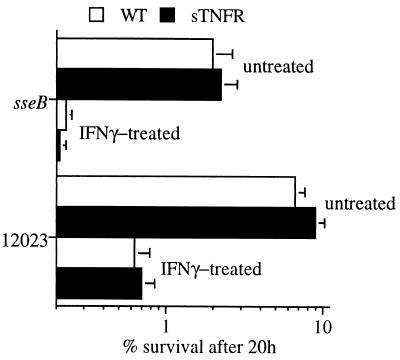
Attenuated sseB mutant S. typhimurium is killed more efficiently by untreated or IFN-γ-treated WT macrophages than its isogenic 12023 parent strain (Fig. 3A). The difference in intracellular survival of these two Salmonella strains is reduced greatly in macrophages from TNFR-KO mice, which were found to harbor as much as 10 times more viable bacteria than WT control macrophages. The reduced bactericidal activity of macrophages from TNFR-KO mice is evident already within 2 h of challenge (Fig. 3B), a phase in which killing of Salmonella by macrophages depends primarily on the NADPH phagocyte oxidase (13, 15, 16). These observations suggest a relationship between TNFR and respiratory burst-dependent killing of Salmonella by macrophages. Macrophages from TNFR-KO mice and congenic WT controls infected with either virulent or attenuated S. typhimurium produce comparable quantities of both superoxide (Fig. 3C) and nitrite (Fig. 3D).
Figure 3.
Absence of TNFR increases the survival of sseB mutant Salmonella in macrophages. Killing of Salmonella by untreated or IFN-γ-treated macrophages from WT and TNFR-KO mice is shown after 20 (A) or 2 h (B) of challenge. Salmonella-infected macrophages from TNFR-KO mice produced similar or greater quantities of superoxide (C) and nitrite (D) as WT control macrophages. The data represent the mean ± SEM of six to 30 independent experiments. *P < 0.001, **P < 0.03 compared with WT controls.
Targeting of NADPH Phagocyte Oxidase-Containing Vesicles to the Salmonella Phagosome Is Defective in Macrophages from TNFR-KO Mice.
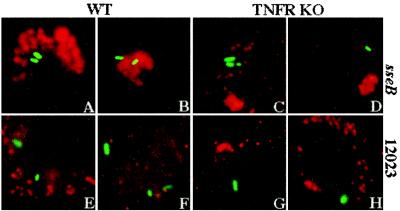
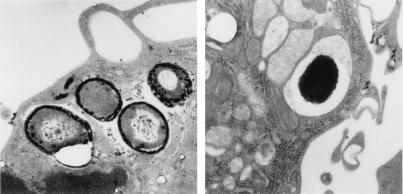
We tested whether the reduced bactericidal activity of TNFRp55-deficient macrophages, despite a robust respiratory burst, results from a failure of vesicles containing active NADPH phagocyte oxidase to target the Salmonella phagosome. S. typhimurium strains expressing GFP were used for fluorescence microscopy. As observed previously (20), the membrane-bound p22phox subunit of the NADPH phagocyte oxidase efficiently localized in the proximity of phagosomes containing SPI2-deficient S. typhimurium but not in 12023-infected macrophages (53 vs. 5%; Fig. 4 A, B, E, and F). In contrast, nearly all (94–100%) vesicles harboring p22phox were found to be remote from Salmonella-containing vacuoles in macrophages from TNFR-KO mice, regardless of whether the internalized bacteria expressed SPI2 (Fig. 4 C, D, G, and H). The localization of p22phox-harboring vesicles was similar in untreated and IFN-γ-treated macrophages infected with GFP-expressing Salmonella (data not shown). The proximity of the NADPH phagocyte oxidase to the Salmonella phagosome was examined further by transmission electron microscopy. Precipitation of cerium perhydroxide, an indicator of the respiratory burst (20), was observed on the surface of 40% of sseB-deficient S. typhimurium in IFN-γ-treated WT macrophages (Fig. 5, Left) but only on 3% of the bacteria within macrophages from TNFR-KO mice (Fig. 5, Right). These results suggest that TNFRp55 is necessary for targeting of NADPH phagocyte oxidase-harboring vesicles to the Salmonella phagosome.
