Abstract
Introduction
In the last few years, various studies have underlined a correlation between thyroid function and male sexual function, hypothesizing a direct action of thyroid hormones on the penis.
Aim
To study the spatiotemporal distribution of mRNA for the thyroid hormone nuclear receptors (TR) α1, α2 and β in the penis and smooth muscle cells (SMCs) of the corpora cavernosa of rats and humans during development.
Methods
We used several molecular biology techniques to study the TR expression in whole tissues or primary cultures from human and rodent penile tissues of different ages.
Main Outcome Measure
We measured our data by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) amplification, Northern blot and immunohistochemistry.
Results
We found that TRα1 and TRα2 are both expressed in the penis and in SMCs during ontogenesis without development-dependent changes. However, in the rodent model, TRβ shows an increase from 3 to 6 days post natum (dpn) to 20 dpn, remaining high in adulthood. The same expression profile was observed in humans. While the expression of TRβ is strictly regulated by development, TRα1 is the principal isoform present in corpora cavernosa, suggesting its importance in SMC function. These results have been confirmed by immunohistochemistry localization in SMCs and endothelial cells of the corpora cavernosa.
Conclusions
The presence of TRs in the penis provides the biological basis for the direct action of thyroid hormones on this organ. Given this evidence, physicians would be advised to investigate sexual function in men with thyroid disorders. Carosa E, Di Sante S, Rossi S, Castri A, D'Adamo F, Gravina GL, Ronchi P, Kostrouch Z, Dolci S, Lenzi A, and Jannini EA. Ontogenetic profile of the expression of thyroid hormone receptors in rat and human corpora cavernosa of the penis. J Sex Med 2010;7:1381–1390.
Keywords: Corpora Cavernosa, Thyroid Hormone Receptor, Erectile Dysfunction, Thyroid Disorders and Sexual Dysfunction
Introduction
Thyroid hormone receptors (TRs) are part of the nuclear receptor superfamily. TRα and TRβ are the products of two distinct genes that are further differentially spliced into TRα1, TRα2[1,2], TRβ1, and TRβ2[3,4]. TRα1 and TRβ1 are widely expressed and act as thyroid hormone-dependent transcription factors, inducing or repressing gene expression in response to triiodothyronine (T3). The differentially spliced product TRα2 does not bind the hormone and exerts a dominant negative effect on the action of other TRs[5,6]. The TR α and β forms are expressed in a distinct but often overlapping pattern, suggesting that they may mediate both individual and common functions. Several specific functions of single TR isoforms have been identified using knockout mice for TRα and/or TRβ. In fact, the importance of TRα1 in post-natal development and cardiovascular functions is well known [7–10], whereas TRβ2 is fundamental in the regulation of pituitary–thyroid axis development [11,12]. The influence of thyroid hormones on mating behavior has also been investigated in knockout mice. TRα1−/− animals showed hyposexual behavior, while TRβ−/− mice showed significantly enhanced sexual behavior [13].
Male reproductive and sexual function appears to be controlled by thyroid hormones in both animals and humans [9,14]. Although this area has been neglected in the past, various studies over the past few years have underlined a correlation between thyroid function and male sexual function. Premature ejaculation and erectile dysfunction (ED) are frequent findings in thyroid disease [15–19], suggesting the direct involvement of thyroid hormones in the physiology of male sexual function. However, despite the now well-established association between thyroid function and male sexual activity, there is a dearth of research exploring the locoregional expression of TRs in the penis. For this reason, we studied the expression pattern of the individual isoforms of TRs in rat and human penis during the development.
Materials and Methods
Animals
Male Wistar rats were reared in our institute's animal facilities, and all experimental protocols were approved by the local ethics committee. The penises from rats of different ages [3–6, 20, 60, and 300 days post natum (dpn)] were excised and separated from the cutis and preputial glands and used for cell cultures, for RNA extraction (stored in liquid N2), or fixed for immunohistochemistry. We used, in separate experiments, sexually immature (under 30 days old) and adult (until 300 days old) male rats [20]; we also focused our attention on peripubertal rats (20 days old), where androgen receptor is expressed in corporal cavernosa SMCs [21].
