Abstract
Study Design
In vivo randomized controlled study in the goat intervertebral disc (IVD) injury model.
Objective
To define the effects of allogeneic bone marrow-derived stromal cell injected into the degenerating goat IVDs.
Summary of Background Data
Transplantation of bone marrow stromal cells to the degenerating disc has been suggested as a means to correct the biologic incompetence of the disc. However, large animal models with IVDs similar in shape and size to those of humans are needed to define the efficacy and safety of this approach.
Methods
Goat IVD degeneration was induced by stabbing with a #15 blade. One month after disc injury, the injured discs were randomly selected to receive goat bone marrow-derived stromal cell (suspended in hydrogel), saline (control), or hydrogel (control) injections. Three and 6 months after stem cell transplantation, goats were euthanized and the IVD were examined for biochemical content and tissue morphology. MR images at 3- and 6-month time points were also examined.
Results
The goat large animal model shows early degenerative changes following disc injury. Degenerating IVDs injected with bone marrow stromal cells showed significantly increased proteoglycan (PG) accumulation within their nucleus pulposus (NP) region. However, collagen content, MRI grade and histology did not show statistically significant differences between the cell-treated and control IVDs.
Conclusions
Following transplantation of bone marrow stromal cells, NP tissue contained more PG than control discs. Although this result was promising, the rate and severity of degeneration in this goat disc injury were modest, suggesting that a more severe injury and a larger sample size is indicated for future studies to better define the utility of cell therapies in this model.
Keywords: bone marrow-derived stromal cells, intervertebral disc (IVD), degenerative disc disease, low back pain
INTRODUCTION
Cell density within the adult human disc is surprisingly low,1 even compared to articular cartilage.2 With aging and degeneration, disc cell death occurs and the cell numbers further decrease.3 In addition, decreasing metabolic activity of the surviving cells leads to an incompetence of the disc to maintain its extracellular matrix structure. In the early stages of degeneration, it remains possible, at least in small animal models, to stimulate matrix production by the disc cells with growth factors introduced directly into the disc by injecting protein or gene expression.4 However, in the later stages of degeneration, new cells may be necessary to repopulate the disc and restore the extracellular matrix and tissue integrity.
Various cell sources have been explored for potential cell therapy, including nucleus pulposus (NP) cells,5–9 articular chondrocytes,10 bone marrow stromal cells or bone marrow-derived stem cells,11–17 and stem cells derived from other sources.18,19 Clinically, the feasibility of intervertebral disc (IVD) repair with a variety of cell sources will offer patients options depending on their disease status and cell availability. Bone marrow-derived stem cells are considered favorable because cells can be isolated from autologous or allogeneic sources with minimal donor site morbidity.
Most studies for disc regeneration have been conducted in small rodents or rabbits. Disadvantages of these smaller animals are: the difficulty of accurately injecting cells into the disc due to the very small size of the IVDs, and persistence of notochordal cells, which may produce a different outcome following intervention compared to humans. The goat offers a number of advantages as a study model for IVD interventions. First, the goat disc is similar in shape and size to that of humans. Goats are considered skeletally mature by the age of four, and are free of notochordal cells at this age.20 It is likely that the degenerative pattern and time course would be more similar between the goat model and humans. Thus, the goat model is attractive for preclinical studies, especially for implantable devices or biomaterials.
METHODS
Isolation of Goat Bone Marrow Stromal Cells
Goat bone marrow stromal cells were produced from a castrated male Boer Cross goat by a bone marrow aspiration. Cells were expanded, passaged twice, and cryopreserved. Immediately prior to implantation, cells were thawed, and re-suspended to 25 × 106 cells/ml of 1 × PBS.
