Abstract
Phase locking in the gamma-band range has been shown to be diminished in patients with schizophrenia. Moreover, there have been reports of positive correlations between phase locking in the gamma-band range and positive symptoms, especially hallucinations. The aim of the present study was to use a new methodological approach in order to investigate gamma-band phase synchronization between the left and right auditory cortex in patients with schizophrenia and its relationship to auditory hallucinations.
Subjects were 18 patients with chronic schizophrenia (SZ) and 16 healthy control (HC) subjects. Auditory hallucination symptom scores were obtained using the Scale for the Assessment of Positive Symptoms. Stimuli were 40-Hz binaural click trains. The generators of the 40 Hz-ASSR were localized using eLORETA and based on the computed intracranial signals lagged interhemispheric phase locking between primary and secondary auditory cortices was analyzed.
Current source density of the 40 ASSR response was significantly diminished in SZ in comparison to HC in the right superior and middle temporal gyrus (p<0.05). Interhemispheric phase locking was reduced in SZ in comparison to HC for the primary auditory cortices (p<0.05) but not in the secondary auditory cortices. A significant positive correlation was found between auditory hallucination symptom scores and phase synchronization between the primary auditory cortices (p<0.05, corrected for multiple testing) but not for the secondary auditory cortices.
These results suggest that long-range synchrony of gamma oscillations is disturbed in schizophrenia and that this deficit is related to clinical symptoms such as auditory hallucinations.
Keywords: EEG, ASSR, gamma-band, 40 Hz, schizophrenia, auditory stimulation, LORETA
Introduction
Synchronous neural oscillations in the gamma band (>30 Hz) of the electroencephalogram (EEG) have been hypothesized to play an important role in the linking of neurons into cell assemblies both locally and in the inter-regional communications of the brain (Singer 1999). While much of this evidence was provided in animal studies, investigations of gamma oscillations have now also been extensively done in healthy controls and patients with neuropsychiatric diseases. Experimental data obtained in both animals and in humans suggest that gamma-band oscillations are involved in perception and cognition (Herrmann et al 2004; Ribary 2005). For example, gamma-band phase synchronization between the inferior temporal lobe and the medial temporal lobe was recently demonstrated to play an important role in working memory using intracranial recordings (Axmacher et al 2008). Interestingly, it was shown that anatomical connectivity rather than physical distance, determines the coupling strength of the oscillating neurons in the gamma-band range (Csicsvari et al 2003).
The long-range synchrony of gamma oscillations has been demonstrated to be dependent on excitatory postsynaptic potentials (EPSPs) of γ-aminobutyric acid (GABA)ergic interneurons (Fuchs et al 2001). Since gamma-band oscillations depend on intact function of the fast-spiking GABAergic (parvalbumin containing) interneurons (Fuchs et al 2001; Hajos et al 2004; Vreugdenhil et al 2003), gamma-band oscillations may provide a means to investigate the function of GABAergic interneurons at a macroscopic level (Lisman et al 2008). This might be especially interesting for the investigation of schizophrenia, since there is much evidence now suggesting disturbances of the GABAergic interneurons in schizophrenia, such as changes in the concentration of particular proteins, notably glutamate decarboxylase (GAD), the enzyme that synthesizes GABA, and the Ca2+-binding protein parvalbumin (Lewis et al 2005; Woo et al 1997). Reductions in the overall number of GABAergic interneurons have also been described by post-mortem analyses (Benes et al 1991). Alterations in other neurotransmitter systems in schizophrenia (e.g. the dopaminergic and the glutamatergic system) can well be integrated in current models about the pathophysiology of schizophrenia suggesting a key-role for the disturbance of the GABAergic interneurons (Lisman et al 2008). Dysfunction of GABAergic interneurons is likely to be a major factor of disturbed gamma-band oscillations and related changes in perception and cognition. In schizophrenia, there is now an increasing number of studies describing altered gamma-band oscillation patterns (Hall et al 2009; Leicht et al 2010; Spencer et al 2004; Symond et al 2005; Uhlhaas and Singer 2010; Woo et al 2010).