Patients
After protocol approval by the local Clinical Investigation Committee, human corpora cavernosa were obtained from three impotent men (age 71 ± 10.4) at the time of penile prosthesis implantation. After surgery, the corpora cavernosa were immediately used for cell culture preparation or frozen in liquid N2 for RNA extraction [22].
Immunohistochemistry
Penises from 60-day-old animals were fixed in 4% paraformaldehyde in PBS (140 mM NaCl, 50 mM phosphate buffer pH 7.2) and embedded in paraffin. Immunostaining was carried out on 4-µm thick sections of 60-day-old rat penis. Sections were deparaffinized in xylene and rehydrated (in isopropylalcohol and ethanol–water). Next, sections were boiled in Target Retrieval Solution High pH (Dako Cytomation, Carpinteria, CA, USA) for 40 minutes at 95°C in a water bath, and then the sections were left for 20 minutes on bench for temperature equilibration. Next, the sections were placed in deionized water. The endogenous peroxidase was blocked in the water solution of hydrogen peroxide (3%) containing 0.1% sodium azide for 30′ and again transferred to water. The TRα1 monoclonal antibody was a kind gift from Dr. Onno Bakker [23]. The primary antibody was diluted in Dako Real, Antibody Diluent at 1:100 dilution. The sections were incubated with the antibody overnight at 4°C. After washing in TBS (50 mM Tris, 150 mM NaCl, pH 7.6) (three times for 3 minutes each), the sections were incubated with a secondary antibody for 30 minutes at room temperature (using the EnVision System HRP from Dako). Finally, the sections were washed again in TBS (three times for 3 minutes), and the antibody was visualized using the DAB Dako Liquid DAB + Substrate Chromogen System (Dako) (5 minutes incubation). Sections were washed shortly afterwards with water. In some cases, nuclei were controstained using Harris Hematoxylin. Sections were dehydrated in alcohol and xylen, then mounted in Solacryl (Penta, Prague, Czech Republic). Slides were observed in Olympus BX60 microscope (Center Valley, PA, USA) equipped with the DP30 CCD camera (Olympus, Center Valley, PA, USA).
Corpus Cavernosum Smooth Muscle Cell Cultures
Primary cultures of rat corpus cavernosum smooth muscle cells were obtained by modifying the method used by Krall et al. [24]. Briefly, small pieces of corpora cavernosa (about 1 mm square) were cut from penises excised from rats of different ages and/or from human corpora cavernosa. Sterile forceps were used to press the fragments onto the bottom of cell culture wells containing Dulbecco's Modified Essential Medium (D-MEM) (Gibco/Invitrogen, Carlsbad, CA, USA) + 20% Fetal Bovine Serum (FBS; Sigma, St. Louis, MO, USA), 2 mmol/L glutamine (Gibco), 100 IU/mL penicillin, and 100 µg/mL Streptomycin (Gibco). The fragments were incubated undisturbed for 4–6 days at 37°C in fully humidified atmosphere with 5% CO2. After 4–6 days, the culture medium was replaced with fresh medium. The cultures were incubated undisturbed until 50% confluence was reached, when the remaining tissue fragments were removed and the medium changed. When the cultures were confluent, the cells were split 1:3 and plated in D-MEM + 10% FBS. This transfer procedure was repeated for subsequent passages. With this method, we obtained a culture of smooth muscle cells from 3 (rCC3) and 20 (rCC20) dpn rat corpora cavernosa and from human corpora cavernosa (hAdult CC). We characterized rat primary cultures by immunostaining with an antibody anti-α-smooth muscle actin and with an antibody against the endothelial-specific marker CD31, as previously described in Carosa et al. [21]. Endothelial cell contamination was less than 10% [21]. We also characterized the human culture with immunostaining with anti-α-smooth muscle actin antibody (data not shown). These cells were used within the 10th passage.
RNA Extraction and Northern Blot
Total RNA was prepared by homogenization from rat penis, human corpora cavernosa and corpus cavernosum cells from different aged rats or humans and extracted with RNeasy Kit with on-column deoxyribonuclease (DNase) digestion (Qiagen, Milan, Italy). RNA purity and integrity was checked spectroscopically and by gel electrophoresis. Human fetal penile smooth muscle cells (hfPSMC) were kindly provided by Prof. Maggi (University of Florence, Italy). hfPSMC were cultured as described in Granchi et al. [25] and used for the RNA preparation.
