Summary
Recent studies have indicated a gene by environment interaction between serotonin transporter gene (5-HTTLPR) polymorphism and childhood abuse on depressive symptoms. In addition, persistent elevation of cerebrospinal fluid (CSF) corticotropin-releasing factor (CRF) concentrations following early-life adversity has been posited to underlie the subsequent development of major depression. This pilot study tested the hypothesis that elevations of juvenile CSF CRF concentrations are, in part, determined by an interaction between polymorphisms of the 5-HTTLPR and early-life stress. Nine juvenile male bonnet macaques (Macaca radiata) had been raised under variable foraging demand (VFD) conditions, a nonhuman primate model of early-life stress, whereas nine subjects were normatively raised under LFD (low foraging demand) conditions. Genotyping revealed that four (44.4%) of the VFD-reared monkeys possessed at least one “s” allele whereas five VFD monkeys were of the l/l genotype. Of the nine LFD subjects, two (22%) had the s/l genotype and seven had the l/l genotype. A “juvenile” CSF sample was obtained at approximately three years of age. CSF CRF concentrations were elevated specifically in the VFD “s/s” and “s/l” allele group in comparison to each of the remaining three groups, indicating a gene by environment (GxE) interaction.
Keywords: Nonhuman primates, corticotropin-releasing hormone, early-life stress, serotonin transporter gene, major depression, anxiety disorders, gene by environment interaction
1. Introduction
A burgeoning database supports the view that corticotropin-releasing factor (CRF), a neuropeptide that orchestrates the mammalian response to stress, plays an important role in mediating long-term vulnerability to affective dysregulation following early-life adversity (Nemeroff et al., 1984). In nonhuman primates, early-life stress leads to persistent elevations of cerebrospinal fluid (CSF) CRF concentrations at both the juvenile and adult phases of development (Coplan et al., 2005). Clinical research has revealed CSF CRF elevations in depressed patients with perceived early-life stress (Carpenter et al., 2004), and in patients with borderline personality disorder exposed to early-life trauma (Lee et al., 2005).
Recent studies, however, indicate that depression consequent to early-life stress is regulated by the presence or absence of specific genes that confer a vulnerability diathesis (Kaufman et al., 2004). One such gene, SLC6A4, encodes the serotonin transporter, a protein critical to the regulation of brain serotonin function. A serotonin transporter polymorphic site that maps to the promoter region, commonly known as 5-HTTLPR, consists of a variable number of tandem repeats. There are two common functionally different alleles at 5-HTTLPR, the short (“s”) allele and the long (“l”) allele. The “s” allele encodes an attenuated promoter segment, and, relative to the “l” allele, is associated with reduced transcription and functional capacity of the serotonin transporter (Lesch et al., 1995). The “s” allele of 5-HTTLPR is associated with the development of depression, but only in individuals with a history of childhood maltreatment or recent stressful life events (Caspi et al., 2003). Additionally, the hypothalamic-pituitary-adrenal (HPA) axis activity has been shown to be affected by 5-HTTLPR polymorphism (Barr et al., 2004). Ressler et al. (2010) reported a gene by gene by environment (GxGxE) interaction between the CRF receptor gene (CRHR1), the 5-HTTLPR polymorphisms, and childhood abuse on depressive symptoms.
A recent meta-analysis failed to validate the existence of an interaction between 5-HTTLPR and stressful life events in depression (Risch et al., 2009). However, this study was challenged by Rutter et al. (2009) and Uher and McGuffin (2010), both citing a number of limitations. These included the failure to integrate biological evidence, the use of stressful life events as risk factor, omitting childhood maltreatment, and, more importantly, the utilization of unstructured self-report of environmental adversity. Those studies relying on self-report measures often showed an excess of negative findings (Uher and McGuffin, 2010).
Exposure to early-life stress in humans can be variable and entails a complex interaction among multiple factors (Kaufman et al., 2004). Using animal models, we can experimentally control the characteristics of early-life stress, provide objective measures of environmental adversity, and ensure non-exposure to unusual stress thereafter (Rosenblum and Paully, 1984). Moreover, the shorter life span of macaques compared to humans facilitates longitudinal assessment. Additionally, the bonnet macaques, like humans and rhesus macaques (M. mulatta), display polymorphism of the 5-HTTLPR site (Barr et al., 2004).