Although different kinds of paradigms have been used during the last few years, some of the most reliable findings so far come from studies of auditory steady state response (ASSR) (Brenner et al 2009; Kwon et al 1999; Spencer et al 2008; Teale et al 2008). The ASSR is an EEG response to periodic auditory stimulation (such as click trains or amplitude-modulated tones) in which the sensory cortex acts as a tuned oscillator, synchronizing to the phase and frequency of the presented stimulus. The ASSR appears to have a “resonant” frequency at ~40 Hz at which the power and phase locking of the ASSR are enhanced in comparison with other stimulation frequencies (Pastor et al 2002). In addition to assessing the frequency response characteristics of sensory neural circuits, the ASSR may be a tool to investigate oscillatory mechanisms which might have a more general significance in the pathophysiology of schizophrenia. The typical finding in patients with schizophrenia is reduced power (related to the amplitude of the response) and phase-synchrony in the gamma-band across trials, measured at scalp electrodes or after source localization in the two auditory areas separately (Spencer et al 2009; Teale et al 2008).
Even more pronounced than in the visual or somatosensory system, there are extensive commissural links between the primary auditory cortices of both hemispheres linking tonotopically and binaurally matched subregions across the representational axis of characteristic frequency (Lee and Winer 2008). Although there is a substantial degree of interhemispheric transfer at subcortical levels of the auditory system, the callosal pathway in hearing is generally assumed to play an important role e.g. due to the left hemisphere supremacy in language perception and the contralateral pathway dominance in auditory signal transmission (Bamiou et al 2007). According to the “callosal relay” model, speech stimuli entering the right hemisphere will require callosal transfer to the left hemisphere in order to be processed (Hugdahl et al 1997; Zaidel 1986). The temporal-callosal pathway has been demonstrated to be related to phonological skills in children (Dougherty et al 2007) and it was shown that an intact posterior third of the corpus callosum (where the fibers of the auditory cortex are crossing) is important for the integration of prosodic information (right hemisphere function) and syntactic information (left hemisphere function) which is necessary for language comprehension (Friederici et al 2007).
During the last few years, structural connectivity in the brain has been investigated by means of Diffusion Tensor Imaging (DTI) (Kubicki et al 2007). Comparing schizophrenic patients with auditory hallucinations versus patients without hallucinations, increased directionality (suggested as representing stronger connectivity) was found in that part of the corpus callosum (CC, see Hubl et al 2004), Fig. 1, where the inter-hemispheric auditory fibers are assumed to cross (posterior part of the middle third and probably adjacent parts of the posterior third) (Bamiou et al 2007a). While structural connectivity can be assessed using DTI, EEG can be used to investigate functional connectivity (Teipel et al. 2009).
Fig. 1.
Diffusion Tensor Imaging (DTI) results comparing schizophrenic patients with auditory hallucinations and schizophrenic patients without hallucinations; a) the blue arrow shows an area in the posterior part of the corpus callosum (CC) with significantly increased Fractional Anisotropy (FA) in patients with auditory hallucinations; b) The Witelson Classification suggests increased FA values in the part of the CC where the auditory fibers cross (Witelson 1989). F: Frontal fibers, M: Motor fibers, Ss: Somatosensory fibers, A: Auditory fibers, T/P: Temporal and parietal fibers, V: Visual fibers. Reprinted and modified with permission from Hubl et al., Archives of General Psychiatry, 2004, 61(7):658–668, Copyright © (2004) American Medical Association. All rights reserved.
Accordingly, the aim of the present study was to investigate the gamma-band phase synchronization between the left and right auditory areas in patients with schizophrenia during auditory steady-state stimulation. We re-analyzed data from a previously-published study (Spencer et al., 2009) which utilized dipole source analysis to examine the ASSR deficit in schizophrenia. Since a positive correlation was found in that study between auditory hallucination symptoms and a left auditory cortex source, and since functional imaging studies have suggested that both unilateral and bilateral activation of the primary auditory cortex is relevant for auditory hallucinations, we also looked for a possible relationship between phase-synchrony coupling of the left and right primary auditory cortex in the gamma-band range and auditory hallucination symptoms. Here we utilized low-resolution tomography (LORETA) (Pascual-Marqui 2002; Pascual-Marqui et al 1994), a linear source localization method that has been widely used during the last few years by us (Mulert et al 2004; Mulert et al 2006; Mulert et al 2007) and others (Babiloni et al 2010; van der Loo et al 2009) in order to estimate oscillatory activity in the left and right auditory cortex as well as interhemispheric phase synchrony. Previous studies have demonstrated substantial congruence between LORETA and fMRI localization (Mulert et al. 2005, Mulert et al. 2010).
Methods
Subjects
This study was approved by the Institutional Review Boards of the VA Boston Healthcare System and Harvard Medical School. After complete description of the study to the subjects, written informed consent was obtained. All subjects were paid for their participation in the study.