Hydrogel Carrier
A chondroitin sulfate based hydrogel consists of a mixture of poly (ethylene glycol)-diacrylate, acrylated chondroitin sulfate, and high molecular weight hyaluronic acid. The hydrogel was crosslinked using a two-part mixture with a redox initiation system.21
Surgical Techniques
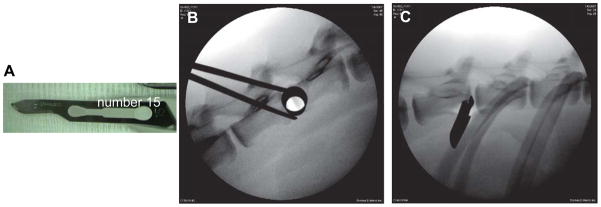
Twenty-four male Nubian goats, four years of age at the initiation of this study, were used. Under general anesthesia, the left lateral flank of the goat was shaved and lateral X-ray images were obtained. Under fluoroscopic guidance, a small skin incision on the left lateral body wall was created directly over the lateral projection of the disc. Next, a series of increasingly larger blunt dilator (METRx Minimal Access Instrument Set, Medtronic Sofamor Danek, Memphis, TN) was gently passed through the skin incision until docked at the lateral anulus of the disc (shown in Figure 1B). Through the tubular dilator, the L1/2 and L3/4 levels were injured with a #15 blade scalpel (Figure 1A), inserted parallel to the endplate over the mid-lateral disc until the blade attachment of the scalpel contacted the lateral margin of the anulus (Figure 1C). Estimated insertion depth was 15 mm. The L2/3, L4/5 and L5/6 discs were reserved as uninjured controls.
Figure 1. Instruments used and sample X-rays obtained during surgery.
A. # 15 blade used to create disc injuries; B & C: lateral X-ray of the goat spine documenting position of the instruments; B. 14 mm tubular retractor docked at the lateral margin of the intervertebral disc (IVD); C. #15 blade inserted into the IVD.
One month post disc injury, the injured discs were randomly assigned to the following injection groups: 1) Bone marrow stromal cell suspended in hydrogel; 2) Hydrogel (control); 3) Saline (control). For the bone marrow stromal cells in hydrogel treatment group, 10 μl of bone marrow stromal cell suspension (2.5 × 105 cells suspended in 1 × PBS) were mixed with 30 μl hydrogel and injected into the center of the NP. For the control groups, 40 μl of hydrogel or saline were injected. The IVD on the right side (opposite the disc injury) was accessed using the dilator technique described above. Through the dilator, a 25 gauge needle was introduced into the disc, midway between and parallel to the endplates to a depth of 15 mm. The solution was injected using a 50 μL Hamilton syringe.
Three and 6 months following the injections, lateral plain radiographic images were obtained under general anesthesia. Subsequently, the goats were euthanized by an overdose of Pentobarbital. The lumbar spines were removed en-bloc. MR images of the isolated lumbar segments were obtained with a 1.5 Tesla clinical imager. The IVDs were then divided in half by cutting in the mid-sagittal plane. Half of the IVD was isolated for biochemical assays and the remaining half was fixed with formaldehyde for histological analysis.
Examination of Extracellular Matrix Content using Biochemical Assays
At the 3-and 6-month post-treatment time points, the biochemical composition [proteoglycan (PG), DNA, and hydroxyproline contents] of the NP and anulus fibrosus (AF) regions of each disc was assessed. First, accurate wet weight of the AF and NP tissues was obtained using a balance with a readability of 1 μg (Mettler-Toledo, OH). The tissues then were digested with papain. DNA and PG contents were measured using the Hoechst dye and the dimethylmethylene blue (DMMB) binding method respectively, as previously described.22,23 Hydroxyproline content, as an indicator of collagen content, was quantified using the dimethylaminobenzaldehyde (DMBA) method.24
Tissue Preparation and Histological Evaluation
The IVDs were dissected with adjacent endplates intact. Subsequently, the vertebral body-disc-vertebral body units were fixed with 10% formaldehyde, decalcified, embedded in paraffin, and sectioned to 7 μM thickness. Adjacent sections were stained with Trichrome with Haematoxylin and Eosin (H&E) counter stain, or H&E stain alone. Histological features were evaluated by a pathologist and physiatrist, based on Masuda et al.25 and Boos et al.26
Statistical analyses
P-values were obtained using the Student two-tail t-test, with statistical significance defined as a p ≤ 0.05.
