Abstract
Purpose
Stroke is the second leading cause of death and a major cause of morbidity and mortality worldwide. Evidence of variations in adiponectin(AdipoQ) genes that are associated with ischemic stroke has not been consistent, and it is unclear whether the same loci contribute to these associations in the Korean population. Using a Korean population, we tested ischemic stroke-associated AdipoQ markers.
Materials and Methods
In a preliminary genome-wide association study using 320 250 k Affymetrix NSP chips, AdipoQ was found to be associated with ischemic stroke in Koreans. To study of AdipoQ, a further 673 ischemic stroke patients and 267 unrelated individuals without a history of stroke or transient ischemic attack were examined in a case-control study.
Results
Six polymorphisms (rs182052G > A, rs16861205G > A, rs822391T > C, rs822396A > G, rs12495941G > T and rs3774261A > G) that had a minor allele frequency of over 1% were strongly associated with stroke (p < 0.05). Two of these, rs822391T > C and rs822396A > G showed this association on both dominant and additive logistic regression analysis after adjusting for age and sex. The haplotypes ht 1 (AGGCGG and AAGTAG) were significantly associated with susceptibility to stroke.
Conclusion
Our findings show that polymorphisms in AdipoQ are associated with risk for ischemic stroke in the Korean population. This study lends further support to the putative role of AdipoQ in stroke.
Keywords: AdipoQ, ischemic, stroke, Koreans
INTRODUCTION
Stroke is the second leading cause of death worldwide and a major cause of morbidity and mortality. According to the Korea National Statistical Office, stroke was the second most common cause of death in 2007 (http://www.nso.go.kr).
The clinical phenotype of stroke is broadly divided into ischemic and hemorrhagic stroke. Ischemic stroke constitutes 80% to 90% of all cases.1-3 Ischemic stroke is divided into 2 principal types: thrombotic and embolic. Thrombotic stroke can also be subdivided into large-vessel occlusion and small-vessel occlusion, correlating with the location of the blockage within the brain. Therefore, stroke does not appear to be defined as a single disease or one distribution.4 The proportion of small-vessel occlusion is relatively high in Korea as compared to Western countries.5 The identification of a reliable genetic risk factor for stroke is important.
Adiponectin is also called GBP-28, apM1, AdipoQ, and Acrp30.6 It was identified as an adipocytokine, a 244-amino-acid protein, the product of the AdipoQ gene, which is highly expressed in adipose cells.7 AdipoQ, a novel adipose tissue-specific protein, is a structural homolog of collagen VIII and X, complement factor C1q, and tumor necrosis factor-α (TNFα).7-10 AdipoQ regulates the homeostasis of glucose, energy storage, and fatty acid metabolism11,12 and acts as an anti-inflammatory13 and antiatherogenic plasma protein.14,15
AdipoQ is associated with obesity, metabolic syndrome, type 2 diabetes mellitus, hypertension, and coronary artery diseases. Furthermore, low levels of plasma adiponectin have been linked to ischemic cerebrovascular disease (CVD).15-23 In comparing type 2 diabetes patients to controls, it can be seen that AdipoQ mRNA levels are reduced in adipose tissue, although they are normal levels in circulating adiponectin.
The adiponectin(AdipoQ) gene is located on chromosome 3q27.24-26 AdipoQ gene variations are associated with changes in plasma adiponectin concentration. Variations in the AdipoQ gene also correlate with ischemic stroke in some populations. In American,27 Japanese,28 Greece29 and European30 populations, AdipoQ is associated with ischemic stroke. The aim of the present study was to investigate this association in a Korean population.
We used 320 250 k Affymetrix NSP chips (Affymetrix Inc., Santa Clara, CA, USA) in a stroke case and control association study. In this genome-wide association chip experiment, many single nucleotide polymorphisms (SNPs) in several genes, including AdipoQ, were associated with ischemic stroke. We queried the GeneCards, Online Mendelian Inheritance in Man (OMIM), and PubMed databases to identify stroke-related genes and compared them with the SNPs from the preliminary experiments.
To test whether AdipoQ was a candidate gene that was linked to the Korean population, we obtained 10 tag SNPs of the AdipoQ gene. Then, we performed an Illumina Golden Gate experiment using samples from 940 Koreans to determine any association with the markers. In this study, we identify the markers that are associated with the AdipoQ gene in the Korean population.