Subjects were 18 patients with chronic schizophrenia (SZ) and 16 healthy control subjects (HC), all right-handed males (see Table 1). The HC were recruited from the local community. They were free of Axis I or II disorders (Structured clinical Interview for DSM III-R Non Patient Edition, Structured Clinical Interview for DSM IV Axis II Personality Disorders) as well as a history of Axis I disorders in first-degree relatives. SZ were diagnosed with schizophrenia according to the DSM-IV criteria (SCID). The diagnostic composition of the SZ group was: 10 paranoid, 5 undifferentiated, 2 schizoaffective, and 1 disorganized.
Table 1.
Comparison of demographic and clinical variables for the HC and SZ groups.
| HC | SZ | Statistic | |
|---|---|---|---|
| Age (years) | 44.4 +/− 6.8 | 39.8 +/− 10.5 | t[32] = 1.47, p = 0.151 |
| Parental socio-economic status | 3.1 +/− 1.3 | 3.2 +/− 0.9 | t[28] = −0.32, p = 0.754 |
| Handedness | 0.78 +/− 0.16 | 0.77 +/− 0.25 | t[32] = 0.17, p = 0.864 |
| Age of onset (years) | 26.1 +/− 8.0 | ||
| Positive symptom total (SAPS) | 9.0 +/− 4.2 | ||
| Negative symptom total (SANS) | 13.9 +/− 4.9 | ||
| Medication dosage (chlorpromazine equivalents) | 450 +/− 306 range 83–1200 | ||
Mean +/− standard deviation are given for each variable.
Subjects were all right-handed, selected without regard for ethnicity, and aged between 18–55 years. There was no history of electroconvulsive treatment, neurological illness, including epilepsy and no history of alcohol or drug dependence, nor abuse within the last year, nor long duration (>1 year) of past abuse (DSM-IV criteria). In addition, there was no present medication for medical disorders that would have deleterious EEG, neurological, or cognitive functioning consequences. For further details see Spencer et al., 2009.
Schizophrenic symptoms were rated with the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen 1984) and the Scale for the Assessment of Negative Symptoms (Andreasen 1983). Auditory hallucination symptoms were rated using the Auditory Hallucinations item of the SAPS. The groups did not differ on age (44 years for HC, 40 for SZ), handedness, or parental socioeconomic status (see Spencer et al., 2009 for details).
All patients received atypical antipsychotic drugs. Antipsychotic medication dosage was calculated in terms of chlorpromazine equivalents (Stoll 2001), and ranged from 83–1200 (mean of 450).
Stimuli and Experimental Design
Subjects were seated in a quiet room in a comfortable chair 1 m in front of a computer monitor. Stimuli were presented through headphones (70 dB sound pressure level). Subjects passively listened to trains of 1 ms clicks presented at 40 Hz for 500 ms. 150 trains were presented binaurally. Subjects were instructed to look at the fixation cross on the monitor and listen to the stimuli.
Electrophysiological Recording and Analysis
The EEG was recorded with Neuroscan Synamp amplifiers (0.01–100 Hz, 500-Hz digitization) with sintered Ag/AgCl electodes in an electrode cap at 60 scalp sites, left and right earlobes, referenced to the right earlobe. The forehead (AFz) served as ground. Bipolar vertical and horizonatal electro-oculograms were recorded from electrodes above and below the right eye and the left and right outer canthi, respectively. Electrode impedances were <10 kΩ.
Single-trial epochs were extracted from −250 to 772 ms relative to stimulus onset and corrected for eye movements and blinks using independent component analysis (Makeig et al 1996). Next, epochs containing artifacts were removed. The artifact criteria were > ± 90 μV change in one time point, and amplitude range within an epoch exceeding 200 μV. These criteria were verified via visual inspection. Finally, artifact-free single epochs were re-referenced to common average reference. There were no differences between the subject groups in the number of trials after artifact rejection (145 for HC and 140 for SZ on average).
All further analyses have been performed using the sLORETA / eLORETA software, as provided by Roberto Pascual-Marqui / The KEY Institute for Brain-Mind Research University Hospital of Psychiatry, Zurich at http://www.uzh.ch/keyinst/NewLORETA/LORETA01.htm, including the software update 2008-August 23. Localization of the neural generators of the ASSR was done using the eLORETA algorithm http://www.uzh.ch/keyinst/eLORETA/index.html. eLORETA is a 3D distributed, linear inverse solution that has no localization error to point sources under ideal (noise-free) conditions. Analysis was focussed on the frequency range 38–42 Hz.
Localization analysis was restricted to the 500 ms stimulation period using a sliding window approach. Time-varying frequency analysis was based on the short time Fourier transfor, using a sliding Bartlett-Hann window function with a width of 40 data points (80 ms). Given the temporal resolution of this approach, results were reported every 50 ms.