RESULTS
Proteoglycan (PG) content in the goat IVD tissues
The goats were sacrificed at the 3-and 6- month time points after treatment with bone marrow stromal cells mixed in a hydrogel carrier, hydrogel only, or saline only. PG content of the NP or AF tissues was divided by DNA content to reflect PG content per cell.
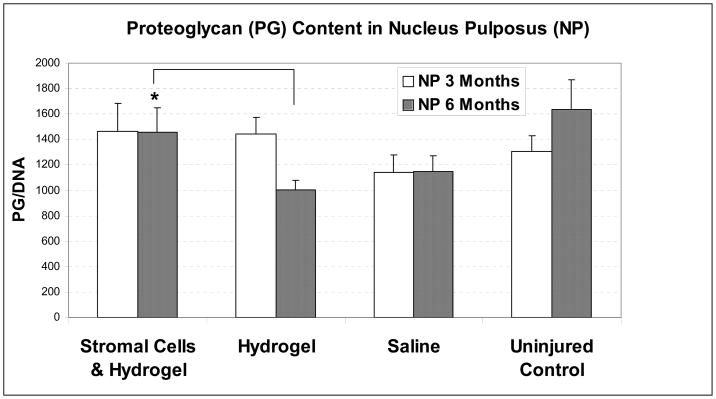
Three months post-injection, the PG content in NP tissue treated with stromal cells (n = 8) did not differ from the PG content in the control groups (hydrogel: p = 0.89, n = 8; saline: p = 0.26, n = 8; uninjured control: p = 0.17, n = 24, Figure 2). In the AF tissues, no difference in PG content was found between the discs treated with bone marrow stromal cells and the various control groups at the 3-month time point (hydrogel: p = 0.26, n = 8; saline: p = 0.16, n = 8; uninjured control at L2/3 and L5/6 levels: p = 0.13, n = 24).
Figure 2. Proteoglycan (PG) content in goat nucleus pulposus (NP) tissues three and six months after transplantation of bone marrow stromal cells, normalized to DNA content.
Error bar stands for standard error of the mean; * indicates statistical significance (p≤0.05).
Six months after stromal cell transplantation, NP tissue treated with bone marrow stromal cells suspended in hydrogel (n = 8) contained 45% more PGs than NP tissues treated with hydrogel alone (p = 0.05, n = 7, shown in Figure 2). NP tissues treated with stromal cells contained 127% of PGs as in saline controls (p = 0.22, n = 7). PG content in NP injected with stromal cells was 89% of those in uninjured control NP tissues at L2/3 and L5/6 levels (p = 0.56, n = 24). In comparison, the NP tissue of injured discs treated with hydrogel or saline only contained 61% or 70% of the PG content of uninjured control tissue (p = 0.02 and 0.08, respectively). In the AF tissues, no difference in PG content was found between the discs treated with bone marrow stromal cells (n = 8) and the various control groups at this time point (hydrogel: p = 0.80, n = 7; saline: p = 0.94, n = 7; uninjured control at L2/3 and L5/6 levels: p = 0.52, n = 24).
Collagen content in the goat IVD tissues
Hydroxylproline content, an indicator of collagen content, was measured in the disc tissues at 3- and 6- month time point after treatment. Hydroxyproline content of the NP or AF tissues was divided by DNA content to reflect collagen content per cell.
Three months post injection, the collagen content in NP tissue treated with stromal cells in the hydrogel carrier (n = 8) did not differ from the content in control groups (101% of hydrogel: p = 0.94, n = 8; 100% of saline: p = 0.98, n = 8; 109% of uninjured control: p = 0.49, n = 22). Similarly, collagen content in the AF tissue treated with stromal cells in the hydrogel carrier (n = 7) did not differ significantly from the collagen contents in control groups (101% of hydrogel: p = 0.97; 91% of saline: p = 0.60; 103% of uninjured control: p = 0.86).