For Northern blot analysis, RNA samples (20 µg) were denatured, separated on 1% formaldehyde–agarose gel, transferred on nylon membranes (Amersham—GE Healthcare Technologies, Milan, Italy), and probed at high stringency with the [32] P-labeled rat TR cDNAs. Specifically, a 442 bp fragment obtained from PCR amplification of TRβ plasmid [26] using TRβ up (5′-CAA TCA CCA GAG TGG TGG ATT TCG CCA-3′) as the upstream primer and TRβ 1574do (5′-ATC CGC AGA TCT GTC ACC TT-3′) as the downstream primer was used to probe the presence of the TRβ. The filter was then hybridized with the full-length cDNA fragment for TRα[26]. Normalization was obtained by hybridizing the filter with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. The probes used for hybridization of human RNA are described in Jannini et al. [27].
Hybridization was carried out in QuikHyb (Stratagene, La Jolla, CA, USA) as recommended by the manufacturer. Autoradiograms were analyzed densitometrically, and the results were expressed as arbitrary units of optical densities. The densitometric analysis was performed using the ImageJ 1.25s program (NIH, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/).
Semiquantitative RT-PCR Analysis
First strand complementary DNA was produced using 2 µg of total RNA for each sample, in the presence of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and poly-d(T)12-18 primer (Invitrogen). The cDNA thus obtained was used as template for PCR amplification of the TRs (Table 1) end GAPDH (GAPDHup 5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′ and GAPDHdo 5′-CAT GTA GGC CAT GAG GTC CAC CAC-3′). GAPDH was used as an internal control to analyze RNA integrity and quantity. The PCR was performed in the presence of Go-Taq DNA polymerase (Promega, Madison, WI, USA; 2.5 units per reaction) in the Px 2 thermal cycler (Thermo Electron Corporation, Basingstoke, UK) for 30 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute for TRs amplification, and 20 cycles at 94°C for 45 seconds, 55°C for 45 seconds and 72°C for 45 seconds for GAPDH amplification. Genomic contamination was excluded by running the PCR without RT and drawing the primers in different exons. The amplified fragments were separated in 1% agarose gel and acquired using the microDOC Gel Documentation System (Cleaver Scientific Ltd, Rugby, Warwickshire, UK). The densitometric analysis was performed using the ImageJ 1.25s program (NIH).
Table 1.
Oligo sequences for TRs amplification
| Gene name | Accession number | Oligo name | Sequence | Fragment length (bp) |
|---|---|---|---|---|
| TRα1 | NM_199334 for human and NM_001017960 for rat | TRα common | 5′-GCG TAA GCT GAT TGA GCA GA-3′ | 758 |
| TRα1do | 5′-CCT CAA AGA CCT CGA GGA AG-3 | |||
| TRα2 | NM_003250for human and NM_031134 for rat | TRα common | 5′-GCG TAA GCT GAT TGA GCA GA-3′ | 824 |
| TRα2do | 5′-GAA CAA CAT GCA TTC CGA GA-3′ | |||
| TRβ | NM_000461for human and NM_012672 for rat | TRβ936up | 5′-GGA ATG GGA GCT CAT CAA AAC-3′ | 639 |
| TRβ1574 | 5′-ATC CGC AGA TCT GTC ACC TT-3′ |
Statistical Analysis
Continuous variables were presented as a mean and standard deviation (SD) and analyzed using Student's t-test for unpaired data. All statistical tests were two-tailed. A P value of <0.05 was considered as statistically significant. The results represent a mean of at least three separate experiments.
Results
Thyroid hormone receptor expression and development-related regulation was evaluated in rat corpora cavernosa by semiquantitative RT-PCR analysis of the penises of rats of different ages. Total RNA extracted from the penises of 4-, 20-, 60-, and 300-day-old rats was amplified using specific primers for TRα1, TRα2, and TRβ1. As shown in Figure 1, the two products of differential splicing TRα1 and TRα2 were both expressed without any significant age-related difference. In contrast, the expression of TRβ1 increased 6.6 times (3.62 ± 1.15 vs. 23.92 ± 7.81 a.u.; P = 0.043) from perinatal rat penis to puberty, remaining high during adulthood (Figure 1). The level of TRα2 was higher than TRα1 in all ages studied, with a constant α2/α1 ratio of 1.88 ± 0.34, demonstrating no changes in the differential splicing machinery.