Lagged phase synchronization across regions was calculated between the left and right primary auditory cortex (Heschl’s gyrus, BA 41) and between the left and right secondary auditory cortex (superior temporal gyrus /STG, BA 42), focussed on the frequency range between 38–42 Hz. Regions of interest were defined using the anatomical definitions provided by the sLORETA / eLORETA software package which are based on the Talairach Daemon (http://www.talairach.org/), see Fig. 2. Regions of interest were selected a priori based on the most consistent reports about activations in the ASSR paradigm.
Fig. 2.
Regions of interest for the lagged phase synchronization analyses. Yellow: Primary Auditory Cortex (BA 41, Heschl’s Gyrus). Green: Secondary Auditory Cortex (BA 42, Superior Temporal Gyrus).
Lagged phase synchronization is defined as the phase synchronization between two signals after the instantaneous, zero phase contribution has been partialled out. Such a correction is necessary when using scalp EEG signals or estimated intracranial signals (EEG-tomography), because zero phase synchronization is often due to non-physiological effects, what might be termed intrinsic physics artefacts: volume conduction and low spatial resolution, see for example (Nolte et al 2004; Stam et al 2007).
The classical phase synchronization definition, which is highly contaminated by the instantaneous artifactual component, is:
| Eq. 1 |
with:
| Eq. 2 |
where xk (t,ω ) and yk (t, ω) denote the discrete Fourier transforms of the two signals of interest for the k-th EEG epoch, k =1...NR, NR being the number of epochs, at time instant t and at frequency ω; Re[c] and Im[c] denote the real and imaginary parts of a complex number c; c denotes the modulus; and the superscript “*” denotes complex conjugate.
Lagged phase synchronization definition [RD Pascual-Marqui: Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: frequency decomposition. arXiv:0711.1455 [stat.ME], 2007-November-09, http://arxiv.org/abs/0711.1455], which statistically partials out the intantaneous component, is:
| Eq. 3 |
In order to assess differences in the lagged phase synchrony between HC and SZ, statistical comparisons were calculated at the maximal values (peaks) of both HC and SZ during auditory stimulation. For the correlation analysis between the SAPS Auditory Hallucination scores and phase synchronization the whole segment information (single trial epochs) was used.
Statistical comparisons between HC and SZ were done using the Statistical non-Parametric Mapping (SnPM) methodology provided in the sLORETA / eLORETA software package, following Nichols and Holmes (Nichols and Holmes 2002). The method is based on empirically estimating the probability distribution of the maximum-statistics under the null hypothesis, via randomizations, thus correcting for multiple testing and without the need to rely on Gaussianity.
Statistical Analysis
Comparisons between the HC and SZ groups were performed with t-tests. Spearman’s Rho was used for correlation analyses (2-tailed). For the correlation analyses between Auditory Hallucination scores as assessed with the SAPS and phase synchronization values between left and right auditory areas (both primary and secondary auditory cortex) a Bonferroni correction with α = 0.05 (hallucination score x 2 phase synchronization values) was applied. Other correlation analyses were also Bonferroni corrected.
Results
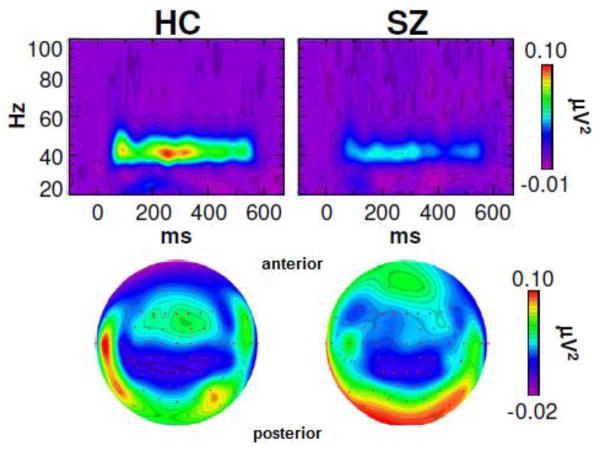
For the description of the scalp data please refer to Fig. 3.
Fig. 3.
Scalp EEG data: Time-frequency and topographic maps of evoked power for healthy controls (HC) and schizophrenia patients (SZ) at electrode Fz. Modified from Spencer et al. 2009 (BMC Neurosci 10:85).
Source localization
Both in HC and in SZ activation was found during the ASSR in superior and middle temporal gyrus and in Heschl’s gyrus (see Table 2). The highest current source density values were found in the middle temporal gyrus in both hemispheres in both groups. While in HC a right > left asymmetry was found, in SZ activation was stronger on the left side.
