Abstract
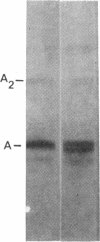
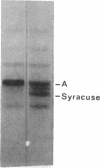
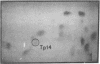
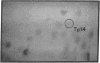
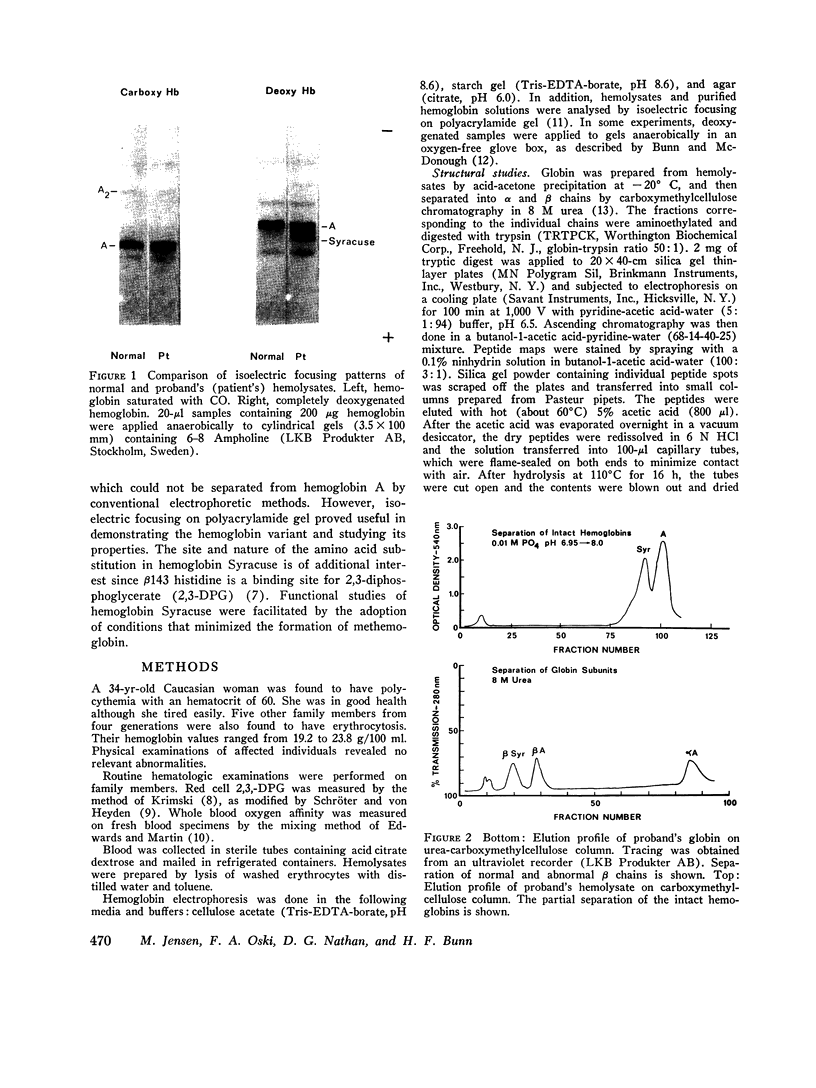
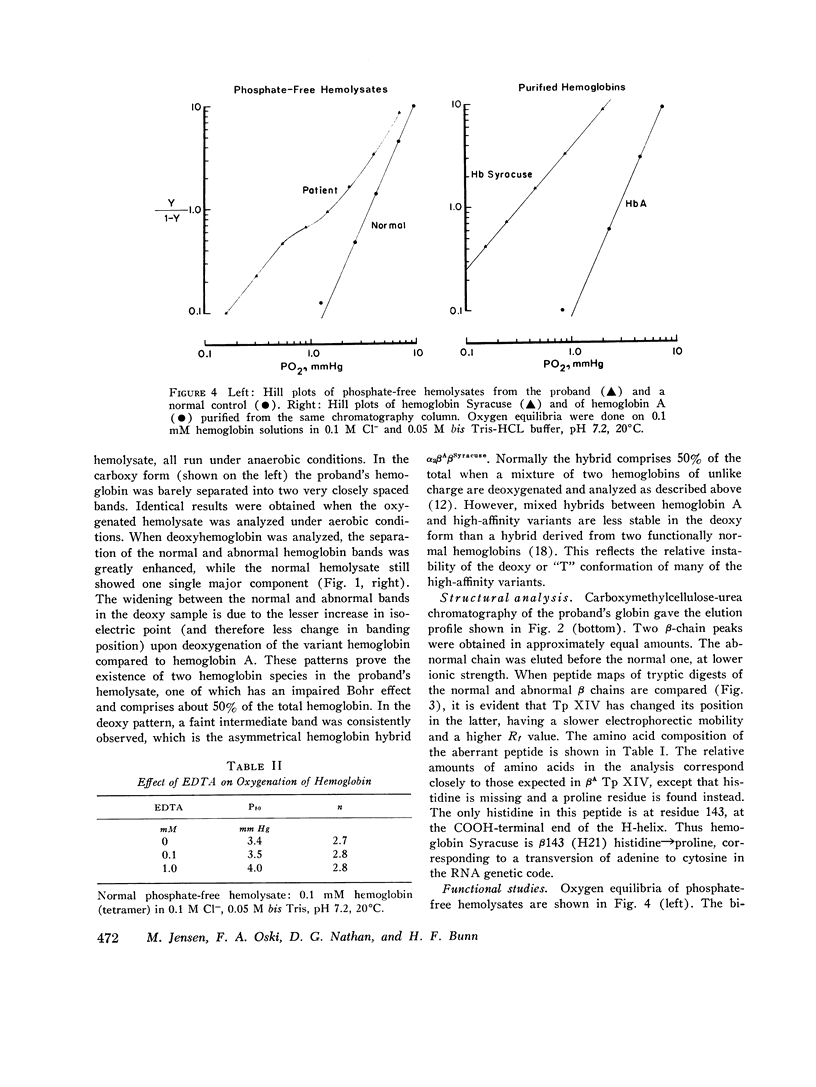
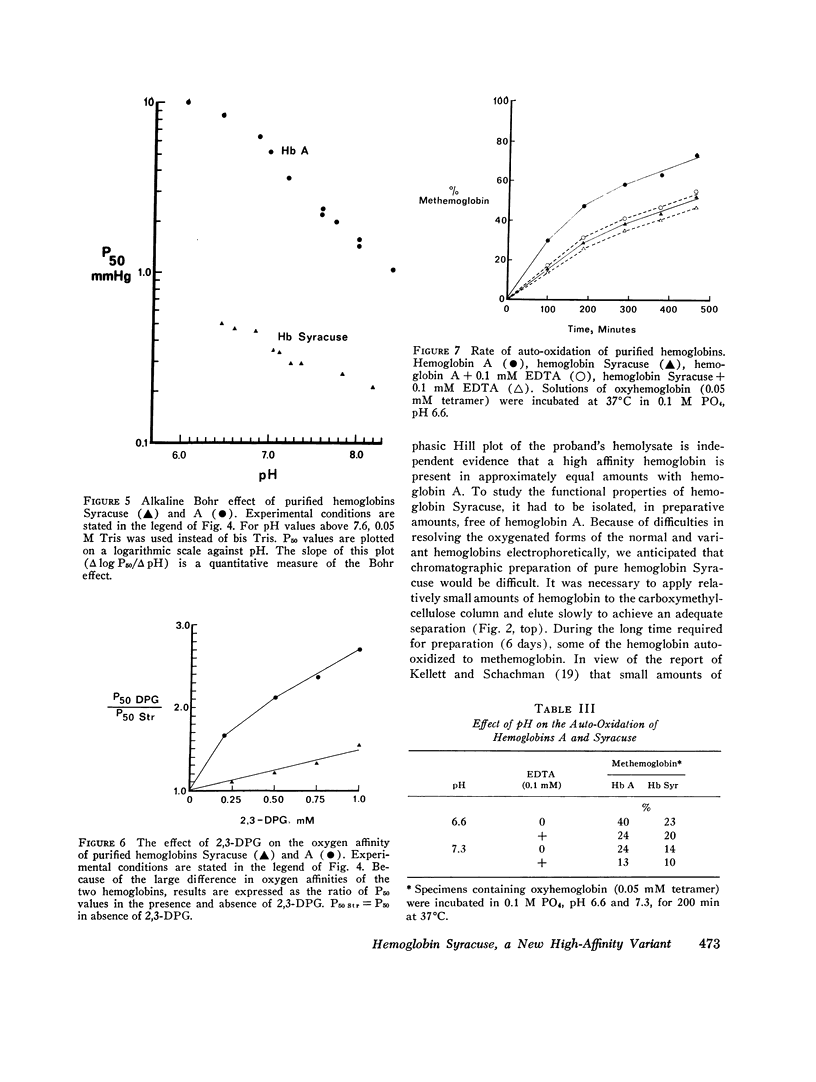
Family members from four generations were found to have polycythemia and increased whole blood O2 affinity (P50; 11 mm Hg; normal, 27 mm Hg). No abnormal hemoglobin bands were seen after electrophoresis on starch gel at pH 8.6 or agar gel at pH 6.0. Analysis of the oxygenated hemolysate by isoelectric focusing on polyacrylamide gel revealed two closely spaced bands. When deoxygenated hemolysate was analyzed in oxygen-free gels, the two components were more widely separated. About 40% of the patient's hemoglobin focused at a more acid pH than hemoglobin A, indicating a hemoglobin variant with impaired Bohr effect. Chromatography of globin in 8 M urea revealed two beta-chain peaks, the first of which was eluted at a lower buffer molarity than normal beta chain. Fingerprints of tryptic digests of the aminoethylated chains were done on silica gel thin-layer plates. Tp 14 from the abnormal beta chain had slower electrophoretic mobility and a greater Rf value. Amino acid analyses of this peptide gave values identical with those of betaTp 14, except that it contained one proline residue and no histidine. Since the one His in betaTp 14 is in position 143, hemoglobin Syracuse in alpha2beta2-143 His leads to Pro. Native Hb Syracuse could be separated from hemoglobin A on a carboxymethylcellulose column. The inclusion of 0.1 mM EDTA in the preparative buffers proved very useful in reducing the formation of methemoglobin. Oxygen equilibria of purified hemoglobin Syracuse showed high oxygen affinity (P50 value 12% that of hemoglobin A) and lack of cooperativity between subunits (Hill's n equals 1.1). The alkaline Bohr effect was about half that of