One paradigm of early-life stress exposure in the nonhuman primate can be achieved through imposing unpredictable foraging conditions on the mother – a procedure termed variable foraging demand (VFD) in contrast to normative low foraging demand (LFD) – thereby disrupting normative patterns of maternal rearing and infant attachment (Rosenblum and Paully, 1984). To test whether the VFD model is susceptible to GxE interactions, we hypothesized that elevations of CSF CRF concentrations would be confined to VFD “s/s” and “s/l” subjects and not evident in the VFD “l/l”, LFD “s/l”, and LFD “l/l” subjects.
2. Methods
2.1. Subjects
Eighteen juvenile male bonnet macaques served as subjects. Nine had been raised under VFD conditions whereas nine subjects were raised under normative LFD conditions. Genotyping (see methods below) revealed that four (44.4%) of the VFD subjects possessed at least one “s” allele (s/s or “s/l”) whereas five VFD subjects possessed the l/l genotype. Of the nine LFD subjects, two (22%) had the “s/l” genotype and seven were l/l. The proportion of subjects with the “s/s” and “s/l” allele did not vary by rearing group (Chi-square = 1.00; p= .31). Juvenile age and body weight data are reported in Table 1. Sixteen of the subjects have been reported upon previously (Coplan et al., 2005). However, none of the data used in the current study were reported before.
Table 1.
Means and Standard Errors for Group, Allele and Group*Allele Effects for Weight, Age and CSF CRF Concentrations in Juvenile Nonhuman Primates
| Group*Allele | Allele | Group | ||||||
|---|---|---|---|---|---|---|---|---|
| df = 1:14 | VFD “s/s” and “s/l” | VFD “l/l” | “LFD “l/l” | LFD “s/s” and “s/l” | “s/s” and “s/l” | “l/l” | VFD | LFD |
| N | 4 | 5 | 7 | 2 | 6 | 12 | 9 | 9 |
| Weight± | 3.62 | 3.38 | 3.83 | 3.71 | 3.67 | 3.60 | 3.50 | 3.77 |
| SE (kg) | 0.35 | 0.32 | 0.27 | 0.50 | 0.31 | 0.21 | 0.24 | 0.28 |
| F | F = 0.23 | F = 0.03 | F = 0.55 | |||||
| p | p = 0.64 | p = 0.86 | p = 0.47 | |||||
| Age± | 952.00 | 900.20 | 1103.43 | 988.50 | 970.250 | 1001.81 | 926.10 | 1045.96 |
| SE (days) | 88.79 | 79.41 | 67.12 | 125.56 | 76.89 | 51.99 | 59.56 | 71.19 |
| F | F = 0.81 | F = 0.12 | F = 1.67 | |||||
| P | p = 0.38 | p = 0.74 | P = 0.22 | |||||
| CRF± | 184.42 | 84.17 | 93.68 | 91.39 | 137.91 | 88.92 | 134.30 | 92.54 |
| SE (pg/ml) | 21.98 | 19.66 | 16.62 | 31.09 | 19.04 | 12.87 | 14.74 | 17.62 |
| F | F = 4.97 | F = 4.54 | F = 3.30 | |||||
| P | p = 0.042 | p = 0.051 | p = 0.090 | |||||
VFD = variable foraging demand rearing. LFD = controls subjects normatively reared; “l” = long allele of the serotonin transporter gene; “s” = short allele of the serotonin transporter gene.
Subjects were housed at the SUNY Downstate Nonhuman Primate Facility. The study was approved by the Institutional Animal Care and Use Committees of SUNY Downstate Medical Center and Yale University School of Medicine. There were no significant post-VFD experimental manipulations of either the VFD or LFD offspring that could confound the VFD-rearing effects.
2.2. VFD rearing
Mother-infant dyads were group-housed in pens of 5–7 dyads each and stabilized for at least four weeks prior to VFD onset. After infants reached at least 2 months of age, dyads were subjected to a standard VFD procedure that involved eight alternating 2-week blocks in which maternal food was either readily accessible (low foraging demand) or more difficult to obtain (high foraging demand).
Difficulty in obtaining food for the mothers was manipulated through the use of a foraging cart, a device in which food rations can be hidden in sawdust, with apertures on the sides of the cart for foraging. No caloric restriction is present in the VFD procedure, and normal maternal and infant weights are maintained (Rosenblum and Paully, 1984).