MATERIALS AND METHODS
Subjects
A preliminary experiment examined 160 normal healthy subjects and 160 stroke patients who were recruited from the Korean Institute of Oriental Medicine (KIOM). The follow-up experiment examined 673 ischemic stroke cases and 267 healthy controls recruited from KIOM. Informed consent was obtained from all participants through KIOM, and our research was approved by the research ethical committee of KIOM.
Candidate gene and marker selection
In the preliminary experiment with 320 Affymetrix 250 k NSP chips, SNPs in genes that were associated with the combined phenotype of ischemic stroke were identified (p < 0.01), and we selected several SNPs within the 100-kb upstream and downstream regions (using the Affymetrix annotation file; Mapping205K_Nsp.na22.annot.csv). We searched the GeneCards, OMIM, and PubMed databases and found 768 stroke-related genes compared with the SNPs in the preliminary experiment.
In a follow-up association analysis (the Illumina Golden Gate experiment), we selected 10 tag SNPs in the AdipoQ gene, which was one of the candidate genes related to ischemic stroke. The 10 tag SNPs were selected if they had an efficiency of more than 80% using Haploview and HapMap Japanese data (http://www.hapmap.org).
Statistical analysis
Differences in clinical characteristics between the 2 groups were determined by a Student's t-test for continuous variables [age, body-mass index (BMI)] and a Chi-squared test for categorical variables (sex, hypertension, diabetes mellitus, and hypercholesterolemia). The Chi-squared test was used to measure Hardy-Weinberg equilibrium (HWE) for comparing allele and genotype frequencies between cases and controls. The relative risk for stroke associated with genotype in the case-control analysis was calculated with odds ratios (ORs) [95% confidence interval (CI)] using logistic regression models and corresponding p values, while controlling for age and sex as covariates [software: PLINK (v1.03, http://pngu.mgh.harvard.edu/~purcell/plink/)].
Three alternative models for the minor alleles-codominant, dominant, and recessive-were applied in our analyses. For each OR, we calculated 95% CIs. Association analysis of the haplotypes was performed using Haploview (v 4.1). Logistic regression models were used to estimate odds ratios that assessed the association between AdipoQ haplotypes and stroke, adjusting for sex and age [software: PLINK (v1.03)]. A 2-tailed p value < 0.05 was significant.
RESULTS
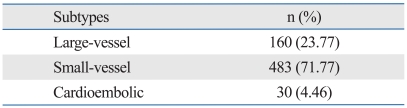
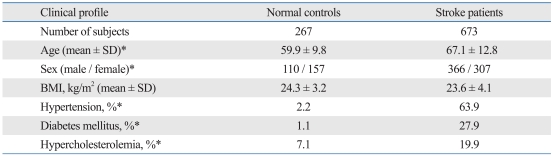
The distribution of stroke types in our study is shown in Table 1. The proportion of small-vessel occlusion cases was relatively high, and that of cardiac embolism was low, as in a previous study.5 The principal characteristics of the subjects in the AdipoQ gene association study are shown in Table 2. Stroke patients experienced a higher prevalence of hypertension, diabetes mellitus, and hypercholesterolemia than the normal controls. This profile was similar to that of a previous study in Koreans, in which hypertension was present in 60% to 70%, diabetes mellitus in 25% to 30%, and hypercholesterolemia in 15% to 20% of stroke patients.5 BMI was not significantly different between the two groups. However, these risk factors were not adjusted for because of the large number of similar missing values within each group.
Table 1.
Ischemic Stroke Subtypes
Table 2.
Clinical Profiles of the Subjects
BMI, body-mass index.
*p value < 0.05 for the difference between stroke patients and normal controls.
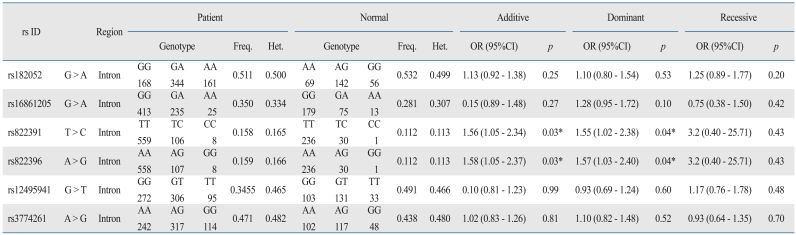
From the selected candidate genes, we identified 10 independent tag SNPs in the AdipoQ gene using public databases. The 10 SNPs had a minor allele frequency (MAF) of ≥ 0.05 and a Hardy-Weinberg equilibrium p value of ≥ 0.00001. Six SNPs were significantly related to strokers 182052, rs16861205, rs822391, rs822396, rs12495941, and rs3774261 (Table 3)-as determined by the chi-squared test (p < 0.05). The 6 polymorphisms were further examined in a more rigorous evaluation of association by multivariable logistic regression analysis, adjusting for sex and age. Genotypes were assessed as either dominant, recessive, or additive. Two SNPs (rs822391T > C and rs822396A > G) were more highly associated with risk of stroke in the dominant and additive analyses.
