Abstract
New and effective treatment strategies are desperately needed for malignant mesothelioma (MM), an aggressive cancer with a poor prognosis. We have shown previously that acid-prepared mesoporous microspheres (APMS) are nontoxic after intrapleural or intraperitoneal (IP) administration to rodents. The purpose here was to evaluate the utility of APMS in delivering chemotherapeutic drugs to human MM cells in vitro and in two mouse xenograft models of MM. Uptake and release of doxorubicin (DOX) alone or loaded in APMS (APMS-DOX) were evaluated in MM cells. MM cell death and gene expression linked to DNA damage/repair were also measured in vitro. In two SCID mouse xenograft models, mice received saline, APMS, DOX, or APMS-DOX injected directly into subcutaneous (SC) MM tumors or injected IP after development of human MMs peritoneally. Other mice received DOX intravenously (IV) via tail vein injections. In comparison to DOX alone, APMS-DOX enhanced intracellular uptake of DOX, MM death, and expression of GADD34 and TP73. In the SC MM model, 3X weekly SC injections of APMS-DOX or DOX alone significantly inhibited tumor volumes, and systemic DOX administration was lethal. In mice developing IP MMs, significant (p<0.05) inhibition of mesenteric tumor numbers, weight, and volume was achieved using IP administration of APMS-DOX at one-third the DOX concentration required after IP injections of DOX alone. These results suggest APMS are efficacious for the localized delivery of lower effective DOX concentrations in MM, and represent a novel means of treating intracavitary tumors.
Keywords: Microparticles, Mesoporous silica, Mesothelioma, Doxorubicin, Intracavitary tumors
Novelty.
Nanomaterials are being advocated for the delivery of cancer chemotherapeutics, but their systemic distribution to other organs including the brain has raised questions regarding their safety. Malignant mesothelioma (MM) is an aggressive cancer with a poor prognosis despite numerous treatment strategies. To overcome these limitations, we developed nontoxic, micron-sized, acid-prepared mesoporous spheres (APMS) to allow administration of drugs or constructs to intracavitary tumors such as MM. Specifically, we suggest the use of APMS to enhance the local delivery and effectiveness of cancer chemotherapeutics and to avoid systemic toxicity.
Impact.
APMS have been engineered in a size range (1–2 μm diameter) that favors tumor cell uptake, yet prohibits entry into the systemic circulation. Studies here show that local administration of APMS preloaded with doxorubicin (DOX) increase sustained delivery of DOX, and inhibit growth of human MM tumors in vitro and in immunocompromised SCID mice without evidence of systemic toxicity. In addition to increasing delivery of chemotherapeutics, APMS can be tagged with targeting moieties such as antibodies and modified to introduce DNA/siRNA constructs, thus facilitating multi-targeted therapeutic approaches.
Introduction
New treatment strategies are desperately needed for the intracavitary tumor, malignant mesothelioma (MM), which typically originates from mesothelial cells of the pleura, peritoneum or pericardium. Current therapies for MM include systemic chemotherapy, gene therapy, surgery, radiation, or palliative procedures.1 The effectiveness of intracavitary chemotherapy is also a topic of intense examination,2, 3 including the administration of intracavitary hyperthermic chemotherapy.4, 5 Single modality therapies have little effect on patient survival, while multiple modality therapies are only slightly more effective.6–11 For example, doxorubicin (DOX), has been utilized in the treatment of a variety of cancers, including MM and breast cancer, but can cause cardiac and systemic toxicity.12 The ineffectiveness of systemic chemotherapy in treating MM may be due to inefficient delivery and uptake of chemotherapeutics. Thus, to increase cell uptake of DOX and to circumvent its systemic toxicity, we explored the use of a novel system to deliver DOX directly to MM cells via local administration.
Vehicles for loading and delivery of chemotherapeutic drugs or molecular constructs have been featured recently in the cancer literature.13–15 Although nanomaterials have been advocated for drug delivery to some tumor types, their limitations include penetration and consequent dysfunction of cellular organelles, and the possibility of systemic toxicity due to entrance into the blood stream and transport to other organs.16, 17 To overcome these limitations and to avoid systemic dissemination, we developed nontoxic, micron-sized particles to allow their injection directly into established tumors, sites of surgical resection of tumors, and peritoneal or thoracic cavities.
Acid-prepared mesoporous spheres (APMS) are porous, amorphous silica microspheres (1–2 μm in diameter) with a pore diameter that is tunable between 40 and 100 Å to allow loading of drugs, functional plasmids,18 or DNA/siRNA constructs.19, 20 In contrast to crystalline silicas, amorphous silicas are less durable and are nontoxic.21 We have shown recently that APMS functionalized with tetraethylene glycol (TEG) are nontoxic in vitro or after intranasal instillation or intrapleural injection into mice, and that this functionalization promotes phagocytosis and uptake by murine lung epithelial and human MM cells in vitro.18 In studies here, we hypothesized that using APMS at sites of MM tumor development might be advantageous as a novel form of localized chemotherapy delivery to avoid systemic toxicity.
We first demonstrated that APMS-TEG loaded with DOX in comparison to DOX alone led to significantly (p<0.05) increased delivery and drug uptake by MMs, increased MM cell death in vitro, and enhanced expression of genes linked to DNA damage and death (cell cycle arrest and apoptosis) pathways. Next, we performed in vivo studies with fluorescently labeled APMS-TEG to confirm particle uptake by subcutaneous (SC) MMs growing in severe combined immunodeficient (SCID) mice, as well as by pleural cells and cells in peritoneal lavage fluid (PLF) after injection of APMS-TEG into the pleural or intraperitoneal (IP) cavities. In the SC human MM xenograft model, we found that localized injection of APMS-TEG loaded with microgram quantities of DOX was more effective in inhibition of SC tumor growth and nontoxic to mice when compared to systemic DOX administration. Lastly, we utilized an IP human MM xenograft model to demonstrate that 3-fold lower concentrations of APMS-TEG-loaded DOX (APMS-DOX) are equally effective in inhibiting IP tumor growth as higher concentrations of DOX alone. Our results suggest that APMS are an effective approach for the localized delivery of chemotherapeutic drugs typically associated with systemic toxicity.
Materials and Methods
Cells and reagents
Human pleural MM cell lines isolated from surgical resection of MMs or at autopsy were kind gifts from Drs. Luciano Mutti (Maugeri Foundation, Pavia, Italy; MO), Maurizio Bocchetta (Loyola University, Mayfair, IL; ME-26) and Harvey Pass (NYU School of Medicine, New York, NY; PPM Mill, PPM Rob, PPM Ada, PPM Gar, PPM Gates, PPM Gord). All isolates were confirmed as MM cells by immunohistochemistry using an antibody to calretinin and verified for lack of mycoplasma contamination using a polymerase chain reaction (PCR) assay prior to use in studies described here. Hmeso cells, originally designated H-MESO-1, were initially isolated by Reale et al,22 and supplied by Drs. Joseph Testa and Deborah Altomare (Fox Chase Cancer Institute, Philadelphia, PA). Cell culture procedures were as described.18
Synthesis of APMS-TEG and preloading with doxorubicin (DOX)
Synthesis of APMS and surface modification with TEG have been described previously.18 Hereafter, APMS-TEG will be referred to simply as APMS. APMS were loaded with DOX via incubation with a saturated aqueous solution of DOX-HCl (Sigma, St. Louis, MO) for 16 h, and then isolated with centrifuge membrane filters (Nanosep MF 0.45 μm, Pall Corporation, Ann Arbor, MI) and dried under vacuum at room temperature in the dark.
