Abstract
Platelets are crucial for hemostasis and are vital regulators of inflammation. Foxp3 is a key transcription factor for T regulatory cell development. Humans with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, x-linked) and the scurfy (Foxp3sf) mouse have mutations in the Foxp3 gene that lead to a host of pathologies including autoimmunity and skin diseases. Scurfy mice and some humans with IPEX are also thrombocytopenic.
Objective
Determine if the absence of functional Foxp3 leads to defects in megakaryocytes and platelets.
Methods and results
We discovered that human and mouse megakaryocytes express Foxp3 mRNA and protein. Using shRNA and Foxp3sf mice, we demonstrated that Foxp3 deficient mouse and human megakaryocyte progenitors exhibited proliferation defects. Striking platelet abnormalities were observed in both an IPEX patient and Foxp3sf mice. Impaired platelet spreading and release of TGF-β and CD40 ligand (CD40L), and abnormal levels of plasma CD40L were observed in a case of IPEX syndrome. Foxp3sf mice were thrombocytopenic and had increased platelet volume and altered serum levels of CD40L, TXB2, and TGF-β.
Conclusion
These findings provide compelling new evidence that Foxp3 is needed for proper megakaryopoiesis and plays a role in regulating platelet function including spreading and release.
Introduction
Platelets play vital roles in the normal hemostatic response to injury and are key cellular elements in diseases such as stroke and myocardial infarction 1. They are also now recognized as contributing to chronic diseases such as type-2 diabetes 2. Platelets contain mRNAs, pre-mRNAs and splicing machinery to synthesize proteins 3. In addition, they release pro-inflammatory eicosanoids and proteins such as CD40 ligand (CD40L; formally called CD154), a potent cytokine that activates immune cells and structural cells such as endothelial cells. These biologic processes make platelets important regulators of the immune system and the inflammatory response. In addition to unwanted plalelet activation, loss of platelet function can also lead to morbidity and mortality. Reduced platelet number through autoimmunity, cancer chemotherapy or ionizing radiation exposure can be fatal.
Normal megakaryopoiesis is necessary for optimal platelet production and function. Megakaryopoiesis is a complex process which involves the differentiation of bipotential erythroid/megakaryocyte progenitors to megakaryocyte progenitors followed by their differentiation to megakaryocyte precursor cells. While megakaryocyte progenitors proliferate, megakaryocyte precursors lack proliferative potential, but instead replicate DNA and increase cellular content to form mature, polyploid megakaryocytes that shed platelets.
Forkhead box protein 3 (Foxp3) is a key transcription factor believed to be restricted to a subset of regulatory T (Treg) cells and is required for their development 4, 5. Genetic mutations in Foxp3 lead to an X-linked often fatal autoimmune disease known as IPEX (immune dysregulation, polyendocrinopathy, enteropathy, x-linked) syndrome in humans 6. A similar disease arising from a Foxp3 mutation occurs in the spontaneous mouse mutant scurfy (Foxp3sf) in which a frameshift mutation in Foxp3 results in a dysfunctional product lacking the forkhead domain 7. The defects observed in IPEX and scurfy have been ascribed to dysfunctional Tregs. Based on the low platelet numbers and gastrointestinal bleeding in the presence of mutant Foxp3 in Foxp3sf mice and IPEX patients 8,9, we hypothesized that Foxp3 plays an intrinsic role in megakaryocyte maturation and thrombopoiesis. In this manuscript, we report the expression of Foxp3 in human and mouse megakaryocytes, demonstrate its importance in megakaryopoiesis, and describe the platelet phenotype in Foxp3sf mice and in a case of IPEX syndrome.
Materials and Methods
Cell culture and treatment conditions
Meg-01 cells, were originally established from the bone marrow of a patient with philadelphia chromosome positive chronic myelogenous leukemia 10, and M07e cells, human leukemic cells with megakaryoblastic features 11, were purchased from the American Type Culture Collection (Rockville, MD). These and Dami cells, established from the blood of a patient with megakaryoblastic leukemia, were cultured as previously described 12. The primary human lung fibroblast strain, L828, were cultured as previously described 13.
Megakaryocyte differentiation from human cord blood-derived CD34+ cells
Human CD34+ cord blood cells were obtained from AllCells (Emeryville, CA). Cells were plated at 2.5×105 cells per well in a 12-well plate and cultured in serum-free medium as previously described 12 and supplemented with 100 ng/mL of recombinant human thrombopoietin (rhTPO) (R&D Systems, Minneapolis, MN). After 14 days in culture, primary human megakaryocytes were identified by staining with a CD61-FITC antibody and analyzed on a BD Biosciences FACSCalibur flow cytometer. Cells were greater than 95% CD61 positive. Data were analyzed using FlowJo software (Treestar, Ashland, OR).
Quantitative real-time PCR analysis
Sequences for PCR primer pairs were as follows: human Foxp3, forward 5′-GAA ACAGCACATTCCCAGAGTTC-3′and reverse 5′-AGGTGGCAGGATGGTTTCTG-3′; human 7S, forward 5′-ACCACCAGGTTGCCTAAGGA-3′ and reverse 5′-CACGGGAGTTTTGACCTGCT-3′. Another set of human Foxp3 primer pairs 14 were used to confirm results from primers listed above: human Foxp3, forward 5′-CGGACCATCTTCTGGATGAG-3′ and reverse 5′-TTGTCGGATGATGCCACAG-3′.
Western blot analysis
Whole cell lysates were prepared using ELB buffer plus protease and phosphatase inhibitors as previously described 15. One hundred micrograms of protein was electrophoresed and membranes probed with hFOXY antibody (1:500) (eBioscience). Human Foxp3 transfected cell lysate (BioLegend, San Diego, CA) was used as a positive control.
Flow cytometric analysis
One ×106 cells were fixed and permeabilized with Foxp3 staining buffer (eBioscience, San Diego) and stained for Foxp3 using the clone PCH101 (eBioscience) or clone 236A/E7 (eBioscience) according to the manufacturer’s instructions. Cells were incubated with isotype control antibodies (eBioscience) and fluorescence was analyzed as described 12. For DNA content analysis, cells were fixed overnight in 95% ethanol at 4°C. Cells were washed and incubated with 3 μM of DAPI for 20 minutes at 37°C.