2.3. 5-HTTLPR Genotyping
Genotypes were determined by PCR amplification followed by size fractionation on 2% agarose gels. Primers used were CAG CAC CTA ACC CCC TAA TGT CCC TG and GAT TCT GGT GCC ACC TAG ACG CCA G. Each 10 µl reaction contained 20ng DNA, 1 µM of each primer, 1 M betaine, 10 µM dNTPs, and 0.1 unit Klentaq polymerase, in manufacturer’s PC2 buffer. Cycling parameters were 95°C for 5 minutes followed by 30 cycles at 95°/72° for 30 and 60 seconds respectively, using an MJR thermal cycler. Genotypes were ascertained in duplicate for quality control, with complete concordance; gels were scored independently by two individuals.
2.4. CSF Sampling
Subjects were socially housed in their natal groups until a juvenile CSF sample was obtained (age 926 ± 60 days for VFD group and 1046 ± 71 days for LFD group). CSF sampling and analysis were the same for all subjects. The time between the end of the VFD procedure and the CSF sampling was about 2–3 years.
In brief, subjects were taken from their home cages and placed in carrying cages, a routine procedure. For CSF sampling, subjects were released into restraint cages and intramuscular ketamine (15mg/kg) was administered. Sedation of subjects was achieved in less than five minutes after exiting the carrying cage. Cisternal CSF sampling was performed immediately following sedation. CSF samples were then placed in Gant tubes and stored in a −70° degree freezer. Assays for CRF were performed according to the methods described in Nemeroff et al. (1984). The assay has a sensitivity of 2.5 pg per tube and intra- and interassay coefficients of variation of 3–6% and 10–13%, respectively. The laboratory personnel conducting the CRF radioimmunoassays were blind to the rearing status of the subjects’ samples.
2.5. Statistical Analyses
Means and standard errors for age and body weight were divided into eight subgroups: four defined by the G*E interaction between 5-HTTLPR and VFD rearing; two comparing “s/s” and “s/l” versus “l/l” genotypes and two subgroups comparing rearing condition: VFD versus LFD (Table 1). We first examined whether a G*E interaction between VFD rearing and the “s/s” and “s/l” genotype of 5-HTTLPR would conspire to induce elevations of CSF CRF concentrations in relationship to the other three control groups. To determine this question, a 2X2 factorial design in a general linear model (Statistica 8.0) was employed. Post-hoc Newman-Keuls testing was only performed on the group by allele interaction effect and separately compared the vulnerable VFD “s/s” and “s/l” group in relationship to each of the three other experimental groups – VFD “l/l”, LFD “l/l”, and LFD “s/s” and “s/l”.
Because CSF CRF concentrations were to be evaluated as the only biological dependent variable, correction for multiple comparisons was not used. A two-tailed probability level of p ≤ 0.05 was defined as significant.
3. Results
Age and body weight were not used as covariates, because there were no rearing, genotype, or genotype×rearing interactions (Table 1). Neither age (r= −.18; N=18 p=.45) nor body weight (r= −.04; N=18; p=.87) correlated with CSF CRF concentrations.