Six months after treatment, NP tissue injected with bone marrow stromal cells in the hydrogel carrier (n = 8) contained 79% of the collagen in NP tissue compared with the hydrogel group (control, p = 0.02, n = 7), 83% of the saline control group (p = 0.18, n = 7), and 86% of uninjured control group (p = 0.12, n = 23). AF tissue treated with bone marrow stromal cells suspended in hydrogel did not show a significant change in collagen content when compared with the control groups (91% of hydrogel control, p = 0.37; 86% of saline control, p = 0.21; 97% of uninjured control, p = 0.75).
DNA content of the goat IVD tissues
DNA content, an indicator of cell number, was measured at 3 and 6 months after treatment. DNA content of the tissues (μg) was normalized to wet weight of the tissues (g).
Three months post treatment, DNA content in the NP tissue treated with bone marrow stromal cells in the hydrogel carrier (n = 8) did not differ from any of the control groups (hydrogel: p = 0.89, n = 8; saline: p = 0.26, n = 8; uninjured control: p = 0.17, n = 24). Similarly, no difference was found in DNA content in the AF tissue treated with stromal cells in the hydrogel carrier (n = 8) from any of the control groups (hydrogel: p = 0.34, n = 8; saline: p = 0.65, n = 8; uninjured control: p = 0.13, n = 24).
Six months after treatment, the DNA content of the NP and AF tissues were measured (Table 1.) Data are expressed as μg of DNA per gram (g) of wet tissue; numbers in parenthesis represent the standard error of the mean. The DNA content in the NP tissue treated with stromal cells (n = 8) did not differ from any of the control groups (hydrogel: p = 0.28, n = 7; saline: p = 0.61, n = 7; uninjured control: p = 0.87, n = 24). Likewise, no difference in AF tissue DNA content at 6 months post injury between IVD treated with bone marrow stromal cells (n = 8) and any of the control groups (hydrogel: p = 0.11, n = 7; saline: p = 0.91, n = 7; uninjured control: p = 0.13, n = 24).
Table 1.
DNA content of the intervertebral disc (IVD) tissues six months after treatment.
| DNA content (μg/g tissue) | Stromal cells in hydrogel | Hydrogel (control) | Saline (control) | Uninjured (control) |
|---|---|---|---|---|
| Nucleus Pulposus (NP) | 99.7 (± 14.3) | 118.2 (± 5.4) | 109.3 (± 11.1) | 101.1 (± 10.1) |
| Anulus Fibrosus (AF) | 463.6 (± 36.3) | 382.9 (± 27.3) | 455.2 (± 71.3) | 395.5 (± 19.9) |
MR Imaging analysis
MRI was obtained after the goats were sacrificed, and the spines were isolated en-bloc. T2 weighted mid-sagittal images were interpreted by three experienced readers (a physiatrist, an orthopedic surgeon, and a rheumatologist) according to Pfirrmann et al.27 The numerical scores by the 3 readers were averaged, and average score of IVDs treated with bone marrow stromal cells in the hydrogel carrier were compared with the control groups.
At the 3-month post-injection time point, MRI scores in the stromal cell suspended in hydrogel treated group (average score = 2.75, n = 8) were not better than the control groups (hydrogel average score = 2.67, p = 0.64, n = 8; saline average score = 2.29, p = 0.02, n = 8). Similarly, at the 6-month post-injection time point, MRI scores in the bone marrow stromal cell in hydrogel treated group (average score = 2.58, n = 8) were not statistically different from the control groups (hydrogel average score = 2.19, p = 0.19, n = 8; saline average score = 2.33, p = 0.31, n = 8).