Immunofluorescence
Cell cytospins were mounted on slides and stained for Foxp3 intracellularly as described above. Cells were visualized using an Olympus BX51 light microscope (Olympus, Melville, NY), photographed with a SPOT camera and analyzed with SPOT RT software (New Hyde Park, NY).
Animals
Blood was obtained from the orbital sinus of male B6.Cg-Foxp3sf (Foxp3sf ) and background strain C57BL/6J mice (Jackson laboratories) at age 24 days. C57BL/6J, age-matched, male mice were used as a comparative control because Foxp3sf/+ females were backcrossed to C57BL/6J males to maintain Foxp3sf mice 16–18. Platelet counts were also performed on healthy C57BL/6J male siblings of the Foxp3sf mice. Platelets were counted, and mean platelet volume (MPV) was determined using the Heska® CBC-Diff Veterinary Analyzer (Fort Collins, CO). Serum was prepared by allowing whole blood clot for 30 minutes at 37° and placing the blood on ice for 2 hours. Bone marrow was harvested from the femora of Foxp3sf and C57BL/6J mice. Two × 104 megakaryocytes were cytospun on glass slides and stained with a Diff-Quik stain set (Dade Behring, Newark, DE).
Analysis of megakaryocytes in Foxp3sf mice
Megakaryocyte number was assessed by counting the number of megakaryocytes in 5 low power (10×) fields. Megakaryocyte progenitor (Meg-CFCs) assay was performed as previously published 19. Meg-CFCs were defined by the expression of GP1bβ (Emfret Analytics, Wurtzburg, Germany) and by their ability to generate colonies containing at least 3 megakaryocytes. Foxp3 expression was analyzed in cells expressing CD41 (BD Biosciences, San Jose, CA) by flow cytometry. Megakaryocytes were stained for Foxp3 using the 150D/EF clone (eBiosciences) according to the manufacturer’s instructions.
siRNA to knock-down Foxp3
Foxp3 shRNA individual clone was obtained from Open Biosystems (Huntsville, AL). One × 106 Meg-01 cells were nucleofected® with 2 μg of pLKO.1 HIV-based lentiviral vector expressing human shRNA Foxp3 plasmid or an empty vector plasmid using Amaxa Biosystem’s Cell Line Nucleofector® Kit C, program V-001. Meg-01 cells were analyzed for Foxp3 expression 24, 48 and 72 hours post-transfection by flow cytometry. Four hours after nucleofection®, cells were washed and proliferation was analyzed using CellTrace CFSE (carboxyfluorescein diacetate, succinimidyl ester) according to the manufacturer’s instructions (Invitrogen). Mitomycin C (Sigma), which crosslinks DNA and inhibits cell division, was used as a proliferation negative control and Meg-01 cells that were not nucleofected® were used as a positive control.
Measuring TXB2, CD40L, TGF-β, 12(S)-HETE, and platelet factor 4 (PF4)
Mouse blood was collected and anti-coagulated with acid-citrate-dextrose (ACD) or activated with either ADP (10 μM) or thrombin (10 U/mL) for 30 minutes. Plasma was obtained by centrifuging anti-coagulated blood at 3000 ×g for 10 minutes. Serum was obtained by centrifuging coagulated blood at 3000 ×g for 10 minutes. TXB2 was measured using a competetive enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI). CD40L, TGF-β, and platelet factor 4 (PF4)were measured using sandwich enzyme-linked immunosorbent assays (ELISAs) (R&D Systems, Minneapolis, MN). 12-hydroperoxyeicosatetraenoic acid (12(S)-HETE) was measured using an EIA (Assay Designs, Ann Arbor, MI).
Human serum collection and blood platelet isolation
Whole blood was obtained under University of Rochester IRB approval from male donors and an IPEX donor using Vacutainer tubes containing buffered sodium citrate or no anticoagulant for serum collection (BD Biosciences, Franklin Lakes, NJ). The IPEX patient was previously revealed to have a G to A transition (1150G>A) in exon 11, resulting in a substitution of Ala to Thr at residue 384, within the DNA binding domain of Foxp3 20. Platelets were isolated as described 21, 22. Complete blood counts were performed on an Abbott Cell-Dyn 1700 (Abbott Park, IL). Platelet purity was typically greater than 99%. Plasma and serum were analyzed for human TGF-β and TXB2 (Cayman Chemical). Human CD40L was analyzed by ELISA as described 21.
Human platelet activation
Washed platelets were spread on fibrinogen-coated (150 μg/mL) slides as described 19. Platelet spreading is critical for platelet-surface contact during wound healing and is used as a measure of platelet function 23. In addition, 3×107 platelet/300μL were incubated (37°C) with platelet activators: Thrombin 0.8 U/mL (Sigma), collagen 10 μg/mL (Chrono-log Corporation, Havertown, PA), and adenosine diphospate (ADP) 10 μM (Chrono-log Corporation). After treatment, platelets were centrifuged (1200×g/1 min), and supernatants were analyzed for human TGF-β, TXB2, platelet factor 4 (PF4) and CD40L.
Statistical analysis
Results are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using a paired, two-tailed Student’s t test with P<.05 deemed as statistically significant. All experiments were repeated at least 3 times unless otherwise stated.
Results
1. Megakaryocytes and megakaryoblastic cell lines express Foxp3 mRNA and protein
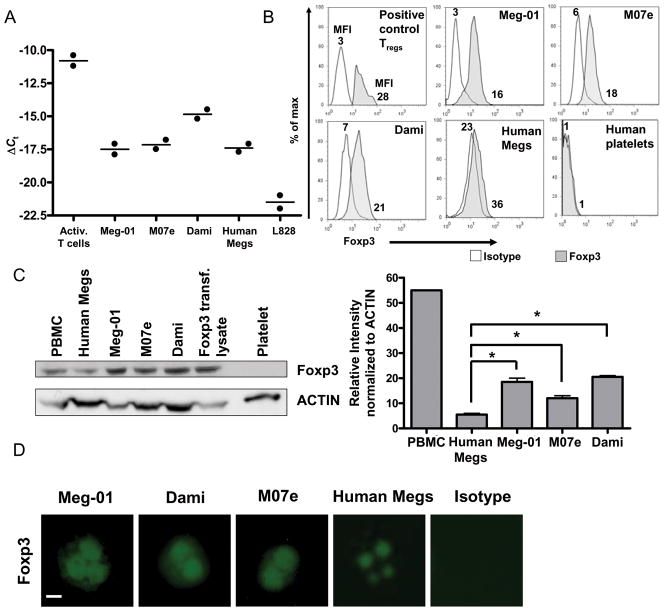
To determine whether Foxp3 mRNA is expressed in megakaryocytes, real-time PCR analysis was used to evaluate 3 different megakaryocytic cell lines (Meg-01, M07e, and Dami) and primary human megakaryocytes. L828 RNA was used as a negative control as human fibroblasts were found to be negative for Foxp3 mRNA 24. Human CD4+CD25+ T cells were isolated from peripheral blood and activated with anti-CD3 and anti-CD8 beads 25. The RNA was isolated and used as a positive control. As shown in Figure 1A, all 3 human megakaryocyte cell lines expressed Foxp3 mRNA, as did primary human megakaryocytes. However, Foxp3 transcript levels were lower compared to activated T cells. Expression of Foxp3 mRNA was confirmed using two different primer sets for Foxp3.