Figure 1.
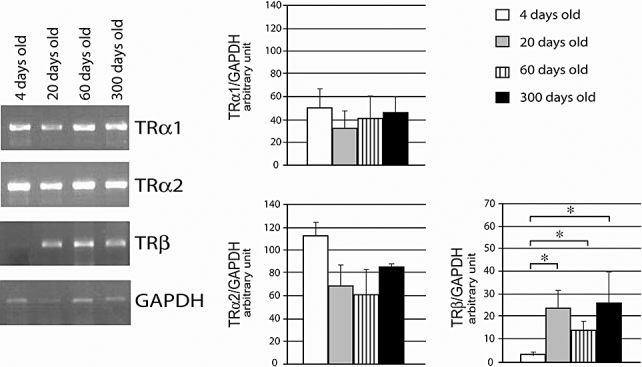
RT–PCR analysis of TRα1, TRα2, and TRβ expression in penises from rats of different ages. The PCR products are derived from total RNA from rat penises of 4, 20, 60, and 300 dpn rats using specific primers for TRs and GAPDH. GAPDH mRNA amplification was performed to verify the integrity of the extracted total RNA. Densitometric evaluation of TRs mRNA expression over the GAPDH housekeeping gene was obtained from band intensity of RT-PCR products. The densitometric analysis of TRα1, TRα2, and TRβ from three independent experiments ± SD (*P < 0.05) is shown.
To analyze the expression pattern of all TR isoforms in more detail, Northern blot experiments were performed on total RNA extracted from whole penises. The full-length cDNA fragment of TRα1, containing the common sequence of TRα1 and TRα2, was used to probe total RNA from the penises of 3–6-, 20-, and 60-day-old rats. The 5.5 kb band signal corresponding to α1 and the 2.8 kb band corresponding to α2 were both present in all samples (Figure 2), with no significant age-related difference. The levels of TRα2 were higher than of TRα1 in all ages studied and the ratio between them was similar to that found with RT-PCR (1.96 ± 0.02). This is in the agreement with the absence of development-related regulation. The same membranes were rehybridized with a PCR fragment containing 442 bp of TRβ1 (Figure 2). A 6.0 kb band corresponding to TRβ1 showed a sharp increase from perinatal to peripubertal age: a very faint band was seen in 3–6 day old rats, increasing threefold in rats of 20 dpn (0.41 ± 0.20 vs. 1.27 ± 0.32 a.u.; P = 0.024). The levels of TRβ1 remained high in the penises of adult, 60-day-old, rats (0.85 ± 0.17 a.u.), further confirming the RT-PCR data. Hybridization to an GAPDH cDNA probe was used for normalization in order to estimate the relative amount of each TR isoform present at different ages.
Figure 2.
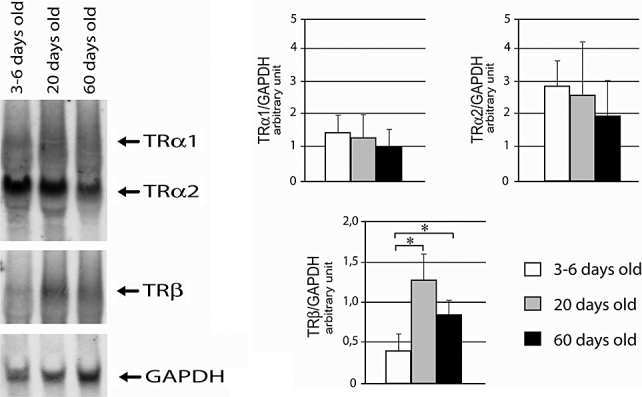
Expression of mRNA from various TR isoforms in rat penis during development and adulthood. Representative Northern blot analysis of 20 µg of total RNA extracted from rat penises of 3–6, 20, and 60 dpn animals. Samples were separated in denaturing gel, blotted on nylon filter, and hybridized with the cDNA fragments corresponding to the indicated TRs or GAPDH. Densitometric evaluation of TRs mRNA expression over the GAPDH housekeeping gene was obtained from band intensity. The densitometric analysis of TRα1, TRα2, and TRβ from three independent experiments ± SD (*P < 0.05) is shown.