Treatment of MM cells with APMS, APMS-DOX, DOX and cisplatin
For initial studies examining differences in DOX delivery via HPLC, the sarcomatoid MM cell line (MO) was plated in complete medium at 37°C. When cells reached 70–80% confluency, the complete medium was aspirated and replaced with maintenance medium (containing 0.5% FBS) for 24 h. APMS were then suspended in maintenance medium at a concentration of approximately 6×107 APMS/100 μl, and sonicated 5X for 2 sec to achieve an even suspension. Fifty μl were then added to cells at a final concentration of 7.5×106 APMS/cm2 surface area dish (~185 APMS/cell). DOX (160 nM) was added directly to maintenance medium and APMS-DOX (160 nM DOX equivalent) was prepared and administered as described above. Cell killing effects of DOX alone, APMS alone, DOX added simultaneously with APMS, and APMS loaded with DOX (APMS-DOX) were assessed using both the MO MM line and an epithelioid MM line (ME26) cultured and treated as described above. Finally, 7 MM cell lines (Hmeso, PPM Mill, PPM Rob, PPM Ada, PPM Gar, PPM Gates, and PPM Gord) were evaluated in dose-response studies to determine their relative sensitivity to DOX and cisplatin. Cells were grown as described above to near confluency, and DOX (0–25 μM) and cisplatin (0–75 μM) were added directly to maintenance medium.
Determination of extracellular and intracellular DOX concentration
After incubation of MO cells with either DOX (160 nM) in medium or APMS-DOX (160 nM DOX equivalent), medium was centrifuged at 10,000 × g at 4°C. The supernatant was then transferred to fresh vials, Daunorubicin (a fluorescent internal standard) added (final concentration of 1 μM), and the vial vortexed prior to incubation at 37°C for 15 min. Proteins were precipitated by adding 250 μl of acetone and 50 μl of aqueous ZnSO4 solution (400 mg/ml) to the sample and vortexing prior to incubation at 37°C for 15 min. Samples were subsequently centrifuged at 20,000 × g (10 min) at 4°C, and supernatants transferred to new vials and dried at room temperature for 2 h under vacuum. The residue was solubilized in 500 μl of MeOH, and a volume of 100 μl used for HPLC analysis. To determine the intracellular DOX concentration, MO cells were trypsinized, centrifuged at 1,000 rpm in cold phosphate-buffered saline (PBS), supernatants removed, and cells resuspended in 190 μl of PBS and 5 μl lysis solution (Triton X-100, 3%). Five μl of proteinase K (10 mg/ml stock solution) then was added. Samples were incubated for 45 min in a water bath at 65°C, and 56.4 μl of 10 μg/ml Daunorubicin was then added to a final volume of 400 μl with PBS. Five μl of phenylmethylsulfonyl fluoride (PMSF) was added for 10 min prior to the addition of 10 μl MgCl2 (0.4 M) and 20 μl DNase I (1 mg/ml). After centrifugation, the sample was incubated in a water bath at 37°C for 30 min, and 450 μl of methanol and 45 μl of ZnSO4 (400 mg/ml) added to 450 μl of each sample. After mixing, the sample was centrifuged for 5 min, and a volume of 100 μl used for HPLC analysis.
High Pressure Liquid Chromatography (HPLC)
A published HPLC method for detection of DOX was modified to allow quantitation of extracellular and intracellular amounts of DOX.23–25 The HPLC system consisted of a 1525 Binary HPLC Pump, a 717 Plus Autosampler, and a 2475 Multi-Wavelength Fluorescence Detector (Waters Corporation, Milford, CT). Chromatography was performed on a BDS Hypersil C18 column (4×150 mm, particle size 5 μm). The mobile phase consisted of water:acetronitrile:tetrahydrofuran (75.5:24:0.5, v/v/v), with an apparent pH adjusted to 2.0 with perchloric acid. The flow rate was maintained at 1.25 ml/min with an injection volume of 40 μl or 100 μl. The effluents were monitored at an excitation wavelength of 480 nm and an emission wavelength of 560 nm at 40°C. Detection and integration of chromatographic peaks was performed using Breeze Chromatography v.3.20 software.
Lactate dehydrogenase (LDH) assay
Cell lysis was measured by determining levels of LDH in the medium of MO or ME26 cells treated with either APMS (7.5×106 APMS/cm2), varying concentrations of DOX (40–800 nM), or APMS-DOX (7.5×106 APMS/cm2; 10–80 nM DOX equivalent) using a Cytotox 96® Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI) per the manufacturer’s recommendations.18 The percentage of LDH release was calculated based on complete lysis induced by a positive control lysis buffer (0.09% Triton-X).
MTS assay
MO cell viability following treatment with medium alone, DOX alone (80 nM), APMS (7.5×106 APMS/cm2), or APMS-DOX (7.5×106 APMS/cm2; 80 nM DOX equivalent), was measured in vitro using the colorimetric MTS assay, CellTiter 9® AQueous One Solution Cell Proliferation Assay (Promega) as per the manufacturer’s recommendations.
Preparation of RNA and PCR Array analyses
To determine if different patterns of gene expression related to cellular DNA damage/repair were observed after addition of DOX alone or APMS-DOX, RNA from MO cells treated with medium alone, APMS alone (7.5×106 APMS/cm2), DOX alone (80 nM), or APMS-DOX (7.5×106 APMS/cm2; 80 nM DOX equivalent) for 24 h was prepared and purified using a RNeas® Plus Mini Kit (Qiagen, Valencia, CA). After quality assessment, 1 μg of RNA was employed for cDNA synthesis using the RT2 First Strand Kit (SABiosciences, Frederick, MD). Quantitative real-time PCR (QRT-PCR) was performed by the Vermont Cancer Center DNA Analysis Facility using RT2 Real-Time™ SYBR Green PCR Master Mix and Human DNA Damage Signaling Pathway RT2 Profiler™ PCR Arrays (SABiosciences) (7900HT Sequence Detection System, Applied Biosystems, Foster City, CA). QRT-PCR (TaqMan) was used to validate selected genes using Assay on Demand (AOD) Primers and Probes (Applied Biosystems). For the purposes of these studies, only gene changes ≥3-fold are provided.