Table 2.
eLORETA localizations of ASSR activation patterns
| localization | X,Y, Z (MNI) | Current Source Density in μA/mm2 (x 10−5) | ||
|---|---|---|---|---|
| Healthy controls | ||||
| L Temporal Lobe, Middle Temporal Gyrus | −65 | −10 | −15 | 3.60 |
| L Temporal Lobe, Superior Temporal Gyrus | −65 | −20 | 0 | 3.16 |
| L Temporal Lobe, Transverse Temporal Gyrus | −60 | −10 | 10 | 2.79 |
| R Temporal Lobe, Middle Temporal Gyrus | 70 | −20 | −10 | 4.27 |
| R Temporal Lobe, Superior Temporal Gyrus | 65 | −10 | 0 | 4.04 |
| R Temporal Lobe, Transverse Temporal Gyrus | 65 | −10 | 10 | 3.84 |
| Patients with Schizophrenia | ||||
| L Temporal Lobe, Middle Temporal Gyrus | −65 | −30 | −20 | 4.94 |
| L Temporal Lobe, Superior Temporal Gyrus | −45 | −20 | −10 | 4.26 |
| L Temporal Lobe, Transverse Temporal Gyrus | −65 | −10 | 10 | 3.77 |
| R Temporal Lobe, Middle Temporal Gyrus | 50 | 10 | −30 | 3.78 |
| R Temporal Lobe, Superior Temporal Gyrus | 50 | −5 | −10 | 3.73 |
| R Temporal Lobe, Transverse Temporal Gyrus | 60 | −10 | 10 | 3.21 |
In the statistical comparison between SZ and HC, there was a significantly diminished activation in the right superior temporal gyrus (MNI coordinates: X, Y, Z: 70, −25, 5; t = 5.45, p<0.05, two-tailed) and right middle temporal gyrus (MNI coordinates: X, Y, Z: 70, −25, −5; t = 5.60, p<0.05, two-tailed) in patients. No region with increased activation in patients was found (see Figure 4).
Fig. 4.

Statistical map demonstrating differences between HC and SZ at p<0.05. There are only regions with increased activation in HC than in SZ (yellow-red): The right superior and middle temporal gyrus. No regions with increased activation in patients can be found. A: Anterior, S: Superior, P: Posterior.
Lagged Phase Synchronization
Primary auditory cortex (Heschl’s gyrus)
In both HC and SZ an increase in the lagged phase synchronization was detected during stimulation. The highest synchronization values were found after 50 ms and after 150 ms in HC and after 500 ms in SZ. In the statistical comparison at the first synchronization-peak (after 50 ms) there was no significant difference to the SZ group. In the statistical comparison at the second synchronization-peak (after 150 ms) there was a significant reduction in the SZ group compared to the HC group (t = 2.42, p<0.05, two-tailed). In contrast, the statistical comparison between HC and SZ at the maximal synchronization of SZ (after 500 ms) showed no significant results (see Fig.5).
Fig. 5.
Phase synchronization during the ASSR in HC (blue) and SZ (green); a) BA 41, b) BA42. Auditory stimulation starts at 0ms and ends at 500ms.
Secondary auditory cortex (STG)
In both HC and SZ an increase in the lagged phase synchronization was detected during stimulation. The highest synchronization was found after 50 ms in HC and after 500 ms in SZ. In both statistical comparisons (at the synchronization peaks of controls and patients) no significant difference across groups could be detected (see Fig. 5).
Correlations with Demographic Variables and Auditory Hallucination Scores
To rule out confounding effects, correlations were calculated between ASSR measures of interest and demographic variables. There were no correlations with age, parental socioeconomic status, handedness, medication dosage or time from admission for both groups.