For TR localization, we performed immunohistochemistry experiments using an anti-TRα1 antibody. Widespread staining for this TR isoform was present in both endothelial and muscular cells of the corpora cavernosa (Figure 3B). A similar expression pattern was found in the corpus spongiosum, with TRα1 abundantly expressed in both muscular muscle and endothelial cells (Figure 3D).
Figure 3.
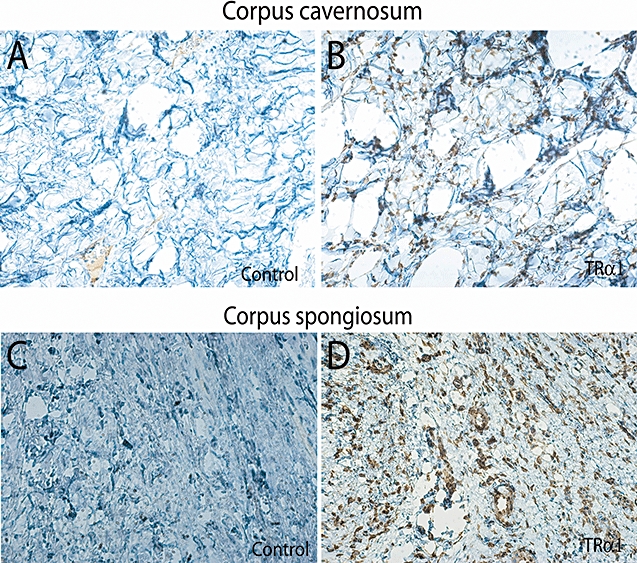
Localization of TRα1 obtained by immunohistochemistry experiment of histological sections of adult (60 dpn) rat penis. A and C represent the negative control. B and D are stained with the anti-TRα1 antibody (original magnification: 20×).
As ED in thyroid diseases has been postulated to be due to a direct effect of thyroid hormones on SMCs [17,18], we focused our attention on these cells. We used SMCs obtained from the penises of 3- (rCC3) and 20-day-old (rCC20) rats (Figure 4), evaluating the presence of TRα1 and TRα2 mRNAs by RT-PCR. TRα1 and TRα2 levels remained unchanged, as did the α2:α1 ratio, which was almost the same as that observed for the whole penis (1.51 ± 0.09). As expected, a low level of TRβ1 was found in SMCs from the corpora cavernosa of perinatal rats, increasing 2.7-fold in peripubertal rats, showing the same expression pattern as seen in penile tissues (9.45 ± 5.81 vs. 25.94 ± 10.71 a.u.; P = 0.046) (Figure 4).
Figure 4.
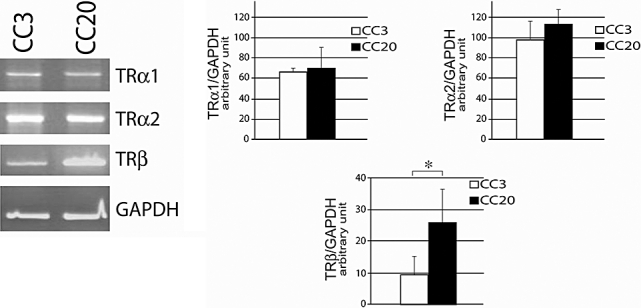
RT–PCR analysis of TRα1, TRα2, and TRβ expression in corpus cavernosum SMCs from rats of different ages. The products are derived from total RNA from rat corpus cavernosum SMCs from 3 (CC3) and 20 (CC20) dpn rats using specific primers for various TRs and GAPDH. GAPDH mRNA amplification was performed to verify the integrity of the extracted total RNA. Densitometric evaluation of TRs mRNA expression over the GAPDH housekeeping gene was obtained from band intensity of RT-PCR products. The histograms are calculated after normalization on GAPDH and on the basis of the amount of volume of PCR amplification loaded in the gel (1/5 of volume for TRα1 and TRα2, and 4/5 for the TRβ isoform). The densitometric analysis of TRα1, TRα2, and TRβ from three independent experiments ± SD (*P < 0.05) is shown.