Figure 1. Primary human megakaryocytes and megakaryoblastic cell lines express Foxp3 mRNA and protein.
(A) RT-PCR analysis of Foxp3 expression in T cells activated with CD3 and CD28 beads, Meg-01 cells, M07e cells, Dami cells, primary human megakaryocytes, and L828 cells (n = 2) for two different sets of primers. Data are representative of one primer set. ΔCt was calculated as Ct (7S) – Ct (Foxp3). (B) Flow cytometric intracellular analysis of Foxp3 protein expression in Meg-01 cells, M07e cells, Dami cells, primary human megakaryocytes and human platelets using the PCH101 antibody clone. PBMCs were stained for Foxp3 and the Foxp3 positive cells were gated and analyzed as a positive control. Experiments were performed 3 times and confirmed using the anti-Foxp3 236A/E7 antibody clone. Data are presented as mean fluorescence intensity (MFI). (C) Western blot analysis of Foxp3 protein expression in Meg-01 cells, M07e cells, Dami cells, primary human megakaryocytes and human platelets. PBMCs and Foxp3 transfected cell lysate were used as positive controls. Experiments were performed 3 times and densitometry indicates that the megakaryoblastic cell lines have significantly higher levels of Foxp3 protein compared to primary human megakaryocytes. Results are presented as mean ± SD *(P =.05, Meg-01; P=.05, M07e; P=.04, Dami). PBMCs show 10× more Foxp3 protein compared with primary human megakaryocytes. (D) Two × 104 megakaryoblastic cells were stained for Foxp3 using an intracellular staining protocol, cytospun onto slides and cover-slipped. Foxp3 expression was analyzed by fluorescence microscopy and photographs were taken of individual cells at 400× magnification. Bar in first picture represents 10 μm.
To determine whether the expression of Foxp3 mRNA resulted in the production of Foxp3 protein, human megakaryocytes were examined by flow cytometry using intracellular staining and by western blotting using antibodies with different specificities for Foxp3. Flow cytometric analysis using the PCH101 antibody clone, which binds the C-terminus of the transcription factor, showed that all 3 megakaryoblastic cell lines and primary human megakaryocytes expressed intracellular Foxp3 protein (Fig. 1B). Foxp3 was not detected in human blood platelets. These results were confirmed using the 236A/E7 antibody clone, which binds to an internal portion of Foxp3 (data not shown). These data reveal that megakaryoblastic cell lines have about 2–4 times as much Foxp3 protein as primary human megakaryocytes.
To support the flow cytometric data, western blotting was performed using the hFoxy antibody clone. Human Foxp3 transfected cell lysate and human PBMC lysate were used as positive controls. Figure 1B demonstrates that megakaryocytes express Foxp3 protein, yet platelets, themselves, lack detectable Foxp3 protein. Western blotting also confirmed that 2–4 times more Foxp3 protein was detected in Meg-01, M07e, and Dami cells compared to primary human megakaryocytes (Fig. 1C).
Next, immunofluorescent staining using the PCH101 antibody was used to determine the subcellular localization of Foxp3. Figure 1D, shows the presence of Foxp3 protein in 3 different megakaryoblastic cell lines and in primary human megakaryocytes, where it was predominantly detected in the nuclei (Fig. 1D).
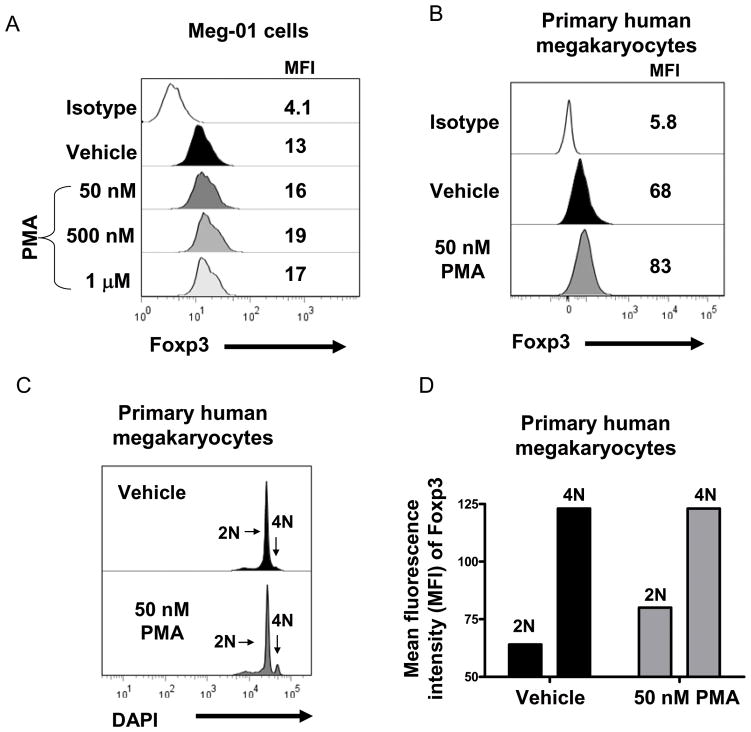
2. Foxp3 protein expression is enhanced with phorbol ester myristate acetate (PMA)-induced megakaryocyte maturation
Meg-01 cells and primary human megakaryocytes were treated with the megakaryocyte differentiation agent, phorbol ester myristate acetate (PMA; 50 nM-1 μM), for 24 hours. Figure 2A demonstrates that PMA dose-dependently increased Foxp3 expression in Meg-01 cells. The optimal dose for Meg-01 cells was 500 nM PMA, increasing the mean fluorescence intensity from 13 to 19 (Fig. 2A). PMA (50 nM) induced Foxp3 expression in primary human megakaryocytes (Fig. 2B) and increased megakaryocyte DNA content (Fig 2C). Cells with a DNA content of 4N had a higher expression of Foxp3 protein compared with cells with a DNA content of 2N (Fig 2D), which correlates with the data describing nuclear localization of Foxp3 in megakaryocytes. However, the PMA-treated 2N primary human megakaryocytes cells had higher Foxp3 expression compared with vehicle-treated 2N megakaryocytes (MFI PMA (50 nM): 83 vs. MFI vehicle: 60). These results show that Foxp3 is induced during megakaryocyte maturation.