Confocal Scanning Laser Microscopy (CSLM) for determination of APMS-Alexa-568 and APMS-Alexa-488 localization in vivo
APMS were covalently labeled with the fluorescent dye, Alexa Fluor 568 (APMS-Alexa-568), by standard peptide-bond forming methodology.18 APMS-Alexa-568 were suspended in PBS at a concentration of approximately 3.3×107 APMS/100 μl PBS. C57BL/6 mice (n = 3; Jackson Laboratories, Bar Harbor, ME) received intrapleural injections and were euthanized after 72 h.18 Rib cage and adjacent diaphragm were removed surgically and either placed in Tissue-Tek O.C.T® compound and snap-frozen, or fixed in 4% paraformaldehyde and paraffin embedded. For determination of tumor uptake, 5×106 Hmeso cells were injected into 4 subcutaneous (SC) sites on the dorsa of 6 week old male Fox Chase strain severe combined immunodeficient (SCID) mice (Jackson Laboratories, Bar Harbor, ME). Tumors appearing at 21 days were injected on day 28 at the distal pole of the tumor with 3×106 APMS labeled with Alexa Fluor 488 succinimide ester (APMS-Alexa-488) for 24 h and removed for processing and step sectioning. Tissue section slides from frozen tissues were prepared,18 and representative images were acquired using a Zeiss LSM 510 META confocal scanning laser microscope (Carl Zeiss Microimaging Inc., Thornwood, NY). All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Inhibition of human MM growth in subcutaneous (SC) and intraperitoneal (IP) mouse xenograft models
In the SC xenograft model, Hmeso cells (5×106 cells/injection site in 50 μl 0.9% NaCl, pH 7.4, hereafter referred to simply as saline) were injected into 4 sites on the dorsa of 6 week-old male Fox Chase strain SCID mice (Jackson Laboratories) under isoflurane anesthesia. This MM line was selected because of its intermediate sensitivity to DOX and cisplatin in vitro (see Results) and its reproducible tumor growth and phenotype (biphasic MM). Tumors were first characterized by a board certified pathologist using previously described criteria26, 27 to confirm their mesothelial origin. In an initial study (Experiment 1) utilizing the SC model (n = 2 mice or 8 MM tumors/group), Hmeso tumors appearing at 19 days post-injection were injected 1X weekly at their distal poles for 3 weeks with 50 μl saline alone, APMS (3×108 APMS/tumor, equivalent to 160 mg/kg APMS) in saline, DOX (6.15 μg DOX/tumor injection, equivalent to a total concentration of 1 mg/kg DOX/mouse) in saline, or APMS-DOX (3×108 APMS; 6.15 μg DOX equivalent/tumor) in saline. Another group of mice received injections of DOX (5 mg/kg) at concentrations known to inhibit MM growth in rats28 via intravenous (IV) tail vein injection. All mice were weighed weekly and examined every other day for tumor growth and morbidity. When the largest axes of the tumors in the control mice reached 1 cm, mice were euthanized as described above, necropsied to determine possible metastases, and major organs removed and stored in 4% paraformaldehyde before processing for histopathology and evaluation by a board-certified pathologist. Tumors were weighed and were measured using digital calipers. Tumor volumes were calculated using the formula (π × long axis × short axis × short axis)/6. Since the 1X weekly injection regimen failed to inhibit tumor growth, a second experiment was performed (n = 4 mice or 16 MM tumors/group) in which agents were administered 3X weekly (Experiment 2).
In the intraperitoneal (IP) xenograft model (Experiment 3), Hmeso cells (5×106 cells in 50 μl saline) were injected into the lower left quadrant of the peritoneal cavity of 6 week-old male Fox Chase strain SCID mice (n = 5 mice/group). After 2 weeks, a time period in which all mice developed non-adherent spheroid MMs (see below), mice were injected IP 3X over the course of 1 week with 500 μl saline alone, DOX (1 mg/kg) in saline, or APMS-DOX (7.8×108 APMS/mouse, equivalent to 0.33 mg/kg DOX) in saline. Based on our results in Experiment 2, in which we did not see a difference in tumor growth between APMS-DOX and DOX alone groups when administered SC at a higher concentration (1 mg/kg; see Results), we administered a lower dose of DOX (0.33 mg/kg) in APMS to demonstrate increased efficiency of this delivery method. In addition, this DOX concentration is similar to the dose of DOX delivered during intracavitary MM chemotherapy in humans (15 mg/m2, equivalent to 0.41 mg/kg).3 An APMS alone group was not included in Experiment 3 since separate studies have demonstrated that IP doses of APMS up to 500 mg/kg (equivalent to 3.8×109 APMS/mouse) are well tolerated in mice (data not shown). Mice were euthanized as described above, and tumors were classified as either “spheroid” (small, free-floating nodules) or larger “mesenteric” tumors attached to the peritoneal mesentery. The total number of spheroid or mesenteric tumors/mouse was determined, along with individual tumor volume and weight. For each mouse, total tumor weight and volume were calculated separately for spheroids and mesenteric tumors by multiplying the average tumor weight and volume by the total number of spheroid and mesenteric tumors present, respectively. A summary of all in vivo xenograft studies and treatment regimens is presented in Table 1.
Table 1.
Summary of in vivo treatment regimens following establishment of Hmeso tumors SC or IP.
| Exp # | Group | # Mice/Group | Route* | Inj/Week | APMS Dose† (mg/kg) | DOX Dose‡ (mg/kg) |
|---|---|---|---|---|---|---|
| 1 | Saline§ | 2 | SC | 1 | — | — |
| APMS | SC | 1 | 160 | — | ||
| DOX | SC | 1 | — | 1 | ||
| DOX | IV | 1 | — | 5 | ||
| APMS-DOX | SC | 1 | 160 | 1 | ||
| 2 | Saline | 4 | SC | 3 | — | — |
| APMS | SC | 3 | 160 | — | ||
| DOX | SC | 3 | — | 1 | ||
| DOX | IV | 3 | — | 5 | ||
| APMS-DOX | SC | 3 | 160 | 1 | ||
| 3 | Saline | 5 | IP | 3 | — | — |
| DOX | IP | 3 | — | 1 | ||
| APMS-DOX | IP | 3 | 104 | 0.33 | ||
IP - intraperitoneal; IV - intravenous; SC - subcutaneous
160 mg/kg APMS equivalent to 3×108 APMS/tumor or 1.2×109 APMS/mouse; 104 mg/kg APMS equivalent to 7.8×108 APMS/mouse (assuming 25 g mouse)
For SC injections, each tumor injected with 6.15 μg DOX, equivalent to ~25 μg/mouse or 1 mg/kg total dose (assuming 25 g mouse)
0.9% NaCl, pH 7.4
Note: Exp – experiment; Inj – injection
Collection and preparation of peritoneal lavage fluid (PLF) for cytospins
To determine the presence of free APMS and those associated with cells in PLF in Experiment 3, the peritoneal cavity of each mouse was instilled with 5 ml of cold sterile PBS using an 18-gauge needle. Leaving the needle in place, the abdomen was gently massaged and PLF was aspirated back into the syringe and placed on ice. Cytospin slides were generated as described,18 and representative photographs were taken using an Olympus BX50 microscope (Olympus America Inc., Lake Success, NY) and QCapture Pro v.6.0 software.
Statistical analysis
For all in vitro experiments, at least 3 independent experiments were performed (n = 2–4 samples/experiment). For in vivo tumor experiments, results are representative of 2–5 mice/group/experiment. Statistical significance was evaluated by ANOVA using the Student Neuman-Keul’s procedure for adjustment of multiple pairwise comparisons between treatment groups or using the non-parametric Kruskal-Wallis, Mann-Whitney or Tukey HSD tests. Values of p<0.05 were considered statistically significant.