Figure 4.
Localization of the NADPH phagocyte oxidase in macrophages from TNFR-KO mice. (A and B) The p22phox membrane-bound subunit of the NADPH phagocyte oxidase (red) localizes in the proximity of GFP-expressing sseB mutant S. typhimurium (green) within IFN-γ-treated macrophages from WT mice. The p22 subunit of the NADPH phagocyte oxidase is found remote from bacteria-containing phagosomes within TNFR-deficient macrophages infected with either attenuated sseB mutant (C and D) or virulent 12023 (G and H) S. typhimurium, as well as in WT macrophages infected with S. typhimurium strain 12023 (E and F). The data are representative of 83 vacuoles examined from three independent experiments.
Figure 5.
Ultrastructural localization of active NADPH phagocyte oxidase. NADPH phagocyte oxidase activity was detected as cerium perhydroxide precipitate on the surface of sseB mutant Salmonella in macrophages from WT (Left) but not TNFR-KO (Right) mice. The examples shown are representative of 91 bacteria examined.
Extracellular TNFα Is Not Needed for Salmonella Killing by Macrophages.
To test the role for extracellular TNFα signaling in macrophage killing of S. typhimurium, the bactericidal activity of macrophages treated with saturating concentrations of TNF-soluble receptor (22) was examined in macrophages from WT mice. Enhanced killing of sseB mutant S. typhimurium by WT macrophages was not reversed by the addition of high concentrations of TNF-soluble receptor (Fig. 6).
Figure 6.
Effect of TNFα neutralization on Salmonella killing by macrophages. Neutralization of extracellular TNFα with 10 μg/ml TNF-soluble receptor (sTNFR) does not alter the killing of virulent 12023 or sseB mutant S. typhimurium by peritoneal macrophages from WT mice. Selected groups of macrophages were treated with 20 units per ml IFN-γ. The data represent the mean ± SEM of three to nine independent experiments.
Effect of TNFα Deletion on Salmonella Killing by Macrophages.
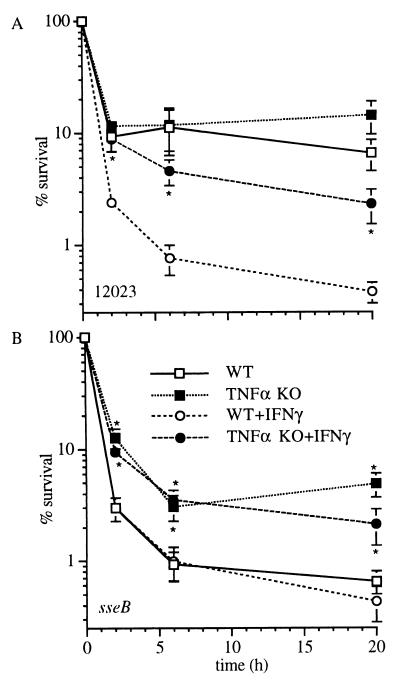
TNFα-KO mice were genotyped by using PCR for the Nramp1 locus (23), and the TNFα deficiency was confirmed by measuring macrophage TNFα synthesis by mouse ELISA (data not shown). Similar to phagocytes from TNFR-KO mice, TNFα-deficient macrophages exhibit decreased killing of S. typhimurium as early as 2 h after challenge (Fig. 7). Moreover, the poor bactericidal activity of TNFα-deficient macrophages cannot be compensated by exogenous IFN-γ administration. Macrophages deficient in TNFα, like those lacking TNFRp55, are capable of producing normal amounts of superoxide and nitrite in response to Salmonella (data not shown).
Figure 7.