Figure 2. Phorbol ester myristate acetate (PMA) upregulates Foxp3 protein expression.
A) Meg-01 cells were treated with vehicle or 50 nM, 500 nM or 1 μM PMA for 24 hours and Foxp3 protein expression was analyzed by flow cytometry. The MFI is shown and data are representative of 3 separate experiments. B) Primary human megakarycytes were treated with PMA (50 nM) for 24 hours and Foxp3 protein expression was analyzed by flow cytometry. C) Primary human megakarycytes were treated with PMA (50 nM) for 24 hours and DNA content was analyzed by flow cytometry. D) Bar graph demonstrates that 2N megakaryocytes have less Foxp3 expression that 4N megakaryocytes and that 2N primary human megakaryocytes treated with PMA (50 nM) have a higher MFI compared with 2N primary human megakaryocytes treated with vehicle.
3. Characterization of megakaryocytes in Foxp3sf mice
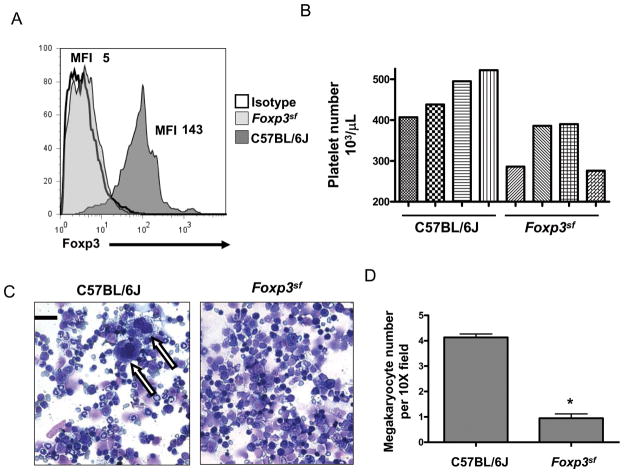
In support of our human megakaryocyte data, mouse bone marrow megakaryocytes were also found to express Foxp3 protein (Fig. 3A). To determine the functional significance of megakaryocytic Foxp3, Foxp3sf mice were studied. To assess the influence of mutant Foxp3 on platelet levels, platelet counts were performed on 4 individual Foxp3sf and C57BL/6J mice. Wildtype, C57BL/6J, healthy siblings of the Foxp3sf mice had platelet counts similar to the C57BL/6J mice (data not shown). Foxp3sf mice have up to 53% fewer platelets compared to C57BL/6J mice (Fig. 3B) and we newly report that Foxp3sf mice have ~4-fold less mature bone marrow megakaryocytes than normal mice (Fig. 3C-D).
Figure 3. Foxp3sf mice have fewer megakaryocytes and platelets.
(A) Bone marrow was harvested and cultured for 4 days in the presence of rhTPO. Foxp3 protein expression was analyzed in CD41-expressing cells. Wildtype megakaryocytes expressed Foxp3 protein whereas Foxp3sf megakaryocytes lacked Foxp3 protein. (B) Platelet number was measured in Foxp3sf mice aged 24 days. Foxp3sf mice are thrombocytopenic. Bar graph demonstrates platelet counts from 4 C57BL/6J mice and 4 Foxp3sf mice. (C) Bone marrow was harvested from the femora of mice. Two × 104 cells were cytospun onto glass slides and stained with a Diff-Quik stain set. Microscopy demonstrates megakaryocytes (indicated by arrows) can be observed in a single 40× field in C57BL/6J mice and cannot be observed in Foxp3sf mice. Megakaryocyte morphology appears normal. Bar in first picture represents 30 μm. (D) Quantitation of bone marrow megakaryocytes in C57BL/6J mice and Foxp3sf mice. Data are presented as mean ± SD *(P=.01)
4. Characterization of megakaryocyte progenitors in Foxp3sf mice
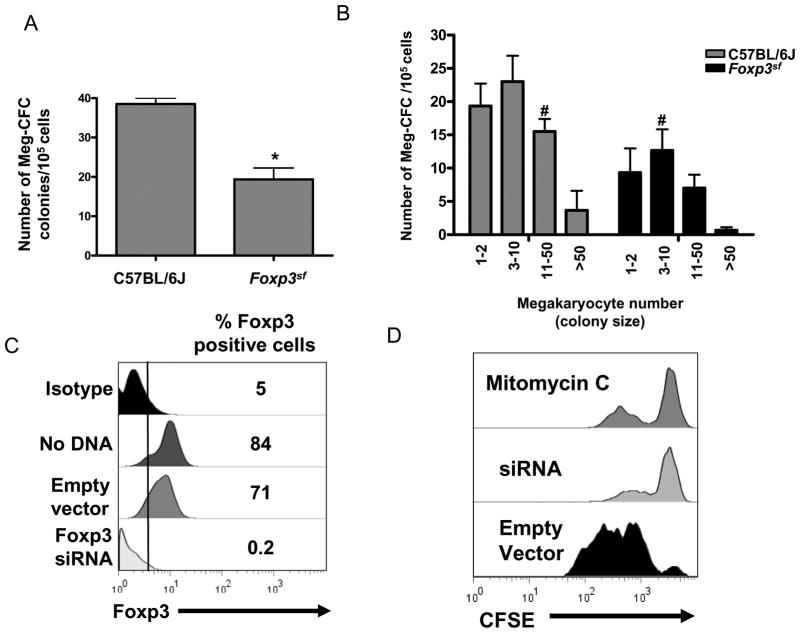
To determine whether this reduction in megakaryocyte number was due to a reduction in megakaryocyte progenitors, we next performed a Meg-CFC colony assay. Meg-CFC-derived colonies were identified by labeling with anti-GPIbβ antibodies. Figure 4A demonstrates that Foxp3sf mice have an ~50% reduction in megakaryocyte progenitors compared to the C57BL/6J mice. In addition, mean colony size was lower in Foxp3sf mice, exhibiting significantly fewer colonies in the 3–50 cell count range, compared to the C57BL/6J mice (Fig. 4B).