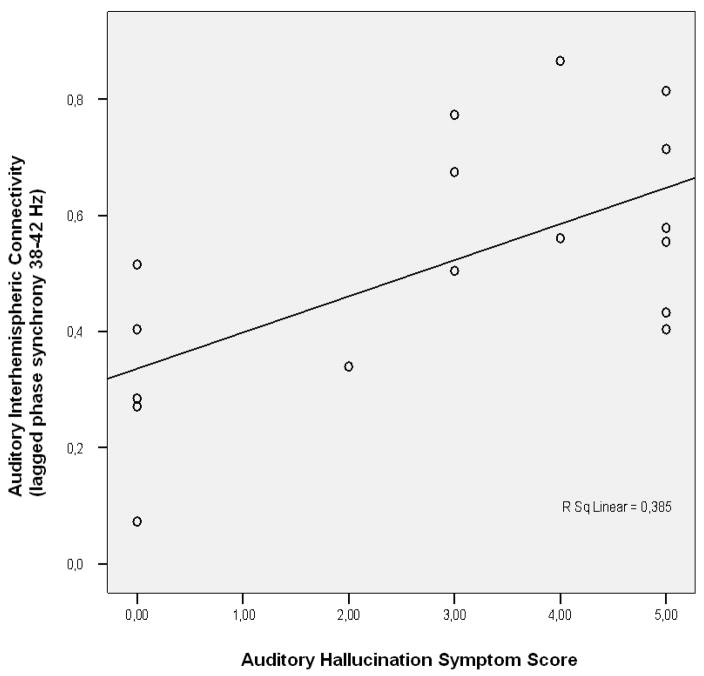
In the correlation analysis between the Auditory Hallucination score as assessed with the SAPS and the lagged phase synchronization values between left and right primary auditory cortex we found a significant positive correlation (Spearman’s rho: 0.56, p<0.05, corrected for multiple comparisons, see Fig. 6).
Fig. 6.
Spearman’s Correlations between SAPS (scale for the assessment of positive symptoms) auditory hallucination scores and phase synchrony between left and right primary auditory cortex.
In the correlation analysis between the SAPS Auditory Hallucination score and the lagged phase synchronization values between left and right secondary auditory cortex, however, there was no significant correlation.
Discussion
This study was intended to use a new methodological approach in order to investigate whether long-range synchrony of gamma oscillations is disturbed in schizophrenia and whether a relationship between inter-hemispheric phase synchronization and auditory hallucination symptoms can be found. The major finding was reduced phase synchronization in schizophrenia only between the left and right primary auditory cortex (Heschl’s gyrus), but not between the bilateral secondary auditory cortices. Furthermore, a positive correlation between auditory hallucination symptom scores and interhemispheric phase synchronization was present only for primary auditory cortices, but not for secondary auditory cortices.
In general, analyzing EEG phase synchronization may be a promising tool in order to investigate disturbed functional connectivity in schizophrenia. However, volume conduction effects have been a problem in the investigation of coherence or phase synchrony reports based on scalp channel data (Stam et al 2007). A considerable strength of our approach was the combination of source localization and the assessment of lagged phase synchrony in a combined approach thus minimizing possible misinterpretations due to volume conduction.
Relevant disturbances in the functional and structural connectivity in schizophrenia have been suggested by several authors (Andreasen et al 1998; Friston and Frith 1995; Innocenti et al 2003; McGlashan and Hoffman 2000) and during the last few years there has been increasing empirical evidence in agreement with these proposals (Bassett et al 2008; Garrity et al 2007; Rotarska-Jagiela et al 2008; Whitford et al 2007). Recently, corresponding reductions have been described for both the gray matter of the Heschl’s gyri and the respective white matter tissue, assessed as the decrease of the Fractional Anisotropy (FA) as measured by means of Diffusion Tensor Imaging (DTI) in patients with adolescent schizophrenia (Douaud et al 2007). Reductions of the Heschl’s gyri volumes have been described earlier (Hirayasu et al 2000) and also a connection between Heschl’s gyrus volumes and the severity of auditory hallucinations (Gaser et al 2004). Alterations of the corpus callosum, which is the major commissural structure for the connection of corresponding auditory areas in each hemisphere are also a common finding in schizophrenia (Arnone et al 2008). Some authors were suggesting a “hyperconnection” in parts of the CC connecting temporal association cortices and suggested a relationship to positive symptoms (John et al 2008). Most important for the current study are findings suggesting increased white matter directionality (supposed to represent increased connectivity) in the posterior part of the middle section of the CC / the posterior third of the CC – the area where the auditory fibers are crossing – in schizophrenic patients with auditory hallucinations in comparison to patients without hallucinations (Hubl et al 2004).
Our results of decreased gamma-phase synchronization between left and right Heschl’s gyri in patients with schizophrenia and a positive correlation between interhemispheric connectivity are well in line with a recent study investigating structural connectivity in patients with schizophrenia. In this DTI study, schizophrenia patients exhibited FA reductions in their frontal fibers crossing via the corpus callosum but at the same time significant positive correlations were observed to the severity of their psychotic symptoms such as hallucinations and delusions (Whitford et al. 2010). These results have been interpreted in such a way that mild asynchronies between the activities of spatially discrete brain regions might give rise to psychotic symptoms. However, severe asynchronies, caused by severe white matter damage might not be incorporable into a coherent phenomenological framework and thus not give rise to psychotic symptoms (Whitford et al. 2010).