Results
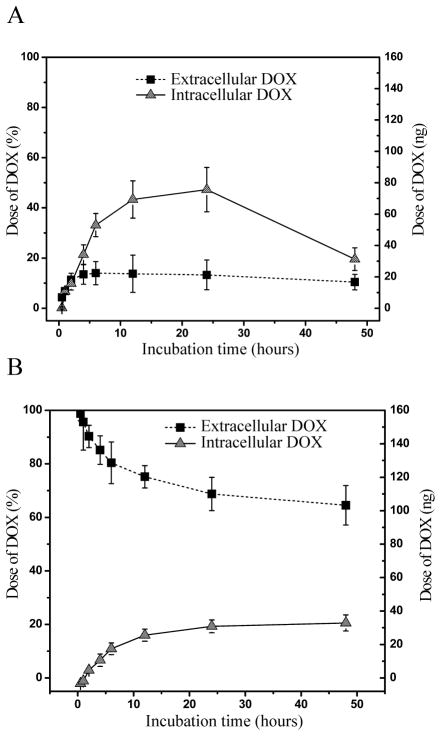
We first studied the amounts of DOX delivered to MO cells in vitro after administration in medium alone or via APMS-DOX. Since DOX becomes rapidly bound to DNA, use of DNase to release DOX from DNA and subsequent addition of MeOH and ZnSO4 were necessary for accurate quantitation by fluorescence HPLC. Similar methodology was used to quantify the amount of extracellular DOX. Whereas the left axis (Figure 1A and 1B) displays the percentage of the total dose of DOX, the right axis shows the actual dose (ng) of DOX/plate of cells. Since the total amount of DOX contained within APMS prior to their addition to MMs was known, the amount of DOX remaining within the APMS over time could be determined. A small amount (10–15%) of DOX was initially detected in medium, presumably through leakage from APMS after sonication (Figure 1A). Over a 24 h period, intracellular DOX increased until a maximum of approximately 50% of the initial amount within the APMS was released. More importantly, the balance of DOX remained within the APMS for 48 h, allowing further release over time. In contrast, the maximum amount of DOX transferred to cells when DOX was added directly to medium was approximately 25% of the initial dose (Figure 1B). The drop in intracellular DOX concentration observed at 48 h in APMS-DOX treated cultures (Figure 1A) is likely due to decreases in the overall number of cells available for analysis as a result of increased APMS-DOX cell death between 24 and 48 h of exposure.
Figure 1.
Determination of DOX uptake in vitro via HPLC following two different methods of administration to sarcomatoid (MO) MM cells for up to 48 h. Extracellular and intracellular DOX levels in MO cells measured by HPLC show greater cell delivery and retention of DOX when (A) loaded and released from APMS (APMS-DOX; 7.5×106 APMS/cm2; 160 nM DOX equivalent) in comparison to (B) direct addition to medium (160 nM DOX). Points represent the mean ± SEM (n = 3 in triplicate experiments).
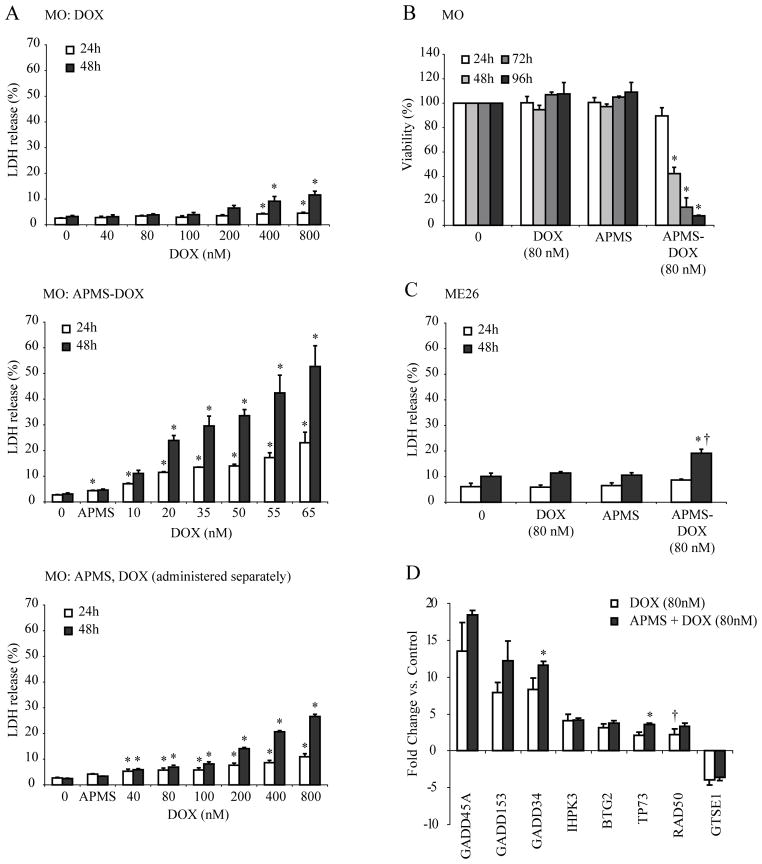
To comparatively assess the effects of DOX alone, DOX added simultaneously (but separately) with APMS, and APMS-DOX, dose-response studies were first performed on the sarcomatoid MO MM line. Initially, a range of concentrations of DOX were incubated with MO cells for 24 or 48 h, and LDH release into the medium was determined as a measure of lytic cell damage. Little LDH release (<10%) was seen with doses of DOX alone ranging from 40 to 800 nM (Figure 2A; top panel). In contrast, addition of APMS-DOX caused a dose-related release of LDH. At 48 h, approximately 50% of total cell lysis was achieved at 65 nM DOX (Figure 2A; middle panel). This potency was not achieved when individual preparations of APMS and DOX were incubated simultaneously with MO cells (Figure 2A; bottom panel). These data also show that APMS alone at the highest concentrations (7.5×106 APMS/cm2 surface area of dish, equivalent to amounts loaded with 65 nM DOX in the APMS-DOX group) were nontoxic to cells over a 48 h period.
Figure 2.
APMS-DOX delivery increases cytotoxicity in both sarcomatoid (MO) and epithelioid (ME26) MM cells in vitro. Cell lysis and viability were determined using the LDH and MTS assays, respectively. (A) LDH release by MO cells was measured under several treatment conditions including exposure to DOX alone (40–800 nM; top panel), exposure to APMS preloaded with varying concentrations of DOX (7.5×106 APMS/cm2, 10–65 nM DOX equivalent; middle panel), and exposure to individual preparations of unloaded APMS (7.5×106 APMS/cm2) and DOX (40–800 nM) simultaneously (bottom panel). (B) MTS viability assay confirming increased cytotoxicity of APMS-DOX (7.5×106 APMS/cm2; 80 nM DOX equivalent) in MO cells. (C) LDH release by ME26 cells. For LDH, percent release was calculated based on a complete lysis induced by a positive control lysis buffer (0.09% Triton-X; data not shown). For (A, B, C), 0 = medium only control; bars represent the mean ± SEM of n = 2–4 treatment groups in duplicate or more experiments; * = p<0.05 in comparison to medium alone at same time point; † = p<0.05 in comparison to DOX alone group. (D) PCR array experiments reveal alteration in several genes related to DNA damage and repair in sarcomatoid (MO) MM cells treated with DOX (80 nM) or APMS-DOX (7.5×106 APMS/cm2; 80 nM DOX equivalent) for 24 h. Upregulation of 6 genes and downregulation of 1 gene was observed following treatment with DOX or APMS-DOX compared to cells treated with medium alone. No significant gene expression alterations were observed with APMS alone. Only gene changes ≥3-fold are provided. * = p<0.05 in comparison to DOX alone group. † = not significant compared to medium control group. Bars represent the mean +/− SEM of n = 3 plates/group.