Figure 4. Lack of Foxp3 influences megakaryocyte proliferation.
(A) Bone marrow was harvested and cultured in the presence of rhTPO, IL-3, IL-6, and IL-11 for 7 days. Bar graph demonstrates that Foxp3sf bone marrow had significantly fewer Meg-CFCs than C57BL/6J bone marrow. Meg-CFCs were defined by their ability to generate colonies containing at least 3 megakaryocytes. Results are presented as mean ± SD *(P=.04). (B) Bar graph demonstrates that Foxp3sf colonies had a lower mean colony size compared to C57BL/6J colonies (mean is 12 colonies for C57BL/6J mice and 9 colonies for Foxp3sf ). Mean colony size is indicated by #. (C) Meg-01 cells were nucleofected® with a Foxp3 shRNA plasmid. After 24 hours Foxp3 expression was analyzed by flow cytometry. Histogram shows the percentage of Foxp3 positive cells. (D) CFSE dye was added to Meg-01 cells 4 hours post-nucleofection® and cell proliferation was analyzed by flow cytometry after 72 hours. Histogram shows cell division in Meg-01 cells treated with mytomycin C or expressing the empty vector plasmid or the Foxp3 shRNA plasmid. Foxp3 knock down Meg-01 cells failed to divide.
5. Meg-01 cells lacking Foxp3 protein have reduced proliferation
Figure 4C demonstrates that nucleofecting® Meg-01 cells with a pLKO.1 lentiviral vector expressing human shRNA Foxp3 plasmid, reduced Foxp3 protein to undetectable levels by 24 hours. This knock down persisted through 72 hours (data not shown). Four hours after nucleofection®, cells were washed and labeled with CFSE to measure the proliferative response of the Foxp3 knock down. CFSE passively diffuses into cells and forms fluorescent conjugates 26. Labeled cells are retained during meiosis and the label is inherited by the daughter cells during cell division 27. Fluorescence was analyzed after 72 hours by flow cytometry. Figure 4D demonstrates that the Meg-01 cells expressing the Foxp3 shRNA plasmid failed to proliferate within the 72 hour period. Histogram peaks of the Foxp3 knock down cells and the mitomycin C treated cells were identical. In contrast, Meg-01 cells expressing the empty vector plasmid underwent one or two cell divisions by 72 hours.
6. Characterization of platelets in Foxp3sf mice
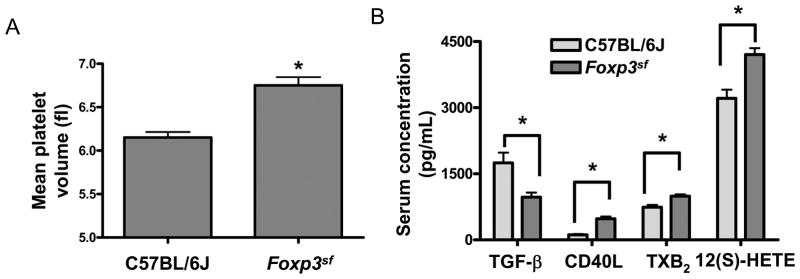
Impaired megakaryopoiesis can lead platelets abnormal in size and function, so we hypothesized that Foxp3sf mice would have platelet dysfunction in addition to thrombocytopenia. Platelet size may reflect altered function 28 and figure 5A demonstrates that mean platelet volumes were significantly increased in Foxp3sf mice (n=4). Each group contained the same 4 animals that had platelet counts depicted in figure 3B. As another measure of platelet function, we next measured levels of key hemostatic mediators released upon platelet activation: transforming growth factor beta (TGF-β), thromboxane B2 (TXB2), CD40 ligand (CD40L) and 12- hydroperoxyeicosatetraenoic acid 12(S)-HETE. Whereas we found no differences in plasma levels of TGF-β, TXB2, CD40L, and 12(S)-HETE (data not shown), Foxp3sf mice (n=6) demonstrated reduced serum levels of TGF-β and elevated serum levels of CD40L, TXB2, and 12(S)-HETE compared to C57BL/6J mice (n=6) (Fig. 5B). These data suggest altered platelet release.
Figure 5. Platelet abnormalities in Foxp3sf mice.
(A) Foxp3sf mice had significantly increased mean platelet volumes compared to C57BL/6J mice. Data are presented as mean ± SD *(P=.01). (B) Foxp3sf mice had significantly reduced serum levels of TGF-β *(P=.03) and significantly elevated serum levels of CD40L *(P=.01), TXB2 *(P=.05), and 12(S)-HETE *(P=.05) compared to C57BL/6J mice.
7. Characterization of platelets in IPEX
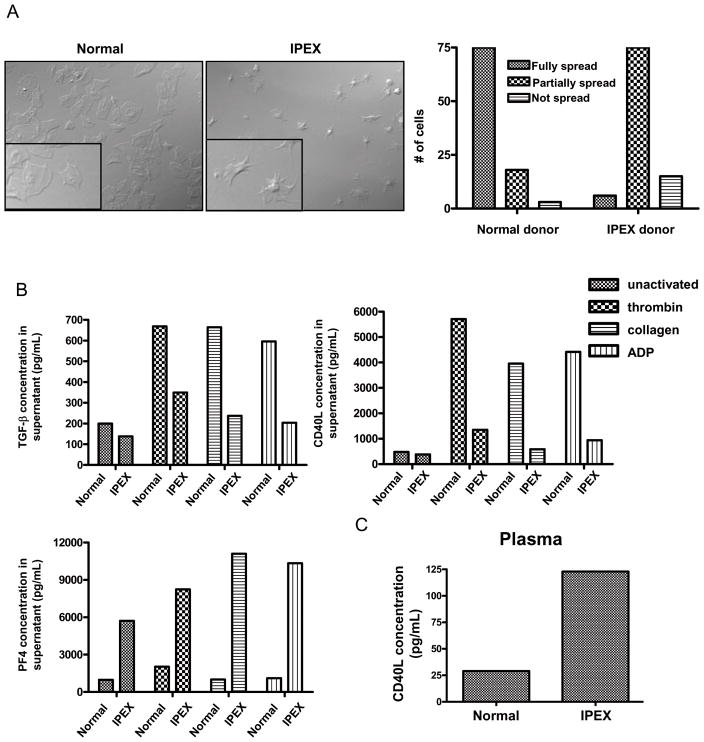
IPEX is a very rare disease involving various Foxp3 mutations 29. Platelet function has not been studied in these patients although thrombocytopenias are reported in some cases. We obtained blood from a 17 year old male with IPEX syndrome. He had a platelet count of 434,000/μL of blood and a mean platelet volume of 10.4 fL, both at the upper end of the normal range. In addition, a subset of his platelets were unusually large (i.e. 32 fL). Strikingly, the IPEX platelets exhibited prominent defects in spreading on fibrinogen (Fig. 6A) and collagen-coated slides (data not shown). Sixty percent of platelets derived from a normal donor fully spread, whereas only 5% of IPEX platelets fully spread.