The interhemispheric auditory pathway has not yet been discussed in relationship to auditory verbal hallucinations (AVH). However, its relationship to phonological awareness and speech perception might help to understand its role in AVH (for review see Bamiou et al 2007b). The interhemispheric auditory pathway, mainly crossing in the posterior third of the corpus callosum, is responsible for the interhemispheric interplay of prosodic information and syntactic information that is necessary for speech comprehension. For example, patients with a lesion in the posterior third of the corpus callosum do not show an event-related N400 potential in a speech comprehension task requiring the interaction of prosodic and syntactic information (Friederici et al 2007). In this paradigm a mismatch between the syntactic and prosodic structure of the initial sentence part is associated with an N400 potential in healthy controls. The N400 pattern has been interpreted as the neural correlate of a successful interaction of the left and right auditory areas. While identification of phonemes, words and the syntactic relation between them is processed mainly in the left hemisphere, emotional prosody is processed in the right hemisphere. Thus left and right auditory areas interact during normal on-line spoken language comprehension.
In the current study we found a disturbance in SZ only of the inter-hemispheric phase synchrony between the primary auditory cortices, but not between the secondary auditory cortices. While this finding has to be seen within the limitations of EEG source localization and needs independent replication, it is never the less interesting, since earlier studies suggest both a pronounced pathology in Heschl’s gyrus (Dierks et al 1999; Gaser et al 2004; Salisbury et al 2007) and in the STG in schizophrenia (Nestor et al 2007; Rajarethinam et al 2000).
In the present study utilizing LORETA we found a reduction of the ASSR in schizophrenia patients in the right temporal lobe. This finding is consistent with the dipole source analysis of the same data set (Spencer et al., 2009), in which ASSR power in a right auditory cortex source was significantly reduced in schizophrenia patients. Thus, two different source localization methods led to the same result. The present study, however, adds the information about disturbed long-range synchronization in the gamma-frequency range.
While disturbed gamma oscillations have been suggested to be related to basic pathopysiological mechanisms in schizophrenia (Uhlhaase and Singer 2010) and AVH are a frequent symptom of this disease, it is nevertheless interesting to notice that gamma oscillations might be related to hallucinations in other modalities such as somatic hallucinations (Baldeweg et al. 1998).
This study focused on the auditory 40 Hz-SSR demonstrating significant findings in temporal areas. However, there also reports of contributions in the frontal lobe, parietal lobe and the cerebellum in the 40 Hz ASSR (Reyes et al. 2005). In addition, there are reports about disturbances in the SSR in schizophrenia in other frequency ranges, such as the 30 Hz response (Spencer et al. 2008). It might be an interesting goal for further investigations to address the question of long-range synchronization between the areas of the extended SSR network and in different frequency-bands.
In summary, the current study reports decreased interhemispheric phase synchrony in SZ between the primary auditory cortices and a positive correlation of this interhemispheric phase synchrony with auditory hallucination scores. The applied technique using a combination of source localization and calculation of lagged phase synchrony might be useful also concerning the investigation of other aspects of disturbed functional connectivity in schizophrenia.