hemoglobin A. The proline substitution at betaH21 disrupts the helical configuration and probably prevents the formation of salt bonds that are important in stabilizing the deoxy structure and contribute to the alkaline Bohr effect. Since beta143 His is a binding site for 2,3-diphosphoglycerate (2,3-DPG), it is not suprising that hemoglobin Syracuse had markedly impaired reactivity with 2,3-DPG. Hemoglobin Syracuse auto-oxidized more slowly than hemoglobin A, probably reflecting a slower rate of dissociation of oxygen from fully liganded hemoglobin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson J. W., Parer J. T., Stamatoyannopoulos G. Erythrocytosis associated with hemoglobin Rainier: oxygen equilibria and marrow regulation. J Clin Invest. 1969 Aug;48(8):1376–1386. doi: 10.1172/JCI106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Riggs A. Hemoglobin Kansas, a human hemoglobin with a neutral amino acid substitution and an abnormal oxygen equilibrium. J Biol Chem. 1968 Mar 10;243(5):980–991. [PubMed] [Google Scholar]
- Bromberg P. A., Alben J. O., Bare G. H., Balcerzak S. P., Jones R. T., Brimhall B., Padilla F. High oxygen affinity variant of haemoglobin Little Rock with unique properties. Nat New Biol. 1973 Jun 6;243(127):177–179. doi: 10.1038/newbio243177a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Bradley T. B., Davis W. E., Drysdale J. W., Burke J. F., Beck W. S., Laver M. B. Structural and functional studies on hemoglobin Bethesda (alpha2beta2 145His), a varient associated with compensatory erythrocytosis. J Clin Invest. 1972 Sep;51(9):2299–2309. doi: 10.1172/JCI107040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., McDonough M. Asymmetrical hemoglobin hybrids. An approach to the study of subunit interactions. Biochemistry. 1974 Feb 26;13(5):988–993. doi: 10.1021/bi00702a024. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Wohl R. C., Bradley T. B., Cooley M., Gibson Q. H. Functional properties of hemoglobin Kempsey. J Biol Chem. 1974 Dec 10;249(23):7402–7409. [PubMed] [Google Scholar]