Figure 6. Platelet abnormalities in IPEX.
(A) Left picture shows microscopy of normal platelets spread on a fibrinogen coated slide. Right picture shows microscopy of platelets derived from an IPEX patient spread on a fibrinogen coated slide. Bar graph demonstrates the number of platelets out of a total 125 platelets that were either fully spread, partially spread, or not spread. Platelets were counted from several 100X fields. (B) IPEX donor insufficiently released CD40L and TGF-β in response to thrombin, collagen, or ADP. IPEX donor exhibited higher levels (6×) of PF4 in the supernatants from both unactivated and activated platelets. (C) IPEX donor exhibited 3X higher plasma levels of CD40L when compared to an age-matched male control.
IPEX platelets failed to release both TGF-β and CD40L after activation with either thrombin, collagen, or ADP (Fig. 6B), while they exhibited no defect in thromboxane B2 (TXB2) release (data not shown). Interestingly, IPEX unstimulated platelets had approximately 6 times more platelet factor 4 (PF4) in the supernatant compared with normal unstimulated platelets. These levels were approximately doubled with activation in both IPEX and normal platelet supernatants (Fig. 6B). No differences in serum TGF-β and CD40L levels were observed between the IPEX patient and an age-matched control suggesting that multiple platelet agonists in conjunction could promote the release of TGF-β and CD40L (data not shown). However, the IPEX patient had plasma CD40L levels 3 times higher than the control (Fig. 6C).
Discussion
The specificity and restriction of Foxp3 expression to a subset of T lymphocytes has provoked controversy in the scientific literature 30–33. However, more recent studies indicate that Foxp3 is expressed in some epithelial cells and some tumor cells 24, 34. The abnormal platelet levels observed and the hemorrhaging that occurs in IPEX syndrome and in Foxp3sf mice prompted us to examine Foxp3 expression in the megakaryocyte lineage and determine if it plays a role in megakaryopoiesis and platelet production. Herein, we demonstrate that primary human megakaryocytes and megakaryoblastic cell lines express Foxp3 mRNA and protein, although to a lesser extent than in Treg cells. Consistent with its putative role as a transcription factor, Foxp3 protein expression in megakaryocytes was predominantly nuclear which may account for the failure to detect Foxp3 in human platelets (Fig. 1C). Nuclear localization of Foxp3 may also explain increased levels of Foxp3 protein expression following PMA treatment (Fig. 2), as PMA increases cellular DNA content in megakaryocytes by inducing endomitosis 35. We also demonstrated that Foxp3 protein expression was greater in megakaryoblastic cell lines compared to primary human megakaryocytes (Fig 1B–C). However, the reason for, and the consequences of this increased expression are unknown. It has been speculated that Foxp3 plays an intrinsic role in malignant transformation and tumor survival 24, 36. Interestingly, the Meg-01 cell line and the Dami cell line were derived from patients with megakaryoblastic leukemias that presented elevated bone marrow blast cells and thrombocytosis 10, 37. Future studies will examine the importance of Foxp3 expression in megakaryoblastic cell lines.
Our findings indicate that Foxp3 plays an important role in megakaryopoiesis. Foxp3sf mice, which lack the full length, functional Foxp3 protein, had 4-fold fewer bone marrow megakaryocytes compared to C57BL/6J mice (Fig. 3D). Further, megakaryocyte colony number was ~ 50% lower in Foxp3sf mice compared with C57BL/6J mice (Fig. 4A). In addition, megakaryocyte mean colony size was lower in Foxp3sf mice compared with that in C57BL/6J suggesting that a reduction in progenitor proliferation contributes to reduced progenitor number (Fig. 4B). These new findings demonstrate that the reduced colony number in Foxp3sf mice is due to a defect in megakaryocyte progenitor proliferation and could also be due to a potential role of Foxp3 in the generation of megakaryocyte progenitors from upstream bipotential or multipotential progenitors. We also observed that platelet count does not necessarily correlate with megakaryocyte number or megakaryocyte progenitor number. The platelet counts from individual mice exhibited more variability which may indicate differences in the ability of mature megakaryocytes to produce platelets. These data however, do not rule out the possibility that the reduced megakaryocyte number is a consequence of profound defects in T regulatory cells. Megakaryopoiesis may be impaired in vivo due to the wide range of autoimmune-associated symptoms which characterize the Foxp3sf mouse. However, Foxp3 knock-down human Meg-01 cells demonstrate a greatly reduced proliferative response, suggesting a direct role for Foxp3 in megakaryopoiesis (Fig. 4D).
The precise mechanism by which Foxp3 regulates megakaryopoiesis remains unknown. Foxp3 functions in T lymphocytes, in part, as a transcriptional repressor by recruiting both histone acetyl transferases and histone deacetylases 38. Foxp3 also functions as a passive transcriptional repressor by physically interacting with proteins such as nuclear factor-kappa B (NF-κB) 39, 40 and acute myeloid leukemia 1 (Aml1)/runt related transcription factor 1 (Runx1) 41. Foxp3 may be playing a similar role in megakaryocytes by suppressing or activating transcription factors.