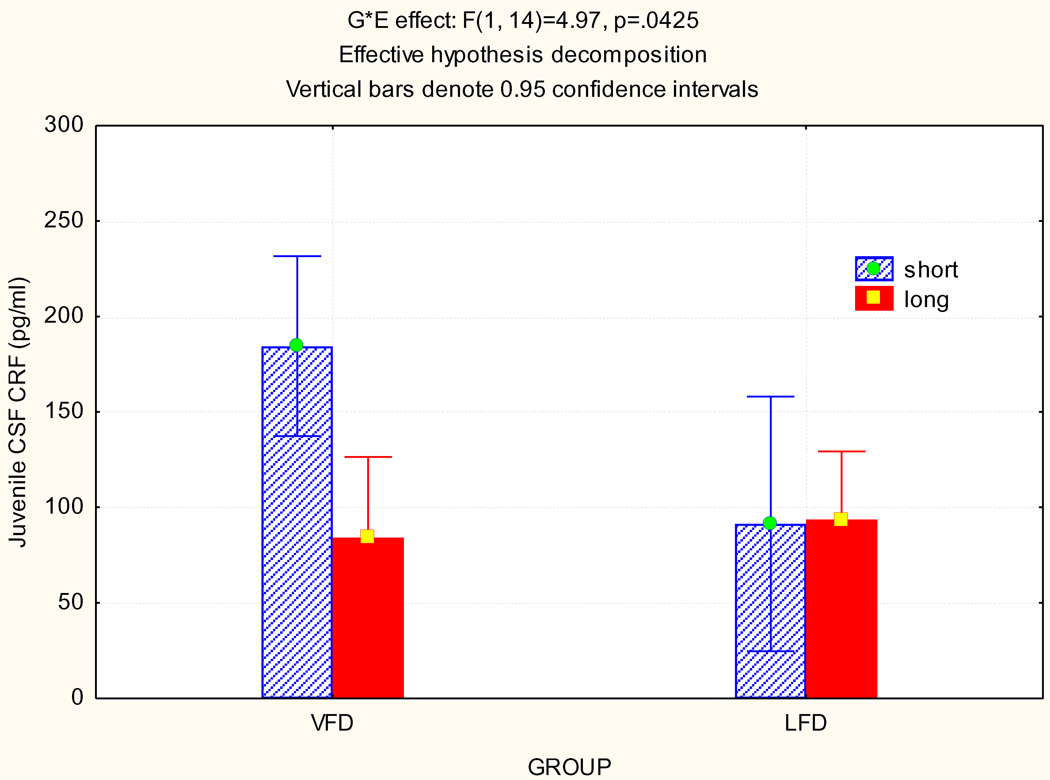
A positive 5-HTTLPR genotype×rearing environment interaction was observed with a trend main effect for rearing and a near-significant main effect for genotype (Table 1). Post-hoc testing revealed elevations for CSF CRF concentrations in the VFD “s/s” and “s/l” group when compared separately in comparison to each of the other three experimental groups (all p<.05). Results were not attributable to age of onset of VFD: (r=−0.21; p > .1) or body weight of subjects at the time of onset of VFD (r= −.05: p > .1).
4. Discussion
The major finding of the current study is the elevations of CSF CRF concentrations in juvenile VFD male subjects with 5-HTTLPR “s/s” and “s/l” alleles in comparison to each of the other three groups [VFD ”l/l”, LFD “s/l” and LFD “l/l”] demonstrating a gene-by-environment (GxE) interaction in bonnet macaques for CNS CRF systems. Results were not attributable to age or weight of the subjects. Although these data should be regarded as preliminary, the potential implication is that increases in the activity of CNS CRF neurons may contribute, in part, to the phenomenon whereby life stressors interact with the “s/s” and “s/l” allele to increase risk for human depression.
The current data are in accordance with rhesus monkey studies by Bennett et al. (2002), indicating a GxE interaction for reduced CSF 5-HIAA in peer-reared “s” subjects in comparison to maternally reared and peer-reared “l” controls, suggesting abnormalities in the 5-HT system in depression vulnerability. Moreover, Barr et al (2004) reported an exaggerated ACTH response in rhesus macaques carrying the “s” allele of the 5-HTTLPR upon exposure to maternal separation. These studies support the utility of certain macaque species to model GxE interactions in the context of early-life stress. In addition, the present results are concordant with the observations of Tyrka et al. (2004), who reported that serotonin depletion in healthy adults is associated with marked increase in CSF CRF concentration.
The findings should be considered as preliminary due to a relatively limited number of subjects. An additional caveat includes the lack of maternal genotypes to be factored in the analysis. The mothers’ genetic status could have influenced their response to stress and subsequently affected the infants, especially because we previously found synchronized mother-infant elevations of CSF CRF in response to VFD (Coplan et al., 2005). Also, future studies should include females, particularly because gender differences have been noted for HPA axis responsivity in short allele females versus males (Barr et al., 2004).
In summary, the data suggest a potential point of access towards an understanding of the mechanisms whereby the serotonin transporter gene interacts with early-life stress to produce a biological diathesis for depression and potentially other psychiatric disorders.
Figure 1. Gene by Environment (GxE) interaction for CSF CRF concentrations in Juvenile Bonnet Macaques.