- Charache S., Weatherall D. J., Clegg J. B. Polycythemia associated with a hemoglobinopathy. J Clin Invest. 1966 Jun;45(6):813–822. doi: 10.1172/JCI105397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Righetti P., Bunn H. F. The separation of human and animal hemoglobins by isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1971 Jan 19;229(1):42–50. doi: 10.1016/0005-2795(71)90315-1. [DOI] [PubMed] [Google Scholar]
- Edwards M. J., Martin R. J. Mixing technique for the oxygen-hemoglobin equilibrium and Bohr effect. J Appl Physiol. 1966 Nov;21(6):1898–1902. doi: 10.1152/jappl.1966.21.6.1898. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Maldonado J. E., Charache S., Boyer S. H., 4th Familial erythrocytosis due to electrophoretically undetectable hemoglobin with impaired oxygen dissociation (hemoglobin Malmö, alpha 2 beta 2 97 gln). Mayo Clin Proc. 1971 Nov;46(11):721–727. [PubMed] [Google Scholar]
- Garel M. C., Cohen-Solal M., Blouquit Y., Rosa J. A method for isolation of abnormal haemoglobins with high oxygen affinity due to a frozen quaternary r-structure: application to Hb Creteil alpha 2 A beta 2 (F5) 89 ASN. FEBS Lett. 1974 Jul 1;43(1):93–96. doi: 10.1016/0014-5793(74)81113-0. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H., Riggs A., Imamura T. Kinetic and equilibrium properties of hemoglobin Kansas. J Biol Chem. 1973 Sep 10;248(17):5976–5986. [PubMed] [Google Scholar]
- Glynn K. P., Penner J. A., Smith J. R., Rucknagel D. L. Familial erythrocytosis. A description of three families, one with hemoglobin Ypsilanti. Ann Intern Med. 1968 Oct;69(4):769–776. doi: 10.7326/0003-4819-69-4-769. [DOI] [PubMed] [Google Scholar]