Genetic lesions in megakaryocytes that cause thrombocytopenia often cause abnormal platelet function. Foxp3sf mice have reduced platelet counts and our new findings demonstrate that they exhibit striking activation abnormalities. TGF-β serum levels were significantly lower in Foxp3sf mice. Since platelet-derived TGF-β is a cytokine mainly involved in wound healing and tissue repair, we speculate that reduced TGF-β could potentiate the dermatitis and the skin lesions which characterize the IPEX disease and the Foxp3sf mice. In addition, TGF-β can suppress T cell responses. TGF-β reduces T cell proliferation by inhibiting IL-2 production and upregulating cell cycle inhibitors 42 and inhibits the differentiation of Th1 to Th2 by down-regulating T-bet and GATA-3 43. TGF-β also inhibits the activation of macrophages and reduces the ability of dendritic cells to present antigens to T cells 42. Interestingly, we found that Foxp3sf serum had elevated levels of CD40L and two arachidonic acid metabolites, TXB2 and 12(S)- HETE, despite having fewer platelets. TXB2 is a more stable metabolite of thromboxane A2 (TXA2), a cyclooxygenase-derived product generated by platelets which induces irreversible platelet aggregation and vascular smooth muscle contraction. 12(S)-HETE is a 12-lipoxygenase-derived product that is produced abundantly in platelets during activation. These data suggest that the Foxp3sf platelets produce more arachidonic acid metabolites during activation. As described in the Introduction, CD40L activates immune and structural cells, as well as platelets. The majority of the circulating soluble CD40L originates from platelets and CD40L levels are elevated during inflammatory disease states 44–46. Collectively, these new data suggest that the platelet phenotype in Foxp3sf mice contributes to the inflammation observed in the ‘scurfy’ disease.
Foxp3sf mice also had increased mean platelet volumes (Fig. 5A). There are many intrinsic and reactive reasons why platelet volume is elevated in disease states. For example, Gata-1 knock out mice have deficiencies in megakaryocyte maturation and as a result, their platelets have elevated volumes 47. The peripheral platelet destruction from circulating anti-platelet antibodies increases platelet volume in immune thrombocytopenic purpura (ITP) patients because a higher percentage of platelets are younger 48, 49. Therefore, the increased platelet size observed in Foxp3sf mice may indicate both impaired platelet production and peripheral platelet destruction or in contrast that the elevated mean platelet volume may be compensating for the decrease in platelet number.
To determine if a similar platelet phenotype was observed in IPEX, the human correlate of scurfy, we examined the platelets of an IPEX patient. IPEX is rare disease involving various Foxp3 mutations and can result in death at an early age 29. About 50% of IPEX patients are reported to be thrombocytopenic and hemorrhage is one of the most common causes of death in untreated patients 9. Gastrointestinal bleeding has occurred in a case with normal platelet counts, suggesting inadequate platelet function 50. The IPEX donor evaluated herein was aged 17 years and had a G to A transition (1150G>A) in exon 11, resulting in a substitution of Ala to Thr at residue 384, within the DNA binding domain of Foxp3 20. His platelets demonstrated striking abnormalities in spreading (Fig. 6A) and a reduced ability to release CD40L and TGF-β in response to potent platelet activators (Fig. 6B). Our IPEX donor also demonstrated a profound elevation in plasma levels of CD40L (Fig. 6C). These data indicate that the IPEX patient did not respond normally to platelet activators and possibly that his platelets already released internal stores of CD40L in vivo.
We also demonstrated that the IPEX patient had 6 times more platelet factor 4 (PF4) in the supernatants of both unactivated and activated platelets compared with the normal donor. This suggests that the IPEX platelets contain higher levels of PF4 or that the release of PF4 from alpha granules is enhanced. Since IPEX platelets release less TGF-β and CD40L than the normal platelets, we speculate that IPEX platelets contain higher levels of PF4. The increased release of PF4 may implicate platelets in the symptomology of IPEX. IPEX disease is characterized by severe atopic dermatitis and recently, plasma levels of PF4 were shown to be elevated in patients with atopic dermatitis and in a mouse model of atopic dermatitits 51–53. In addition, PF4 is a negative regulator of megakaryopoiesis suggesting that elevated PF4 may be a mechansism for inhibiting megakaryocyte proliferation 54. Collectively, these new findings demonstrate that the defect in Foxp3 observed in IPEX influences platelet function.
Our study adds considerable new information to the ongoing discussion of the presence of Foxp3 in cell types other than Tregs. Overall, we have shown that Foxp3 deficiency results in a lesion of megakaryocyte proliferation that is associated with platelet dysfunction. These new findings support the concept that genetic disorders that cause thrombocytopenia also cause abnormal platelet function such as occurs in myelodysplasias. Therefore, we have elucidated an underlying mechanism of megakaryopoiesis that contributes to the pathophysiology of IPEX syndrome and the ‘scurfy’ disease.
Acknowledgments
This work was supported by NIH Grants T32ES07026, DE011390, ES01247, HL078603, HL086367, EY017123, DK09361, an EPA STAR grant RD832415 and the PhRMA Foundation.
We gratefully acknowledge the support of Dr. Lisa Beck, and Steve Pollock for assistance with donor recruitment.
Footnotes
J.J. Bernard designed and performed the research, analyzed the data and wrote the paper. K.E. Seweryniak performed research. A.D. Koniski performed research. N. Blumberg, C.W. Francis, M.B. Taubman, S.S. Spinelli and J. Palis analyzed data and edited the manuscript. R.P. Phipps designed research, analyzed data and edited the manuscript. All the authors disclose no conflict of interest.
All the authors disclose no conflict of interest.