To further verify the more potent toxic effects of APMS-DOX on MM cells, we assessed cell viability using an MTS assay over 96 h (Figure 2B). When MO cells were exposed to either 80 nM DOX or APMS alone (7.5×106 APMS/cm2 surface area of dish, equivalent to amounts loaded with 80 nM DOX), there were no significant differences in cell viability in comparison to untreated cells. However, when cells were treated with APMS-DOX, significant (p<0.05) decreases in cell viability occurred at 48 h and thereafter. We next verified the increased potency of APMS-DOX in the LDH assay using ME26 cells (Figure 2C). By 48 h, the APMS-DOX (80 nM DOX equivalent) group showed a significantly (p<0.05) higher amount of lytic cell death, i.e., ~20% of total release, in contrast to 80 nM DOX alone. Because the greatest cytotoxic effects of APMS-DOX were achieved in sarcomatoid MMs, which have the worst prognosis,29 we performed further mechanistic studies on the MO MM line.
To shed light on the mechanisms of DNA damage by DOX and whether these were exacerbated in DOX delivery by APMS, we performed PCR array analysis on genes associated with apoptosis, cell cycle regulation, and various forms of DNA repair. Treatment of MO cells with DOX (80 nM) or APMS-DOX (80 nM DOX equivalent) caused at least 3-fold (p<0.05) increased expression of 6 genes (GADD45A, GADD153, GADD34, IHPK3, BTG2, TP73) in comparison to untreated MO cells (Figure 2D). In contrast, mRNA levels of GSTE1 were significantly (p<0.05) decreased. Messenger RNA levels of RAD50 were increased (p<0.05) in response to APMS-DOX (80 nM DOX equivalent), but not DOX alone (80 nM). In addition, GADD34 and TP73 gene expression was significantly (p<0.05) elevated in response to APMS-DOX when compared to the DOX alone group. No significant gene expression alterations were observed with APMS alone. QRT-PCR was used to confirm significantly (p<0.05) increased gene expression of GADD45A and GADD34 in both DOX and APMS-DOX-treated cells (data not shown). These data indicate increased responses to DNA damage after delivery of APMS-DOX versus DOX alone to MM cells.
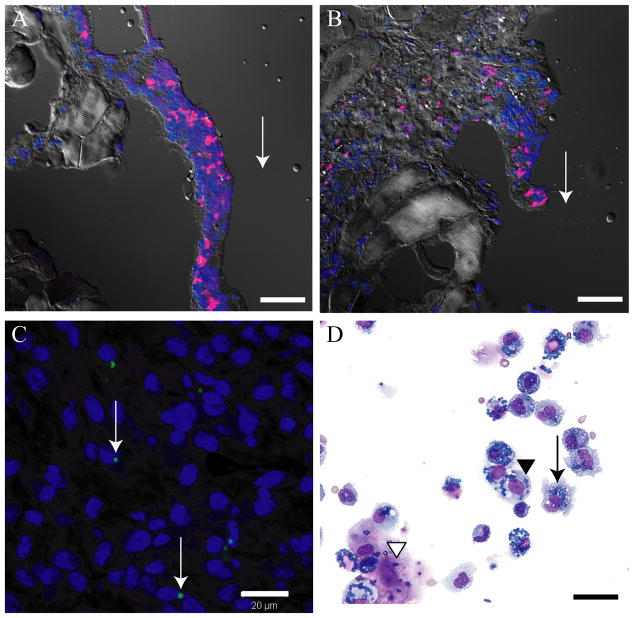
To determine whether APMS could enter and remain in tissues in vivo adjacent to sites of APMS injection, 3.3×107 APMS-Alexa-568 were injected into the pleural cavities of C57BL/6 mice (n = 3). Using CSLM, APMS-Alexa-568 were located adjacent to sites of injection in the soft tissue surrounding the ribs (Figure 3A and 3B). As reported previously,18 APMS-Alexa-568 were seen occasionally in the diaphragm, lung, and spleen using fluorescence microscopy. We then used SCID mice developing MMs after SC injection of Hmeso cells to determine whether APMS-Alexa-488 injected at the distal pole of the tumor migrated to the interior of the tumor. As shown in Figure 3C, APMS-Alexa-488 (green) were detected in the cytoplasm of MM cells in tumor masses. When cytospins were evaluated following IP administration of APMS-DOX (Experiment 3), APMS were detected in PLF samples, indicating these particles remain within the peritoneal cavity up to 10 d (Figure 3D). Further, it appears as if most APMS are associated with MMs and macrophages, although additional studies are required to confirm this observation.
Figure 3.
Several in vivo experiments demonstrate that APMS are taken up by a variety of cell types and remain adjacent to sites of injection. (A, B) APMS-Alexa-568 (red) injected into the pleural cavity of C57BL/6 mice were found in cells lining the rib at 72 h post-injection. Cell nuclei are stained blue (TOTO-3). Arrows indicate the approximate site of APMS injections. Approximately 3.3×107 APMS-Alexa-568 were injected into individual mice (× 400, scale bars = 20 μm). (C) APMS-Alexa-488 (green) injected at the distal pole of Hmeso SC tumors grown in SCID mice were detected within the cytoplasm of individual MM cells towards the center of the tumor mass. Tumor cell nuclei are stained blue (DAPI). Arrows indicate the presence of APMS-Alexa-488 signal. Approximately 3×106 APMS-Alexa-488 were injected into individual tumors (× 400, scale bar = 20 μm). (D) Representative PLF cytospin showing co-localization of APMS with macrophages (arrow), sloughed mesothelial cells (black arrowhead), and MM cells (white arrowhead) (× 400, scale bar = 20 μm).
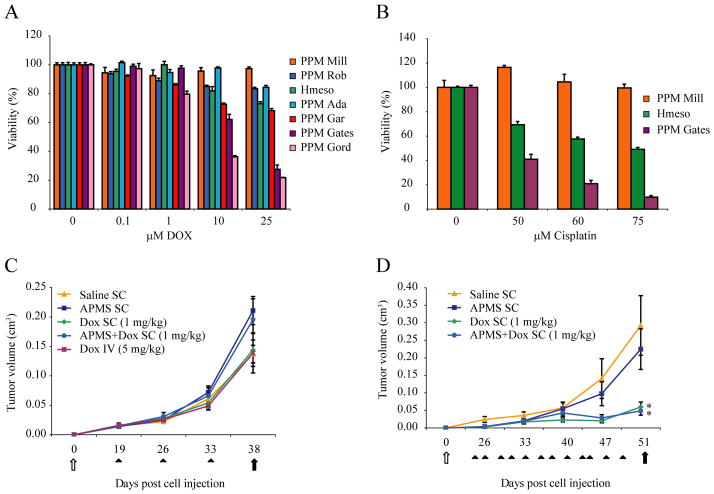
Our final goal was to demonstrate the efficacy of APMS-DOX in a SCID mouse xenograft model. We first tested a number of human MM lines for their sensitivity to DOX or cisplatin over a range of concentrations in vitro and for reproducible tumor growth in SCID mice. The maximum concentration of DOX used in these dose-response studies (25 μM) was approximately equal to DOX concentrations achieved in peritoneal fluids (18.4 μM or 10 μg/ml) following intracavitary DOX chemotherapy (15 mg/m2 or 0.41 mg/kg) in patients with MMs.3 Dose-response experiments with cisplatin were also performed in each cell line to determine if patterns of drug resistance were the same as DOX in individual MM lines.4, 30 These studies revealed that different human MMs were heterogeneous in their sensitivity to either DOX or cisplatin (Figure 4A and 4B). Moreover, the patterns of sensitivity to both drugs were similar in individual MMs. Since the Hmeso MM line expressed intermediate sensitivity to drugs in comparison to other MM lines and grew reproducibly after injection into SCID mice, this line was selected for in vivo studies.