The expression of all TRs was also studied in human corpora cavernosa by Northern blot (Figure 5A), confirming the presence of all these forms and isoforms in the adult penis. RT-PCR was then used to analyze the presence of TRs in hfPSMC cells from humans fetus (cell line) and in hAdultCC cells obtained from patients undergoing prosthesis implantation surgery (primary cultures). Total RNA obtained from hfPSMC and hAdultCC cells was amplified with the same oligomers used to study TRs expression in rat tissues (Table 1) in fact, the oligomers were designed in receptor regions found to have almost 98% shared identity between human and rat. As observed in rats, all TR isoforms were found in hAdultCC cells, with the α2 isoform predominant, with an α2/α1 ratio of 1.28 ± 0.01 (Figure 5). Both α isoforms were present in hfPMSC cells, with greater α1 than α2 expression (α2/α1 ratio 0.87 ± 0.01). TRβ1 expression increased between fetal and adult corpora cavernosa cells (Figure 5) (0.026 ± 0.021 vs. 0.71 ± 0.07 a.u.; P = 0.0027), reproducing the same ontogenetic profile as observed in rats.
Figure 5.
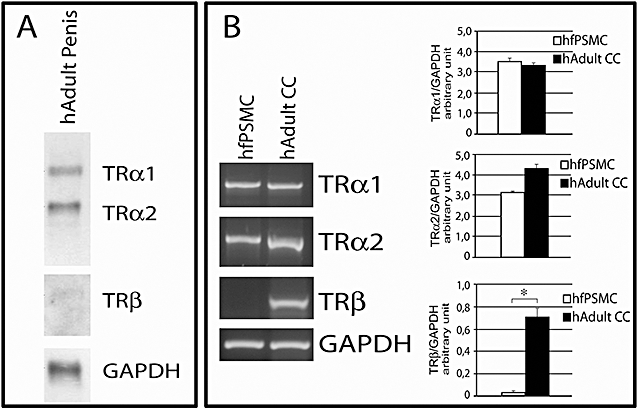
(A) Expression of mRNA from various TR isoforms in adult human corpora cavernosa. Representative Northern blot analysis of 20 µg of total RNA extracted from the penis of a 65-year-old man. Samples were separated in denaturing gel, blotted on nylon filter, and hybridized with the cDNA fragments corresponding to the indicated TRs or GAPDH probe to assess their integrity and concentration. (B) RT–PCR analysis of TRα1, TRα2, and TRβ expression in human corpus cavernosum SMCs. The products are derived from total RNA from human fetal (hfPSMC) and adult (hAdutCC) corpus cavernosum SMCs using specific primers for various TRs and GAPDH. GAPDH mRNA amplification was performed to verify the integrity of the extracted total RNA. Densitometric evaluation of TRs mRNA expression over the GAPDH housekeeping gene was obtained from band intensity of RT-PCR products. The histograms are calculated on the basis of the amount of volume of PCR amplification loaded in the gel (1/5 of volume for TRα1 and TRα2, and 4/5 for the TRβ isoform). The densitometric analysis of TRα1, TRα2, and TRβ from three independent experiments ± SD (*P < 0.05) is shown.
Discussion
In this study, we demonstrate for the first time the presence of TRα1, TRα2, and TRβ in rat and human corpora cavernosa. Although TR expression in animal [28–32] and human [9,27] male genital tissues is dramatically regulated by development, we were unable to demonstrate this in the penis. In fact, we found no difference in penile expression of TRα1 and TRα2 mRNA at any studied age. However, TRβ1 mRNA did increase from the perinatal to adult age. The same expression pattern was observed with both RT-PCR and Northern blot experiments. The absence of an absolute quantification of TRs expression is a limitation of this study, but the complete parallelism between RT-PCR, Northern blot, and immunohistochemistry analysis gives consistency to our data.