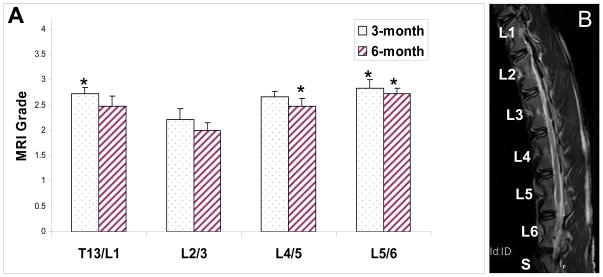
Interestingly, at the 3-month observation time point, uninjured (and untreated) control discs were less degenerative at the L2/3 level (average score = 2.22, n = 12) compared with T13/L1 level (average score = 2.72, p = 0.05, n = 12) or the L5/6 level (average score = 2.83, p = 0.03, n = 12, Figure 3). The MRI score at L2/3 level did not differ significantly compared with L4/5 level (uninjured, average score = 2.67, p = 0.07, n = 12). A similar observation was made at the 6-month time point: L2/3 discs (average score = 2.00, n = 12) were less degenerative than L4/5 (average score = 2.48, p = 0.01, n = 12) or L5/6 discs (average score = 2.73, p < 0.01, n = 12, Figure 3). The MRI score at L2/3 did not differ significantly compared with T13/L1 (average score = 2.48, p = 0.07, n = 12). In summary, L2/3 discs were less degenerative by MRI at 3-month and 6-month observation time points compared with L5/6. At the 6-month time point, the L2/3 IVD is less degenerative than all the remaining uninjured IVDS (T13/L1, L4/5, and L5/6).
Figure 3. MRI grades of uninjured control discs.
Panel A on the left shows MRI grades according to Pfirman et al. Panel B on the right is a representative T2-weighted MR image of a goat spine. Error bar stands for standard error of the mean, * indicates statistical significance (p≤0.05) compared to L2/3. Note that a higher score indicates more severe degeneration.
DISCUSSION
This study is the first to explore the effects of transplantation of allogeneic bone marrow stromal cells in a hydrogel carrier in a goat disc injury model. Hoogendoorn et al. have used a goat model to study IVD degeneration following the injection of chondroitinase ABC.28,29 However, this method induces more severe and rapid IVD degeneration, as opposed to the mild and slow injury-induced degeneration which more closely resembles the human disease. On the other hand, our injury model clearly needs to be refined: degeneration induced with a #15 scalpel stabbing is at a mild degree (or perhaps early stage) of degeneration by the 6 month period. It was surprising that a full thickness injury with a #15 blade scalpel did not produce a more severe degeneration over the 6 month follow-up time course. This indicates that the goat model presents a much slower and perhaps less severe response to injury when compared with the small animal models such as the rabbit. The difference in response to stabbing injury may be explained, in part, by the consistency of NP tissue: rabbit NP has a gel-like consistency that often herniates immediately after a needle stab injury, whereas the goat NP is more firm and did not herniate with a #15 scalpel stab.
The present study found changes isolated to the NP that were manifested biochemically at the 6-month, but not at 3-month, time point. This suggests that the overall course of both degeneration and repair in the goat model is relatively slow compared to rodents and rabbits, but is probably more closely related to the human where it is know that degeneration usually occurs over the course of years.
The current study does suggest that different disc levels in the goat spine appear to have differing predilections towards degenerative changes: there is a trend towards a higher MRI grade (more degeneration) in the uninjured levels adjacent to the relatively immobile thoracic spine and the sacrum. A similar observation has been made for humans, which show the more common and severe degenerative changes at the L4/5 and L5/S1 levels of the spine.30,31 In the future, this finding could be utilized to evaluate biologic interventions with the spontaneous (as opposed to induced) degeneration of the lower lumbar spine which may be more similar to human degeneration. Also, this variable should be controlled when performing a study of multiple disc levels with various interventions.