References
- 1.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 2.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 3.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 5.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura M, Morishima Y, Ohno R, Kato Y, Hirabayashi N, Nagura H, Saito H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985;66:1384–1392. [PubMed] [Google Scholar]
- 11.Avanzi GC, Brizzi MF, Giannotti J, Ciarletta A, Yang YC, Pegoraro L, Clark SC. M-07e human leukemic factor-dependent cell line provides a rapid and sensitive bioassay for the human cytokines GM-CSF and IL-3. J Cell Physiol. 1990;145:458–464. doi: 10.1002/jcp.1041450310. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien JJ, Spinelli SL, Tober J, Blumberg N, Francis CW, Taubman MB, Palis J, Seweryniak KE, Gertz JM, Phipps RP. 15-deoxy-{Delta}12,14-PGJ2 enhances platelet production from megakaryocytes. Blood. 2008 doi: 10.1182/blood-2008-05-158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baglole CJ, Sime PJ, Phipps RP. Cigarette smoke-induced expression of heme oxygenase-1 in human lung fibroblasts is regulated by intracellular glutathione. Am J Physiol Lung Cell Mol Physiol. 2008;295:L624–636. doi: 10.1152/ajplung.90215.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25- T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien JJ, Baglole CJ, Garcia-Bates TM, Blumberg N, Francis CW, Phipps RP. 15-deoxy-Delta12,14 prostaglandin J2-induced heme oxygenase-1 in megakaryocytes regulates thrombopoiesis. J Thromb Haemost. 2009;7:182–189. doi: 10.1111/j.1538-7836.2008.03191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Means GD, Toy DY, Baum PR, Derry JM. A transcript map of a 2-Mb BAC contig in the proximal portion of the mouse X chromosome and regional mapping of the scurfy mutation. Genomics. 2000;65:213–223. doi: 10.1006/geno.2000.6173. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 18.Russell WL, Russell LB, Gower JS. Exceptional Inheritance Of A Sex-Linked Gene In The Mouse Explained On The Basis That The X/O Sex-Chromosome Constitution Is Female. Proc Natl Acad Sci U S A. 1959;45:554–560. doi: 10.1073/pnas.45.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien JJ, Spinelli SL, Joanna T, Blumberg N, Francis CW, Taubman MB, Palis J, Seweryniak KE, Gertz JM, Phipps RP. 15-deoxy-delta 12,14 -PGJ2 enhances platelet production from megakaryocytes. Blood. 2008 doi: 10.1182/blood-2008-05-158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieves DS, Phipps RP, Pollock SJ, Ochs HD, Zhu Q, Scott GA, Ryan CK, Kobayashi I, Rossi TM, Goldsmith LA. Dermatologic and immunologic findings in the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Arch Dermatol. 2004;140:466–472. doi: 10.1001/archderm.140.4.466. [DOI] [PubMed] [Google Scholar]
- 21.Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 22.Ray DM, Spinelli SL, Pollock SJ, Murant TI, O’Brien JJ, Blumberg N, Francis CW, Taubman MB, Phipps RP. Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 24.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronner-Fraser M. Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J Cell Biol. 1985;101:610–617. doi: 10.1083/jcb.101.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med. 1993;13:937–950. [PubMed] [Google Scholar]
- 29.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–538. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Chang X, Gao JX, Jiang Q, Wen J, Seifers N, Su L, Godfrey VL, Zuo T, Zheng P, Liu Y. The Scurfy mutation of FoxP3 in the thymus stroma leads to defective thymopoiesis. J Exp Med. 2005;202:1141–1151. doi: 10.1084/jem.20050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liston A, Farr AG, Chen Z, Benoist C, Mathis D, Manley NR, Rudensky AY. Lack of Foxp3 function and expression in the thymic epithelium. J Exp Med. 2007;204:475–480. doi: 10.1084/jem.20062465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Chen GY, Chen C, Wang L, Chang X, Zheng P, Liu Y. Cutting edge: Broad expression of the FoxP3 locus in epithelial cells: a caution against early interpretation of fatal inflammatory diseases following in vivo depletion of FoxP3-expressing cells. J Immunol. 2008;180:5163–5166. doi: 10.4049/jimmunol.180.8.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth BJ, Sledge GW, Jr, Straneva JE, Brandt J, Goheen M, Hoffman R. Analysis of phorbol ester stimulated human megakaryocyte development. Blood. 1988;72:202–207. [PubMed] [Google Scholar]
- 36.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, Grutzmann R, Pilarsky C, Ungefroren H, Saeger HD, Kloppel G, Kabelitz D, Kalthoff H. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–8350. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg SM, Rosenthal DS, Greeley TA, Tantravahi R, Handin RI. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988;72:1968–1977. [PubMed] [Google Scholar]
- 38.Li B, Greene MI. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle. 2007;6:1432–1436. [PubMed] [Google Scholar]
- 39.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc Natl Acad Sci U S A. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon HK, So JS, Lee CG, Sahoo A, Yi HJ, Park JN, Lim SY, Hwang KC, Jun CD, Chun JS, Im SH. Foxp3 induces IL-4 gene silencing by affecting nuclear translocation of NFkappaB and chromatin structure. Mol Immunol. 2008;45:3205–3212. doi: 10.1016/j.molimm.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 42.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 43.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 45.Freedman JE. CD40 ligand--assessing risk instead of damage? N Engl J Med. 2003;348:1163–1165. doi: 10.1056/NEJMe030012. [DOI] [PubMed] [Google Scholar]
- 46.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 47.Orkin SH, Shivdasani RA, Fujiwara Y, McDevitt MA. Transcription factor GATA-1 in megakaryocyte development. Stem Cells. 1998;16 (Suppl 2):79–83. doi: 10.1002/stem.5530160710. [DOI] [PubMed] [Google Scholar]
- 48.Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Opin Hematol. 2007;14:511–514. doi: 10.1097/MOH.0b013e3282ba5552. [DOI] [PubMed] [Google Scholar]
- 49.Grossi A, Vannucchi AM, Casprini P, Guidi S, Rafanelli D, Pecchioli MG, Rossi Ferrini P. Different patterns of platelet turnover in chronic idiopathic thrombocytopenic purpura. Scand J Haematol. 1983;31:206–214. doi: 10.1111/j.1600-0609.1983.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 50.Lucas KG, Ungar D, Comito M, Bayerl M, Groh B. Submyeloablative cord blood transplantation corrects clinical defects seen in IPEX syndrome. Bone Marrow Transplant. 2007;39:55–56. doi: 10.1038/sj.bmt.1705542. [DOI] [PubMed] [Google Scholar]
- 51.Kasperska-Zaj CA, Nowakowski M, Rogala B. Enhanced platelet activation in patients with atopic eczema/dermatitis syndrome. Inflammation. 2004;28:299–302. doi: 10.1007/s10753-004-6054-z. [DOI] [PubMed] [Google Scholar]
- 52.Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int. 2008;57:391–396. doi: 10.2332/allergolint.O-08-537. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe O, Natori K, Tamari M, Shiomoto Y, Kubo S, Nakamura Y. Significantly elevated expression of PF4 (platelet factor 4) and eotaxin in the NOA mouse, a model for atopic dermatitis. J Hum Genet. 1999;44:173–176. doi: 10.1007/s100380050136. [DOI] [PubMed] [Google Scholar]
- 54.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110:1153–1160. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]