- Hamilton H. B., Iuchi I., Miyaji T., Shibata S. Hemoglobin Hiroshima (beta-143 histidine--aspartic acid): a newly identified fast moving beta chain variant associated with increased oxygen affinity and compensatory erythremia. J Clin Invest. 1969 Mar;48(3):525–535. doi: 10.1172/JCI106010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Stamatoyannopoulos G., Yoshida A., Adamson J. Haemoglobin Rainier: beta-145 (HC2) tyrosine leads to cysteine and haemoglobin Bethesda: beta-145 (HC2) tyrosine leads to histidine. Nat New Biol. 1971 Apr 28;230(17):264–267. doi: 10.1038/newbio230264a0. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Berglund S. Thin-layer isoelectric focusing for haemoglobin screening and its application to haemoglobin Malmö. Clin Chim Acta. 1972 Aug;40(1):153–158. doi: 10.1016/0009-8981(72)90263-x. [DOI] [PubMed] [Google Scholar]
- Jones R. T., Osgood E. E., Brimhall B., Koler R. D. Hemoglobin Yakina. I. Clinical and biochemical studies. J Clin Invest. 1967 Nov;46(11):1840–1847. doi: 10.1172/JCI105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett G. L., Schachman H. K. Dissociation of hemoglobin into subunits. Monomer formation and the influence of ligands. J Mol Biol. 1971 Aug 14;59(3):387–399. doi: 10.1016/0022-2836(71)90306-8. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Interaction of hemoglobin with hydrogen ions, carbon dioxide, and organic phosphates. Physiol Rev. 1973 Oct;53(4):836–890. doi: 10.1152/physrev.1973.53.4.836. [DOI] [PubMed] [Google Scholar]
- Lokich J. J., Moloney W. C., Bunn H. F., Bruckheimer S. M., Ranney H. M. Hemoglobin brigham (alpha2Abeta2100 Pro--Leu). Hemoglobin variant associated with familial erythrocytosis. J Clin Invest. 1973 Aug;52(8):2060–2067. doi: 10.1172/JCI107390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Gibson Q. H., Charache S. Relation between structure and function in Hemoglobin Chesapeake. Biochemistry. 1967 Aug;6(8):2395–2402. doi: 10.1021/bi00860a015. [DOI] [PubMed] [Google Scholar]