There are two potential mechanisms for the increase in PG content seen in this study. First, stem cells may produce trophic factors that stimulate the local NP cells (via a paracrine mechanism) leading to the additional production of extracellular matrix by the resident NP cell population. Indeed, matrix content or gene expression by IVD cells increases when the cells are co-cultured with mesenchymal stem cells.16,32 Further study into this mechanism may be helpful in providing the trophic factors, by an alternative (perhaps cell-free) route of administration. Second, stem cells may assume an IVD-like phenotype after implantation and, in turn, produce extracellular matrix, as suggested by Ganey et al..33 Evidence has been provided by several independent groups that suggests IVD cells induce stem cell differentiation into a NP cell-like phenotype.16,34,35 Additional interventions to induce the cells along a desirable (chondrogenic) lineage prior to implantation may be beneficial. In the current study, we did not detect a significant increase in cell density. The Hoechst dye binding method for DNA content may not be sensitive enough to detect the amounts of DNA contained in the transplanted stromal cells. Also, division of transplanted stem cells may have been limited by nutrient supply.36
Limitations of the current study include the sample number and the observed differences in degeneration among different regions of the spine. The sensitivity of the current MRI and histological grading systems were insufficient for detecting differences among the treatment categories, while biochemical changes were able to be detected. A reliable interpretation of the histological sections was difficult due to excessive artifacts related to tissue preparation, and lack of published histological scoring system for large animal IVDs. Clearly, improving grading systems for MRI and histology for goat disc degeneration would be beneficial in the future to provide more quantifiable results. Another limitation is that transplanted cells were not tracked to confirm cell survival. Finally, the modest differences between cellular intervention discs and control discs indicate that a longer observation period in a more severely injured IVD may be needed for future studies.
Key Points
The goat large animal model shows early degenerative changes following disc injury.
Following transplantation of bone marrow stromal cells, nucleus pulposus tissues accumulated more proteoglycans than control discs.
A more severe injury, larger sample size, and longer observation period is indicated for future studies utilizing this model.
Acknowledgments
Dr. Yejia Zhang is supported by the NICHHD (1K08 HD049598-01). This work was supported, in part, by Medtronic Spinal Biologics. We would like to thank Dr. Jade Borneman at Cognate Therapeutics for providing goat bone marrow stromal cells, Dr. Jennifer Elisseeff at Johns Hopkins University for providing the hydrogel carrier. The authors would like to thank Ms. Yiding Shen for performing biochemical assays, Dr. Chadi Tannoury assistance in goat surgeries, tissue dissection, and photographs for X-ray and MR images. The authors would also like to thank Dr. Gunnar Andersson for valuable insights and discussions, Dr. Ana Chee for manuscript preparation, and Dr. Alvaro Sandroni for statistical advice.
Footnotes
DISCLOSURES
Dr. Yejia Zhang is supported by the NICHHD (1K08 HD049598-01). This work was supported, in part, by Medtronic Spinal Biologics.
References
- 1.Maroudas A, Stockwell RA, Nachemson A, et al. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–7. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, An HS, Tannoury C, et al. Biological treatment for degenerative disc disease: implications for the field of physical medicine and rehabilitation. Am J Phys Med Rehabil. 2008;87:694–702. doi: 10.1097/PHM.0b013e31817c1945. [DOI] [PubMed] [Google Scholar]
- 5.Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–20. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 6.Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11 (Suppl 2):S206–14. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruber HE, Johnson TL, Leslie K, et al. Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–33. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 8.Meisel HJ, Ganey T, Hutton WC, et al. Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006;15 (Suppl 3):S397–405. doi: 10.1007/s00586-006-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel HJ, Siodla V, Ganey T, et al. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5–21. doi: 10.1016/j.bioeng.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Li Z, Thonar EJ, et al. Transduced bovine articular chondrocytes affect the metabolism of cocultured nucleus pulposus cells in vitro: implications for chondrocyte transplantation into the intervertebral disc. Spine. 2005;30:2601–7. doi: 10.1097/01.brs.0000187880.39298.f0. [DOI] [PubMed] [Google Scholar]