Figure 4.
Anti-tumor effect of APMS-DOX in a SC xenograft model of MM. Preliminary in vitro studies demonstrated human pleural MM cell lines are differentially sensitive to (A) DOX or (B) cisplatin as measured by the MTS assay when drugs are added at a range of concentrations to maintenance medium. Because the Hmeso MM line exhibited intermediate sensitivity to DOX or cisplatin and grew reproducibly when injected SC or IP in a SCID mouse xenograft model, it was used for (C) 1X weekly (Experiment 1) and (D) 3X weekly (Experiment 2) injection studies to evaluate the effects of DOX when administered systemically or loaded into APMS (APMS-DOX) on inhibition of MM growth. Systemic administration of 5 mg/kg DOX IV 3X weekly resulted in death of mice after 2 sequential doses (data not shown) * = p<0.05 in comparison to saline control groups. White arrows indicate time of injection of Hmeso cells (5×106), and black arrows indicate days at which mice were euthanized in respective experiments. Black arrowheads indicate times at which saline, APMS alone (160 mg/kg), DOX alone (1 mg/kg), and APMS-DOX (160 mg/kg APMS; 1 mg/kg DOX equivalent) were injected into mice. Each point represents the mean volume of all tumors within a particular treatment group ± SEM.
As shown in Experiment 1 (Figure 4C), 1X weekly SC injections of APMS alone, DOX (1 mg/kg), APMS-DOX (1 mg/kg) or IV administration of DOX (5 mg/kg) over a 3 week period did not affect Hmeso tumor growth (n = 2 mice/group). Although histopathological analysis of organs revealed diffuse hepatocytic ballooning in the livers of mice administered DOX IV, no other adverse events were observed in organs evaluated from other treatment groups. However, when agents were administered 3X weekly (Experiment 2), tumor volume failed to increase after day 40 following SC injections of DOX (1 mg/kg) or APMS-DOX (1 mg/kg), and was significantly (p<0.05) lower in these groups in comparison to saline or APMS treated groups at the termination of the experiment (n = 4 mice/group) (Figure 4D). In these experiments, systemic administration of DOX (5 mg/kg) was lethal to all mice after 2 sequential IV doses (data not shown).
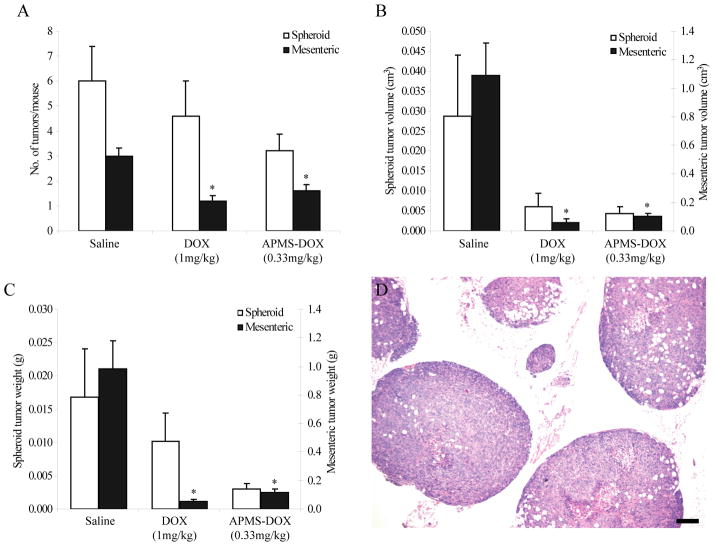
In Experiment 3, using an IP xenograft model (n = 5 mice/group), we reduced the concentration of DOX loaded in APMS (0.33 mg/kg) to determine if we could demonstrate increased or equivalent efficacy as DOX alone at 3-fold higher concentrations (1 mg/kg). In comparison to saline controls, mesenteric MM tumor numbers (Figure 5A), volumes (Figure 5B), and weights (Figure 5C) were significantly (p<0.05) less in mice administered DOX (1 mg/kg) or APMS-DOX (0.33 mg/kg) IP 3X weekly. Although not statistically significant, it appears administration of DOX and APMS-DOX also decreased growth of spheroid tumors. Hematoxylin and eosin staining of representative spheroid (Figure 5D) and mesenteric (data not shown) tumors indicate a biphasic MM tumor type.
Figure 5.
Anti-tumor effect of APMS-DOX in an IP xenograft model of MM. DOX (1 mg/kg) and APMS-DOX (104 mg/kg APMS; 0.33 mg/kg DOX equivalent) administered IP 3X weekly for 1 week were equally effective in decreasing Hmeso IP mesenteric tumor (A) numbers, (B) volumes, and (C) weights. Trends suggest that DOX and APMS-DOX were also effective in decreasing IP spheroid tumor numbers, volumes, and weights, although statistical significance was not achieved. In (B) and (C), the left axis corresponds to spheroid tumors and the right axis corresponds to mesenteric tumors. * = p<0.05 in comparison to saline control group. For each mouse, total tumor weight and volume were calculated separately for spheroids and mesenteric tumors by multiplying the average tumor weight and volume by the total number of spheroid and mesenteric tumors present, respectively. Bars represent the mean value for each treatment group ± SEM of n = 5 mice/group. (D) Representative H&E staining of spheroids from IP xenografts indicates a biphasic MM tumor type (× 40, scale bar = 200 μm).