Table 3.
Association Analyses of stroke with AdipoQ Polymorphism
CI, confidence interval.
*p value < 0.05 for the difference between stroke patients and normal controls.
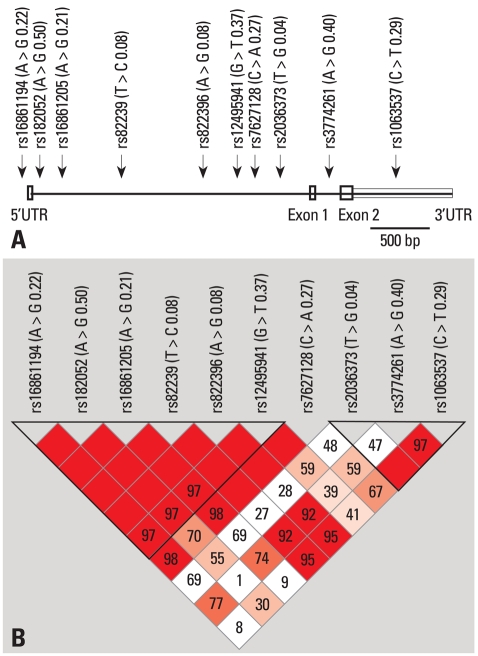
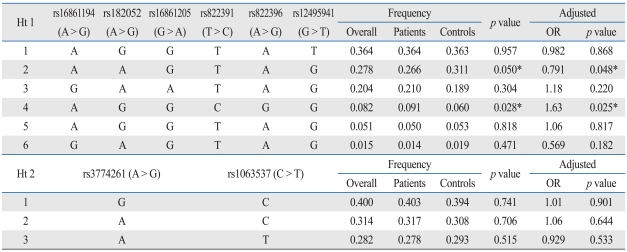
The genomic positions of the 10 SNPs in the AdipoQ gene are shown in Fig. 1A. We performed haplotype-based conditional logistic regression analysis, adjusting for sex and age (Table 4). The haplotypes AGGCGG and AAGTAG in ht1 showed the most preventive effect in stroke (p = 0.029 and 0.050, respectively).
Fig. 1.
Gene map and linkage disequilibrium blocks in the AdipoQ gene. (A) Map of AdipoQ gene on chromosome 3q27. (B) LD Blocks.
Table 4.
Haplotype of LD Blocks
LD, linkage disequilibrium; OR, odds ratio.
*p value < 0.05 for the difference between stroke patients and normal controls.
DISCUSSION
There are few studies of the association between the AdipoQ gene and stroke. Several studies have linked AdipoQ gene variations to the risk of stroke. Five AdipoQ genetic variations (rs266729, rs182052, rs822396, rs2241766, and rs1501299) were examined for their association with incident myocardial infarction or ischemic stroke in a Caucasian population;27 in this study, rs266729 and rs182052, and the haplotype G-A-G (comprising rs266729, rs182052, and rs822396) were correlated with a risk of ischemic stroke, irrespective of diabetes. The rs266729C > G polymorphism of the AdipoQ gene has been associated with atherothrombotic cerebral infarction in Japanese individuals with a metabolic syndrome.28
AdipoQ gene variations are associated with plasma adiponectin concentrations, and metabolic syndrome is a risk factor for cardiovascular disease and also associated with plasma adiponectin concentrations. Therefore, AdipoQ gene variations may be linked to susceptibility to ischemic stroke.
In our study, we found evidence for the association of AdipoQ gene variations/haplotypes with ischemic stroke in the Korean population. We found that rs822391T > C and rs822396A > G were related to an increased risk for ischemic stroke in the dominant and additive analyses. Even though we do not know the exact inheritance mode of AdipoQ gene, according to the association results, we may drop the AdipoQ recessive inheritance in stroke causation or susceptibility. Furthermore, a haplotype block (comprising rs182052, rs16861205, rs822391, rs822396, rs12495941, and rs3774261) that contained the haplotypes AGGCGG and AAGTAG was associated with ischemic stroke.