- 11.Helm GA, Gazit Z. Future uses of mesenchymal stem cells in spine surgery. Neurosurg Focus. 2005;19:E13. doi: 10.3171/foc.2005.19.6.14. [DOI] [PubMed] [Google Scholar]
- 12.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29:2627–32. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 13.Sakai D, Mochida J, Iwashina T, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 14.Sobajima S, Vadala G, Shimer A, et al. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–96. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Song H, Kwon K, Lim S, et al. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19:402–7. [PubMed] [Google Scholar]
- 16.Vadala G, Studer RK, Sowa G, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine. 2008;33:870–6. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YG, Guo X, Xu P, et al. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005:219–26. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Lee JP, Balian G, et al. Modulation of chondrocytic properties of fat-derived mesenchymal cells in co-cultures with nucleus pulposus. Connect Tissue Res. 2005;46:75–82. doi: 10.1080/03008200590954104. [DOI] [PubMed] [Google Scholar]
- 19.Murrell W, Sanford E, Anderberg L, et al. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 2009 doi: 10.1016/j.spinee.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Hoogendoorn RJ, Helder MN, Smit TH, Wuisman PIJM. Notochordal Cells in Mature Caprine Intervertebral Discs. European Cells and Materials. 2005;10(Suppl 3):59. [Google Scholar]
- 21.Li Q, Williams CG, Sun DD, et al. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68:28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- 22.Mok SS, Masuda K, Hauselmann HJ, et al. Aggrecan synthesized by mature bovine chondrocytes suspended in alginate. Identification of two distinct metabolic matrix pools. J Biol Chem. 1994;269:33021–7. [PubMed] [Google Scholar]
- 23.Zhang Y, An HS, Song S, et al. Growth factor osteogenic protein-1: differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83:515–21. doi: 10.1097/01.phm.0000130031.64343.59. [DOI] [PubMed] [Google Scholar]
- 24.Creemers LB, Jansen DC, van Veen-Reurings A, et al. Microassay for the assessment of low levels of hydroxyproline. Biotechniques. 1997;22:656–8. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- 25.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 26.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 28.Hoogendoorn RJ, Helder MN, Kroeze RJ, et al. Reproducible long-term disc degeneration in a large animal model. Spine. 2008;33:949–54. doi: 10.1097/BRS.0b013e31816c90f0. [DOI] [PubMed] [Google Scholar]
- 29.Hoogendoorn RJ, Wuisman PI, Smit TH, et al. Experimental intervertebral disc degeneration induced by chondroitinase ABC in the goat. Spine. 2007;32:1816–25. doi: 10.1097/BRS.0b013e31811ebac5. [DOI] [PubMed] [Google Scholar]
- 30.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–8. [PubMed] [Google Scholar]
- 31.Spangfort EV. The lumbar disc herniation. A computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1–95. doi: 10.3109/ort.1972.43.suppl-142.01. [DOI] [PubMed] [Google Scholar]
- 32.Gaetani P, Torre ML, Klinger M, et al. Adipose-derived stem cell therapy for intervertebral disc regeneration: an in vitro reconstructed tissue in alginate capsules. Tissue Eng Part A. 2008;14:1415–23. doi: 10.1089/ten.tea.2007.0330. [DOI] [PubMed] [Google Scholar]
- 33.Ganey THW, Moseley T, Hedrick M, Strem B, Meisel HJ. Intervertebral Disc Repair Using Adipose tissue-Derived Stem and Regenerative Cells: Experiments in a Canine Model. Proceedings for the International Society for the Study of Lumbar Spine. 2009:67. doi: 10.1097/BRS.0b013e3181a54157. [DOI] [PubMed] [Google Scholar]
- 34.Tapp H, Deepe R, Ingram JA, et al. Adipose-derived mesenchymal stem cells from the sand rat: transforming growth factor beta and 3D co-culture with human disc cells stimulate proteoglycan and collagen type I rich extracellular matrix. Arthritis Res Ther. 2008;10:R89. doi: 10.1186/ar2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei A, Chung SA, Tao H, et al. Differentiation of Rodent Bone Marrow Mesenchymal Stem Cells into Intervertebral Disc-Like Cells Following Co-Culture with Rat Disc Tissue. Tissue Eng Part A. 2009 doi: 10.1089/ten.TEA.2008.0458. [DOI] [PubMed] [Google Scholar]
- 36.Stairmand JW, Holm S, Urban JP. Factors influencing oxygen concentration gradients in the intervertebral disc. A theoretical analysis. Spine. 1991;16:444–9. doi: 10.1097/00007632-199104000-00010. [DOI] [PubMed] [Google Scholar]