Discussion
Limited effective treatment strategies exist for patients with MM. Many chemotherapeutic drugs, including DOX, have been used in single or combination therapies with most current treatments resulting in a mean survival time of 12–15 mos.1, 6–11, 31, 32 Most cancer chemotherapeutic drugs cause adverse systemic side effects because of their lack of tumor specificity and necessary administration at high doses. Thus, we hypothesized that using APMS at sites of MM tumor development might be advantageous as a novel form of localized chemotherapy delivery to avoid systemic toxicity. We show here in preclinical studies that APMS are an attractive vehicle to administer chemotherapeutic drugs and to enhance their delivery, cellular uptake, and cytotoxic effects. The APMS surface can be modified to contain a myriad of functional moieties, including fluorescent molecules for detection, gadolinium for MRI tracking (Steinbacher et al., unpublished data; Lathrop et al., unpublished data), and antibodies for targeting specific tumor types (Cheng et al., unpublished data). Functionalizing the exterior surface of APMS with molecules such as TEG enables rapid uptake by target cells by a process evading lysosomal degradation.18 Other important characteristics of mesoporous microparticles are their large surface areas (up to 1100 m2/g) and pore volumes (>1.0 cm3/g) that are advantageous in loading a large amount of ‘cargo’.33–37
Cisplatin and DOX have proven to be more effective than a variety of single chemotherapeutic drugs in single modality treatment of MM.1, 6–10 Because DOX is more chemically stable and quantifiable using HPLC it was selected for our studies. In addition to showing increased drug delivery and toxicity of APMS-DOX to MMs in vitro, we reveal the increased expression of a number of genes influencing DNA damage/repair. DNA damage is generally regarded as the primary cause of DOX-induced cell death in tumor cells. The molecular events linking DOX-induced DNA signaling to cell death have not been defined, but may involve cell signaling cascades, free radical-mediated events, and DOX-DNA adduct formation.38, 39 Upregulation of 3 GADD (growth arrest and DNA damage response) genes was observed in DOX or APMS-DOX-treated MM cells. Although GADD45 is an oxidative stress responsive gene induced by DOX in a number of cell types, expression of GADD153 (DNA-damage-inducible, alpha or DDIT3), a redox-activated gene that plays a role in cell cycle arrest, apoptosis, and activation of signal transduction pathways following DNA damage, is a novel finding that may be important in combination chemotherapy of MMs. Increased mRNA expression of IHPK3 (inositol hexaphosphate kinase 3), a gene linked to the development of apoptosis, and BTG2 (BTG family member 2), a less well-characterized p53-mediated DNA damage response gene associated with DNA repair, were also increased by DOX or APMS-DOX administration in our studies. Moreover, GADD34 and TP73 mRNAs were increased (p<0.05) more dramatically in MM cells after exposure to APMS-DOX in comparison to DOX alone, presumably because of the increased delivery of DOX when loaded into APMS. The increased expression of TP73 (encoding Tumor protein p73) in MMs treated with APMS-DOX was another novel observation in our gene array studies. This gene has been previously implicated in regulating a p53-dependent apoptotic pathway in tumors treated with chemotherapeutic drugs.40 In contrast to the well-studied p53 gene that encodes a tumor suppressor gene mediating cell cycle arrest or apoptosis in response to DNA damage, the TP73 gene encodes an array of isoforms not possessing typical tumor suppressor gene functions (reviewed in41, 42).
Significant (p<0.05) decreases in GTSE-1 expression, which encodes a cell cycle-regulated protein (hGSTE-1 or human G(2) and S-phase expressed-1), were also observed in both DOX and APMS-DOX treated MM cells. Since hGSTE-1 is able to down-regulate p53 levels and activity,43 its decreased expression in drug-treated MM cells may represent a repair mechanism whereby p53 function is kept intact.
Overall, studies have demonstrated that APMS are a novel and effective tool for enhanced delivery of DOX. Localized administration of APMS-DOX inhibits tumor cell growth in mouse models of MM via SC or IP injection in the absence of systemic toxicity. Most importantly, 3-fold lower effective concentrations of DOX were achieved after loading in APMS as opposed to injection of DOX alone in an IP mouse xenograft model of MM. In this model, APMS-DOX or DOX was injected when all of the mice had pre-established MM spheroids as demonstrated in preliminary experiments. Although DOX has been incorporated into RGD-modified44 and polyethylene glycol-derivatized liposomes45 and nanoparticles46 to increase its stability in the intravascular compartment, our studies are unique in that APMS were engineered in a size range (1–2 μm diameter) favoring tumor cell uptake (via TEG functionalization) at sites of local and intracavitary injection and prohibiting entrance into the systemic circulation, a major problem in DOX-associated cardiotoxicity.47 Infusion of APMS-DOX or other chemotherapeutic drugs into the pleural or peritoneal cavity could potentially inhibit the growth of premalignant MM cells in pleural/peritoneal fluids. Additionally, the ability to functionalize drug-loaded APMS with targeting moieties such as anti-mesothelin antibodies (Cheng et al., unpublished data), and the ability of APMS to release DNA20 and introduce functional plasmids into tumor cells,18 are exciting approaches that could potentially result in more effective treatment regimens for MMs and other cancers.
Acknowledgments
We thank Jennifer Díaz and Trisha Barrett for technical assistance with this manuscript, and Drs. Sean McCarthy and Albert van der Vliet (Department of Pathology, UVM) for help with HPLC analyses. Dr. Pamela Vacek (Department of Medical Biostatistics, UVM) was valuable in performing statistical analyses. We also appreciate the technical assistance of the Vermont Cancer Center DNA Facility staff with PCR Array and QRT-PCR analyses. This work was supported by the Mesothelioma Applied Research Foundation (MARF to BTM), a Small Business Technology Transfer (STTR) grant from the National Cancer Institute (R41 CA126155 to CCL), and a training grant from the National Institute of Environmental Health Sciences (T32 ES007122 to JMH).
Abbreviations used
- APMS
Acid-prepared mesoporous spheres
- MM
Malignant mesothelioma
- DOX
Doxorubicin
- IP
Intraperitoneal
- APMS-DOX
Doxorubicin loaded APMS
- SC
Subcutaneous
- IV
Intravenous
- TEG
Tetraethylene glycol
- SCID
Severe combined immunodeficient
- PCR
Polymerase chain reaction
- MO
Sarcomatoid MM cell line
- PMSF
Phenylmethylsulfonyl fluoride
- HPLC
High Pressure Liquid Chromatography
- LDH
Lactate dehydrogenase
- QRT-PCR
Quantitative real-time PCR
- AOD
Assay on Demand
- PLF
Peritoneal lavage fluid
- ME26
Epithelioid MM cells
References
- 1.Vogelzang NJ. Emerging insights into the biology and therapy of malignant mesothelioma. Semin Oncol. 2002;29:35–42. doi: 10.1053/sonc.2002.37469. [DOI] [PubMed] [Google Scholar]
- 2.Hesdorffer ME, Chabot JA, Keohan ML, Fountain K, Talbot S, Gabay M, Valentin C, Lee SM, Taub RN. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol. 2008;31:49–54. doi: 10.1097/COC.0b013e3180684181. [DOI] [PubMed] [Google Scholar]
- 3.Van der Speeten K, Stuart OA, Mahteme H, Sugarbaker PH. A pharmacologic analysis of intraoperative intracavitary cancer chemotherapy with doxorubicin. Cancer Chemother Pharmacol. 2009;63:799–805. doi: 10.1007/s00280-008-0800-0. [DOI] [PubMed] [Google Scholar]
- 4.Richards WG, Zellos L, Bueno R, Jaklitsch MT, Janne PA, Chirieac LR, Yeap BY, Dekkers RJ, Hartigan PM, Capalbo L, Sugarbaker DJ. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol. 2006;24:1561–7. doi: 10.1200/JCO.2005.04.6813. [DOI] [PubMed] [Google Scholar]