Acknowledgments
This work was supported by a US Department of Veterans Affairs Research Enhancement Award Program and Schizophrenia Center (RWM); US National Institute of Mental Health Grants R01 40799 (RWM), R03 076760 (KMS), and R01 MH080187 (KMS); and a NARSAD Young Investigator Award (KMS). CM was supported by a grant of the German Society for Psychiatry, Psychotherapy and Neurology (DGPPN)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2008;101:124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. 2008;28:7304–7312. doi: 10.1523/JNEUROSCI.1778-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Buffo P, Buttiglione M, Cibelli G, Rossini PM. Cortical responses to consciousness of schematic emotional facial expressions: A high-resolution EEG study. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;22;352(9128):620–1. doi: 10.1016/S0140-6736(05)79575-1. [DOI] [PubMed] [Google Scholar]
- Bamiou DE, Sisodiya S, Musiek FE, Luxon LM. The role of the interhemispheric pathway in hearing. Brain Res Rev. 2007;56:170–182. doi: 10.1016/j.brainresrev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical Organization of Human Cortical Networks in Health and Schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, et al. Activation of Heschl’s gyrus during auditory hallucinations [see comments] Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci U S A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA. Role of the corpus callosum in speech comprehension: interfacing syntax and prosody. Neuron. 2007;53:135–145. doi: 10.1016/j.neuron.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, et al. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc Natl Acad Sci U S A. 2001;98:3571–3576. doi: 10.1073/pnas.051631898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Volz HP, Buchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14:91–96. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, et al. The Early Auditory Gamma-Band Response Is Heritable and a Putative Endophenotype of Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, et al. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Carlsson G, Uvebrant P, Lundervold AJ. Dichotic-listening performance and intracarotid injections of amobarbital in children and adolescents. Preoperative and postoperative comparisons. Arch Neurol. 1997;54:1494–1500. doi: 10.1001/archneur.1997.00550240046011. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- John JP, Shakeel MK, Jain S. Corpus callosal area differences and gender dimorphism in neuroleptic-naive, recent-onset schizophrenia and healthy control subjects. Schizophr Res. 2008;103:11–21. doi: 10.1016/j.schres.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia [see comments] Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Winer JA. Connections of cat auditory cortex: II. Commissural system. J Comp Neurol. 2008;507:1901–1919. doi: 10.1002/cne.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Moller HJ, et al. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol Psychiatry. 2010;67:224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Bell A, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst. 1996;8:145–151. [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Medvedev AV. Temporal binding at gamma frequencies in the brain: paving the way to epilepsy? Australas Phys Eng Sci Med. 2001;24:37–48. doi: 10.1007/BF03178284. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jager L, Schmitt R, Bussfeld P, Pogarell O, Moller HJ, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22:83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Propp S, Karch S, Störmann S, Pogarell O, Möller HJ, Juckel G, Hegerl U. Sound level dependence of the primary auditory cortex: Simultaneous measurement with 61-channel EEG and fMRI. Neuroimage. 2005;15;28(1):49–58. doi: 10.1016/j.neuroimage.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Mulert C, Juckel G, Giegling I, Pogarell O, Leicht G, Karch S, et al. A Ser9Gly polymorphism in the dopamine D3 receptor gene (DRD3) and event-related P300 potentials. Neuropsychopharmacology. 2006;31:1335–1344. doi: 10.1038/sj.npp.1300984. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Pogarell O, Mergl R, Karch S, Juckel G, et al. Auditory cortex and anterior cingulate cortex sources of the early evoked gamma-band response: relationship to task difficulty and mental effort. Neuropsychologia. 2007;45:2294–2306. doi: 10.1016/j.neuropsychologia.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Hepp P, Kirsch V, Karch S, Pogarell O, Reiser M, Hegerl U, Jäger L, Moller HJ, McCarley RW. Single-trial coupling of the gamma-band response and the corresponding BOLD signal. Neuroimage. 2010;49(3):2238–47. doi: 10.1016/j.neuroimage.2009.10.058. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G, Bai O, Wheaton L, Mari Z, Vorbach S, Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA) technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Penuelas I, Masdeu JC. Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci. 2002;22:10501–10506. doi: 10.1523/JNEUROSCI.22-23-10501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41:303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Reyes SA, Lockwood AH, Salvi RJ, Coad ML, Wack DS, Burkard RF. Mapping the 40-Hz auditory steady-state response using current density reconstructions. Hear Res. 2005;204(1–2):1–15. doi: 10.1016/j.heares.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Ribary U. Dynamics of thalamo-cortical network oscillations and human perception. Prog Brain Res. 2005;150:127–142. doi: 10.1016/S0079-6123(05)50010-4. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Schonmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–25. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL. The Psychopharmacology Reference Card. Belmont, Massachusetts: McLean Hospital; 2001. [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42:1481–1489. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Pogarell O, Meindl T, Dietrich O, Sydykova D, Hunklinger U, Georgii B, Mulert C, Reiser MF, Möller HJ, Hampel H. Regional networks underlying interhemispheric connectivity: an EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Hum Brain Mapp. 2009;30(7):2098–119. doi: 10.1002/hbm.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van der Loo E, Gais S, Congedo M, Vanneste S, Plazier M, Menovsky T, et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One. 2009;4:e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164:1082–1089. doi: 10.1176/ajp.2007.164.7.1082. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O’Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus Callosum Abnormalities and Their Association with Psychotic Symptoms in Patients with Schizophrenia. Biol Psychiatry. 2010 May 20; doi: 10.1016/j.biopsych.2010.03.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112 ( Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TU, Spencer KM, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010 doi: 10.3109/10673221003747609. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel E. Callosal dynamics and right hemisphere language. In: Lepore F, Ptito M, Jasper HH, editors. The Blackwell Dictionary of Neuropsychology. Oxford: Blackell; 1986. pp. 279–285. [Google Scholar]