Bactericidal activity of macrophages from TNFα-KO mice. The reduced killing of WT (A) or sseB mutant (B) Salmonella by untreated and IFN-γ-treated macrophages from TNFα-KO mice is manifest as early as 2 h after infection. The data represent the mean ± SEM of six to nine independent experiments. * P < 0.02 compared with WT controls.
Discussion
TNFα can activate phagocytes to produce oxyradicals and nitrogen oxides (3, 4, 8, 10–12). However, this cytokine seems dispensable for the synthesis of oxygen-dependent radicals by primary peritoneal macrophages infected with Salmonella. In fact, Salmonella-infected macrophages from TNFR-KO or TNFα-KO mice produce amounts of reactive oxygen and nitrogen species comparable to WT controls. These data are in agreement with previous studies that have described NADPH phagocyte oxidase and inducible NO synthase (iNOS) activity in the absence of TNFα signaling (24–27). Despite being able to produce oxyradicals and nitrogen oxides, macrophages from TNFα-KO and TNFRp55-KO mice exhibit poor killing of Salmonella, a defect manifested as early as 2 h after infection during a phase dependent on the NADPH phagocyte oxidase (13). These observations suggest that a defective innate effector mechanism related to the NADPH phagocyte oxidase, but distinct from oxidase or iNOS activation, contributes to the greatly enhanced susceptibility of TNFRp55-deficient mice to Salmonella infection.
The high incidence of invasive salmonellosis in patients carrying mutations in any of the membrane-bound or cytosolic components of the NADPH phagocyte oxidase demonstrates the critical importance of the respiratory burst in innate host resistance to Salmonella (23). We present here several lines of evidence supporting the hypothesis that TNFRp55 contributes to early resistance to Salmonella infection by optimizing the antibacterial actions of the NADPH phagocyte oxidase. First, TNFR-KO mice succumb to Salmonella during a phase of infection dominated by the NADPH phagocyte oxidase (13, 15, 16). Second, SPI2-deficient S. typhimurium, which is attenuated in the presence of the NADPH phagocyte oxidase, is able to cause lethal infection in TNFR-KO mice. Third, macrophages from TNFR-KO mice fail to target NADPH phagocyte oxidase-containing vesicles to the proximity of the Salmonella phagosome. Vesicles harboring the p22phox subunit of the NADPH phagocyte oxidase are distributed within the cytoplasm of TNFRp55-deficient macrophages at locations remote from Salmonella-containing vacuoles. Furthermore, electron microscopy revealed that an SPI2-deficient strain of S. typhimurium is protected from the actions of the NADPH phagocyte oxidase in macrophages from TNFR-KO mice, in contrast to WT macrophages.
Unexpectedly, saturating concentrations of TNF-soluble p55 receptor failed to reduce macrophage killing of Salmonella. However, macrophages from TNFα-KO mice, similar to those from TNFR-KO mice, exhibited poor killing of Salmonella despite abundant production of reactive oxygen and nitrogen species. These results are consistent with a model in which intracellular TNFα can signal via the p55 receptor to stimulate trafficking of NADPH phagocyte oxidase-containing vacuoles to the Salmonella phagosome. The Salmonella SPI2 locus appears to ameliorate the cytotoxic actions of the respiratory burst by preventing the trafficking of the NADPH phagocyte oxidase to the phagosome (20). Therefore, it is conceivable that intracellular TNFα–TNFRp55 complexes regulate vesicular trafficking and serve as targets for effector proteins exported by SPI2 type III secretory system.
Among its many functions, TNFα contributes to the recruitment of leukocytes to the foci of infection. Previous studies using neutralizing antibodies against TNFα in Salmonella-vaccinated mice have shown a key role for this cytokine in granuloma formation during late phases of the Salmonella infection (5, 6). However, our studies indicate that TNFRp55 is dispensable for either leukocyte recruitment or early granuloma organization during the earliest stage of primary salmonellosis.