A positive 5-HTTLPR genotype x rearing environment interaction was observed without main effects for rearing but a significant main effect for genotype (Table 1). Newman-Keuls post-hoc testing revealed elevations for CSF CRF concentrations in the VFD “s/s” and “s/l” group in comparison to each of the other three groups (p< 0.05).
Acknowledgments
Role of funding source: Supported in part by NIMH grants R21MH066748, MH-42088, MH-52899.
The authors thank Bruce Scharf, Douglas Rosenblum, and Shirn Baptiste for their invaluable contributions to this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Dr Coplan receives grant support from GlaxoSmithKline, Pfizer, and Alexza Pharmaceuticals. He is on the Pfizer advisory board and gives talks for BMS, AstraZeneca, GSK, and Pfizer. No biomedical financial interests or potential conflicts of interest are reported for Drs Abdallah, Kaufman, Gelernter, Smith, Perera, Dwork, or Kaffman. Dr. Gorman is an employee of Comprehensive NeuroScience, Inc., a corporation that receives funding from the pharmaceutical industry for some of its projects. Dr. Owens receives research grants from Eli Lilly, Pfizer, GlaxoSmithKline, Merck, Lundbeck, Cyberonics, Ortho-McNeil Janssen and served as consultant for Pfizer, Lundbeck, Sepracor, Johnson & Johnson, Sanofi-Aventis, Forest Labs and as speaker’s honoraria for GlaxoSmithKline. He is an inventor on the patent “A method to estimate transporter occupancy”. Currently, Dr. Nemeroff serves on the Scientific Advisory Board for the American Foundation for Suicide Prevention (AFSP); NARSAD, PharmaNeuroboost and CeNeRx. He serves on the Board of Directors of AFSP; NovaDel Pharma, and Mt Cook Pharma, Inc. He owns equity or is stock holder in Revaax; NovaDel Pharma; CeNeRx, and PharmaNeuroboost. He is an inventor on the following patents: method and devices for transdermal delivery of lithium (US 6,375,990 B1) and method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (US 7,148,027 B2).
Contributors:
Jeremy D. Coplan: Lead author, developed the hypothesis and oversaw all aspects of the manuscript; Chadi G. Abdallah: Developed the manuscript, and managed the literature review and references; Joan Kaufman and Arie Kaffman: Developed the concept of gene by environment interaction for variable foraging demand (VFD) reared monkeys; Joel Gelernter: Developed novel testing for serotonin transporter gene in bonnet macaques; Eric L.P. Smith: Supervised and carried out the rearing of VFD monkeys; Tarique D. Perera and Andrew J. Dwork: Contributed to the concept of VFD abnormalities following early life stress and the neurobiology of CRF; Jack M. Gorman: Encouraged and supervised the examination of the impact of early life stress; Leonard A. Rosenblum: As co-director of primate lab, oversaw all aspects of lab procedures; Michael S. Owens and Charles B. Nemeroff: Responsible for running assays for CRF and advancing the intellectual concept of linking early life stress, CRF neurobiology, and affective disorders.
References
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation y a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Altemus M, Mathew SJ, Smith EL, Sharf B, Coplan PM, Kral JG, Gorman JM, Owen MJ, Nemeroff CB, Rosenblum LA. Synchronized maternal-infant elevations of primate CSF CRF concentrations in response to variable foraging demand. CNS Spectr. 2005;10:530–536. doi: 10.1017/s109285290001018x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Jr, Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry. 2005;162:995–997. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gross J, Franzek E, Wolozin BL, Riederer P, Murphy DL. Primary structure of the serotonin transporter in unipolar depression and bipolar disorder. Biol Psychiatry. 1995;37:215–223. doi: 10.1016/0006-3223(94)00147-U. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemeroff CB, Cubells JF, Binder EB. Polymorphisms in CRHR1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:812–824. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS. The effects of varying environmental demands on maternal and infant behavior. Child Dev. 1984;55:305–314. [PubMed] [Google Scholar]
- Rutter M, Thapar A, Pickles A. Gene-environment interactions: biologically valid pathway or artifact? Arch Gen Psychiatry. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Carpenter LL, McDougle CJ, Kirwin PD, Owens MJ, Nemeroff CB, Strong DR, Price LH. Increased cerebrospinal fluid corticotropin-releasing factor concentrations during tryptophan depletion in healthy adults. Biol Psychiatry. 2004;56:531–534. doi: 10.1016/j.biopsych.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]