- Nute P. E., Stamatoyannopoulos G., Hermodson M. A., Roth D. Hemoglobinopathic erythrocytosis due to a new electrophoretically silent variant, hemoglobin San Diego (beta109 (G11)val--met). J Clin Invest. 1974 Jan;53(1):320–328. doi: 10.1172/JCI107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. M. Isoelectric focusing and the study of interacting protein systems: ligand binding, phosphate binding, and subunit exchange in hemoglobin. Ann N Y Acad Sci. 1973 Jun 15;209:237–257. doi: 10.1111/j.1749-6632.1973.tb47532.x. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Reed C. S., Hampson R., Gordon S., Jones R. T., Novy M. J., Brimhall B., Edwards M. J., Koler R. D. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood. 1968 May;31(5):623–632. [PubMed] [Google Scholar]
- Rifkind J. M. Copper and the autoxidation of hemoglobin. Biochemistry. 1974 Jun 4;13(12):2475–2481. doi: 10.1021/bi00709a003. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Nute P. E., Adamson J. W., Bellingham A. J., Funk D. Hemoglobin olympia ( 20 valine leads to methionine): an electrophoretically silent variant associated with high oxygen affinity and erythrocytosis. J Clin Invest. 1973 Feb;52(2):342–349. doi: 10.1172/JCI107190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. J., Maxwell J. C., Caughey W. S. The mechanisms of hemoglobin autoxidation. Evidence for proton-assisted nucleophilic displacement of superoxide by anions. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1104–1110. doi: 10.1016/0006-291x(74)90810-9. [DOI] [PubMed] [Google Scholar]
- White J. M., Szur L., Gillies I. D., Lorkin P. A., Lehmann H. Familial polycythaemia caused by a new haemoglobin variant: Hb Heathrow, beta 103 (G5) phenylalanine leads to leucine. Br Med J. 1973 Sep 29;3(5882):665–667. doi: 10.1136/bmj.3.5882.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak S. J., Brimhall B., Jones R. T., Kaplan M. E. Hemoglobin Andrew-Minneapolis alpha 2 A beta 2 144 Lys leads to Asn: a new high-oxygen-affinity mutant human hemoglobin. Blood. 1974 Oct;44(4):543–549. [PubMed] [Google Scholar]