To the plethora of activities already associated with TNFα–TNFRp55 signaling, our studies identify targeting of the NADPH phagocyte oxidase to the phagosome as a function of this cytokine. The critical importance of this process for innate resistance to bacterial infection must be considered carefully in the implementation of strategies designed to antagonize TNFα for the treatment of chronic inflammatory disorders (28).
Acknowledgments
We are grateful to M. C. Dinauer for providing anti-p22 antibodies, B. Hybertson for use of a chemiluminometer, D. Holden for the gift of SPI2 mutant Salmonella and constructive review of the manuscript, and A. Jones for assistance with the electron microscopy. This work was supported by a National Institutes of Health postdoctoral fellowship, National Institutes of Health Grants AI15614, AI39557, and AI44486, the James Biundo Foundation, and an educational grant from Amgen, Inc.
Abbreviations
- TNF
tumor necrosis factor
- TNFR
TNF receptor
- KO
knockout
- WT
wild type
- GFP
green fluorescent protein
- SPI2
Salmonella pathogenicity island 2
References
- 1.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassalli P. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 2.Everest P, Roberts M, Dougan G. Infect Immun. 1998;66:3355–3364. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green S J, Crawford R M, Hockmeyer J T, Meltzer M S, Nacy C A. J Immunol. 1990;145:4290–4297. [PubMed] [Google Scholar]
- 4.Liew F Y, Li Y, Millott S. J Immunol. 1990;145:4306–4310. [PubMed] [Google Scholar]
- 5.Mastroeni P, Arena A, Costa G B, Liberto M C, Bonina L, Hormaeche C E. Microb Pathog. 1991;11:33–38. doi: 10.1016/0882-4010(91)90091-n. [DOI] [PubMed] [Google Scholar]
- 6.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Microb Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 7.Netea M G, van Tits L J, Curfs J H, Amiot F, Meis J F, van der Meer J W, Kullberg B J. J Immunol. 1999;163:1498–1505. [PubMed] [Google Scholar]
- 8.Skerrett S J, Martin T R. Infect Immun. 1996;64:3236–3243. doi: 10.1128/iai.64.8.3236-3243.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazzoni F, Beutler B. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 10.Oswald I P, Wynn T A, Sher A, James S L. Proc Natl Acad Sci USA. 1992;89:8676–8680. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva J S, Vespa G N, Cardoso M A, Aliberti J C, Cunha F Q. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuortes M, Melchior M, Han H, Lyon G J, Nathan C. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang F C. J Exp Med. 2000;192:227–236. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiloh M U, Ruan J, Nathan C. Infect Immun. 1997;65:3193–3198. doi: 10.1128/iai.65.8.3193-3198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiloh M U, MacMicking J D, Nicholson S, Brause J E, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 16.Mastroeni P, Vázquez-Torres A, Fang F C, Xu Y, Khan S, Hormaeche C E, Dougan G. J Exp Med. 2000;192:237–247. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchmeier N A, Lipps C J, So M Y, Heffron F. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 18.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 19.De Groote M A, Ochsner U A, Shiloh M U, Nathan C, McCord J M, Dinauer M C, Libby S J, Vázquez-Torres A, Xu Y, Fang F C. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vázquez-Torres A, Xu Y, Jones-Carson J, Holden D W, Lucia S M, Dinauer M C, Mastroeni P, Fang F C. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 21.Ochman H, Soncini F C, Solomon F, Groisman E A. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards C K., III Ann Rheum Dis. 1999;58, Suppl. 1:I73–I81. doi: 10.1136/ard.58.2008.i73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacMicking J D, North R J, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nashleanas M, Scott P. Infect Immun. 2000;68:1428–1434. doi: 10.1128/iai.68.3.1428-1434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanos-Velez E, Maerlan S, Osorio L M, Aberg F, Biberfeld P, Orn A, Rottenberg M E. Infect Immun. 1998;66:2960–2968. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 27.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland S M, Wagner H, Pfeffer K. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis B, Faulds D. Drugs. 1999;57:945–966. doi: 10.2165/00003495-199957060-00014. [DOI] [PubMed] [Google Scholar]