We identified associated genes in a genome-wide commercial SNP chip experiment and compared them with candidate genes from public databases in non-Korean populations, such as GeneCards, OMIN, and PubMed. Furthermore, we verified these results by analyzing 10 SNPs in the AdipoQ gene in 940 subjects. Similar, significant genetic risk factors for ischemic stroke were also observed in American,27 Japanese,28 Greek,29 and general European30 populations.
Stroke is the second leading cause of death in Koreans and a major cause of morbidity and mortality around the world, and the role of inflammation may be important.27 AdipoQ acts as an anti-inflammatory13 and antiatherogenic plasma protein.14,15 AdipoQ gene variations are associated with obesity, metabolic syndrome, type 2 diabetes mellitus, hypertension, coronary artery disease, and ischemic CVD. The five population studies-in Americans,27 Japanese,28 Greece,29 European30 and Koreans (our study)-suggest that polymorphisms in AdipoQ increase susceptibility to stroke. Therefore, further functional molecular biology and genetic studies are needed to explain the mechanics of this link.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Cerebrovascular Disease Oriental Medicine Project of KIOM (Korean Institute of Oriental Medicine) and in part by a grant (2010-0012810) from the National Research Foundation of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Warlow CP, Tennis MS, van Gijn J, Hankey GJ, Sandercock PAG, Bamford JM, et al. A practical guide to management. NJ: Wiley-Blackwell; 1996. Stroke. [Google Scholar]
- 2.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 3.Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 4.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35(2):131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS. Stroke in Korea. Int Congr Ser. 2004;1262:348–351. [Google Scholar]
- 6.Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 8.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 10.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 14.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 16.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 17.Stefan N, Bunt JC, Salbe AD, Funahashi T, Matsuzawa Y, Tataranni PA. Plasma adiponectin concentrations in children: relationships with obesity and insulinemia. J Clin Endocrinol Metab. 2002;87:4652–4656. doi: 10.1210/jc.2002-020694. [DOI] [PubMed] [Google Scholar]
- 18.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Hirose H, Saito I, Tomita M, Taniyama M, Matsubara K, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002;103:137–142. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 20.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 21.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 22.Lihn AS, Østergard T, Nyholm B, Pedersen SB, Richelsen B, Schmitz O. Adiponectin expression in adipose tissue is reduced in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2003;284:E443–E448. doi: 10.1152/ajpendo.00358.2002. [DOI] [PubMed] [Google Scholar]
- 23.Ryan AS, Macko RF, Peters MN, Ivey FM, Prior SJ, Joseph LJ, et al. Plasma adiponectin levels are associated with insulin sensitivity in stroke survivors. J Stroke Cerebrovasc Dis. 2009;18:214–220. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M, Arita Y, Yamagata K, Matsukawa Y, Okutomi K, Horie M, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord. 2000;24:861–868. doi: 10.1038/sj.ijo.0801244. [DOI] [PubMed] [Google Scholar]
- 25.Kissebah AH, Sonnenberg GE, Myklebust J, Goldstein M, Broman K, James RG, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci U S A. 2000;97:14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumvoll M, Tschritter O, Fritsche A, Staiger H, Renn W, Weisser M, et al. Association of the T-G polymorphism in adiponectin (exon 2) with obesity and insulin sensitivity: interaction with family history of type 2 diabetes. Diabetes. 2002;51:37–41. doi: 10.2337/diabetes.51.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Hegener HH, Lee IM, Cook NR, Ridker PM, Zee RY. Association of adiponectin gene variations with risk of incident myocardial infarction and ischemic stroke: a nested case-control study. Clin Chem. 2006;52:2021–2027. doi: 10.1373/clinchem.2006.074476. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, et al. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med. 2008;21:801–808. [PubMed] [Google Scholar]
- 29.Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005;36:1915–1919. doi: 10.1161/01.STR.0000177874.29849.f0. [DOI] [PubMed] [Google Scholar]
- 30.Patel S, Flyvbjerg A, Kozàkovà M, Frystyk J, Ibrahim IM, Petrie JR, et al. Variation in the ADIPOQ gene promoter is associated with carotid intima media thickness independent of plasma adiponectin levels in healthy subjects. Eur Heart J. 2008;29:386–393. doi: 10.1093/eurheartj/ehm526. [DOI] [PubMed] [Google Scholar]