- 5.van Ruth S, van Tellingen O, Korse CM, Verwaal VJ, Zoetmulder FA. Pharmacokinetics of doxorubicin and cisplatin used in intraoperative hyperthermic intrathoracic chemotherapy after cytoreductive surgery for malignant pleural mesothelioma and pleural thymoma. Anticancer Drugs. 2003;14:57–65. doi: 10.1097/00001813-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Pass H, Carbone M, Vogelzang N. Malignant mesothelioma: advances in pathogenesis, diagnosis, and translational therapies. New York: Springer Science+Business Media, Inc; 2005. p. 854. [Google Scholar]
- 7.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10:266–83. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 8.Grondin SC, Sugarbaker DJ. Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest. 1999;116:450S–4S. doi: 10.1378/chest.116.suppl_3.450s. [DOI] [PubMed] [Google Scholar]
- 9.Krug LM. An overview of chemotherapy for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1117–36. vii. doi: 10.1016/j.hoc.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Tomek S, Emri S, Krejcy K, Manegold C. Chemotherapy for malignant pleural mesothelioma: past results and recent developments. Br J Cancer. 2003;88:167–74. doi: 10.1038/sj.bjc.6600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukohara T, Civiello G, Johnson BE, Janne PA. Therapeutic targeting of multiple signaling pathways in malignant pleural mesothelioma. Oncology. 2005;68:500–10. doi: 10.1159/000086994. [DOI] [PubMed] [Google Scholar]
- 12.Steele JPC, Rudd RM. Systemic chemotherapy for malignant pleural mesothelioma. In: O’Byrne K, Rusch V, editors. Malignant Pleural Mesothelioma. Oxford: Oxford University Press; 2006. pp. 297–313. [Google Scholar]
- 13.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:15549–54. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feazell RP, Nakayama-Ratchford N, Dai H, Lippard SJ. Soluble single-walled carbon nanotubes as longboat delivery systems for platinum(IV) anticancer drug design. J Am Chem Soc. 2007;129:8438–9. doi: 10.1021/ja073231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–60. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004:16–7. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 17.Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumen SR, Cheng K, Ramos-Nino ME, Taatjes DJ, Weiss DJ, Landry CC, Mossman BT. Unique uptake of acid-prepared mesoporous spheres by lung epithelial and mesothelioma cells. Am J Respir Cell Mol Biol. 2007;36:333–42. doi: 10.1165/rcmb.2006-0319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallis KW, Landry CC. Mesoporous silica and method of making same United States. 2002. [Google Scholar]
- 20.Solberg SM, Landry CC. Adsorption of DNA into mesoporous silica. J Phys Chem B. 2006;110:15261–8. doi: 10.1021/jp061691+. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie GD, Jr, Mossman BT. Health Effects of Mineral Dustsed. 28. Washington, DC: Mineralogical Society of America; 1993. p. 584. [Google Scholar]
- 22.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res. 1987;47:3199–205. [PubMed] [Google Scholar]
- 23.de Bruijn P, Verweij J, Loos WJ, Kolker HJ, Planting AS, Nooter K, Stoter G, Sparreboom A. Determination of doxorubicin and doxorubicinol in plasma of cancer patients by high-performance liquid chromatography. Anal Biochem. 1999;266:216–21. doi: 10.1006/abio.1998.2943. [DOI] [PubMed] [Google Scholar]
- 24.Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta. 1998;1400:139–54. doi: 10.1016/s0167-4781(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 25.Swift LP, Rephaeli A, Nudelman A, Phillips DR, Cutts SM. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006;66:4863–71. doi: 10.1158/0008-5472.CAN-05-3410. [DOI] [PubMed] [Google Scholar]
- 26.Rdzanek M, Fresco R, Pass HI, Carbone M. Spindle cell tumors of the pleura: differential diagnosis. Semin Diagn Pathol. 2006;23:44–55. doi: 10.1053/j.semdp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Martarelli D, Catalano A, Procopio A, Orecchia S, Libener R, Santoni G. Characterization of human malignant mesothelioma cell lines orthotopically implanted in the pleural cavity of immunodeficient mice for their ability to grow and form metastasis. BMC Cancer. 2006;6:130. doi: 10.1186/1471-2407-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linden CJ. Response to doxorubicin and cyclophosphamide of a human pleural mesothelioma clinically and as a xenograft in nude rats. In Vivo. 1990;4:55–9. [PubMed] [Google Scholar]
- 29.Kobayashi M, Takeuchi T, Ohtsuki Y. Establishment of three novel human malignant pleural mesothelioma cell lines: morphological and cytogenetical studies and EGFR mutation status. Anticancer Res. 2008;28:197–208. [PubMed] [Google Scholar]
- 30.Matsuzaki Y, Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Induction of apoptosis by intrapleural perfusion hyperthermo-chemotherapy for malignant pleural mesothelioma. Ann Thorac Cardiovasc Surg. 2008;14:161–5. [PubMed] [Google Scholar]
- 31.Mossman BT, Gee JB. Asbestos-related diseases. N Engl J Med. 1989;320:1721–30. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- 32.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 33.Beck J, Vartuli J, Roth W, Leonowicz M, Kresge C, Schmitt K, Chu C, Olson D, Sheppard E. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc. 1992;114:10834–43. [Google Scholar]
- 34.Kresge C, Leonowicz M, Roth W, Vartuli J, Beck J. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–2. [Google Scholar]
- 35.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–88. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46:7548–58. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 37.Vinu A, Hossain KZ, Ariga K. Recent advances in functionalization of mesoporous silica. J Nanosci Nanotechnol. 2005;5:347–71. doi: 10.1166/jnn.2005.089347. [DOI] [PubMed] [Google Scholar]
- 38.Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif. 2005;38:223–43. doi: 10.1111/j.1365-2184.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, You H, Yang Y, Wei L, Zhang X, Yao L, Fan D, Yu Q. Distinctive regulation and function of PI 3K/Akt and MAPKs in doxorubicin-induced apoptosis of human lung adenocarcinoma cells. J Cell Biochem. 2004;91:621–32. doi: 10.1002/jcb.10751. [DOI] [PubMed] [Google Scholar]
- 40.Sabatino MA, Previdi S, Broggini M. In vivo evaluation of the role of DNp73alpha protein in regulating the p53-dependent apoptotic pathway after treatment with cytotoxic drugs. Int J Cancer. 2007;120:506–13. doi: 10.1002/ijc.22362. [DOI] [PubMed] [Google Scholar]
- 41.Benard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum Mutat. 2003;21:182–91. doi: 10.1002/humu.10172. [DOI] [PubMed] [Google Scholar]
- 42.Stiewe T, Putzer BM. Role of p73 in malignancy: tumor suppressor or oncogene? Cell Death Differ. 2002;9:237–45. doi: 10.1038/sj.cdd.4400995. [DOI] [PubMed] [Google Scholar]
- 43.Monte M, Benetti R, Collavin L, Marchionni L, Del Sal G, Schneider C. hGTSE-1 expression stimulates cytoplasmic localization of p53. J Biol Chem. 2004;279:11744–52. doi: 10.1074/jbc.M311123200. [DOI] [PubMed] [Google Scholar]
- 44.Xiong XB, Huang Y, Lu WL, Zhang X, Zhang H, Nagai T, Zhang Q. Intracellular delivery of doxorubicin with RGD-modified sterically stabilized liposomes for an improved antitumor efficacy: in vitro and in vivo. J Pharm Sci. 2005;94:1782–93. doi: 10.1002/jps.20397. [DOI] [PubMed] [Google Scholar]
- 45.Gabizon AA, Barenholz Y, Bialer M. Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharm Res. 1993;10:703–8. doi: 10.1023/a:1018907715905. [DOI] [PubMed] [Google Scholar]
- 46.Barraud L, Merle P, Soma E, Lefrancois L, Guerret S, Chevallier M, Dubernet C, Couvreur P, Trepo C, Vitvitski L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J Hepatol. 2005;42:736–43. doi: 10.1016/j.jhep.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Lu P. Monitoring cardiac function in patients receiving doxorubicin. Semin Nucl Med. 2005;35:197–201. doi: 10.1053/j.semnuclmed.2005.02.005. [DOI] [PubMed] [Google Scholar]