In the primary cultures of cells obtained from rat corpora cavernosa of different ages, the expression of α1, α2 and β showed the same ontogenetic pattern, with similar levels of TRα1 and TRα2 in cells from both perinatal and prepubertal animals. This would seem to make TRα regulation in the penis by steroid hormones very unlikely. In contrast, the β1 form is expressed at low levels in perinatal rats and increases in peripubertal rats, suggesting possible regulation by androgens; this is currently under exploration in our laboratories. However, the importance of TRβ1 in the corpora cavernosa is unclear. In fact, while the TRα1 gene is widely expressed from early developmental stages, the TRβ gene is restricted until late embryogenesis, when it is induced in brain, pituitary and other tissues [33–35]. Furthermore, the β form is virtually absent in other male genital tissues, such as the testis [9]. On the other hand, while adult hyper- and hypothyroidism have some variable effects on gonadotropin secretion, testosterone transportation and testicular function [9], it is unlikely that the effect of thyroid diseases on sexual function can be solely due to androgen derangement.
The existence of two distinct genes encoding TRs suggests that both are important for the T3-signaling pathway. However, the specific function of each form is not known, as they are often expressed with an overlapping pattern. TR knockout experiments have provided only a few hints of the function of a specific receptor, such as the importance of TRα1 on cardiac function or the development of the small intestine [8,10], or TRβ1 in development of the ear [12]. Further studies are needed to explore the importance of TRs in the contraction of corpora cavernosa SMCs, which is essential for male sexual and reproductive function.
Finally, we analyzed the expression of TRα1, TRα2 and TRβ in human SMCs from adult and fetal penis. All three forms are present in the adult corpora cavernosa; however, although TRα1 and TRα2 are expressed at the same level as in adults in human fetal corpus cavernosum cells, the β form was almost absent. Although these data have been obtained in tissues characterized by vasculogenic impotence, we observed the same ontogenetic regulation in both rats and humans.
Several studies have underlined a correlation between thyroid function and male sexual function. In fact, a high prevalence of premature ejaculation has been found in hyperthyroid patients, whereas in hypothyroid subjects the main sexual complaint is delayed ejaculation [16,17,36]. While evidence of hypo- or hyperthyroidism is relatively rare in ED [15], we were the first to demonstrate that ED is a frequent symptom of male thyroid disease [17]. This result has been fully replicated by other researchers [18,19]. Both ejaculatory disorders and ED revert after achievement of euthyroidism. This suggests the direct involvement of thyroid hormones in the physiology of male sexual function.
Penile erection is largely due to the relaxation of SMCs within the corpora cavernosa. For this reason, we used immunohistochemistry to evaluate the cellular expression and localization of the TR prevalently expressed in the penis, the α1 isoform. Despite same limitations (data obtained only from 60-day-old animals), TRα1 has been demonstrated to be present in both endothelial cells and SMCs inside the corpora cavernosa and spongiosa, underlying its importance in SMCs contraction. The fact that we analyzed only TRα1 at protein level is another limitation of our study. However, the importance of the α isoforms is also confirmed by the fact that the α2/α1 ratio remains constant. In fact, it has been demonstrated that the alterations described in TRα2−/− mice are correlated with the modification of the α2/α1 ratio. Moreover, it seems that the tissue-specific difference in thyroid hormone responsiveness may depend on the relative amount of TRα1 expressed in each tissue [37].
The role of thyroid hormones in vascular function has been largely studied, but it is still not clear how thyroid hormones can impair relaxation of corpora cavernosa SMCs. In rabbits, hyperthyroidism impairs both the neurogenic and endothelium-dependent relaxation of corpus cavernosum smooth muscle. Alteration in SMCs function can be accompanied by adaptive changes in the smooth muscle contractile system. In a study on rabbits treated with thyroxine, Giuriato et al. [38] reported intimal thickening and up-regulation of non-muscle myosin in the aorta. It has been found that the thyroid state influences the density of α- and β-adrenoreceptors in smooth muscle, correlated with changed responses to catecholamines [39]. Also, hyperthyroidism leads to increased acetylcholine- and potassium chloride-induced contractions of urinary bladder strips [40] and increased acetylcholine-mediated relaxation of blood vessels [41]. Lofgren et al. [42] demonstrated that thyroid hormone treatment alters in vivo the isoform composition of myosin in fast and slow smooth muscles and that this change is sufficient to modify SMCs function. Studies of vascular smooth muscle (VSM) cells demonstrated the presence of iodothyronine deiodinase type II [43], suggesting that SMCs are a direct target for the actions of thyroid hormones. The identification of TR in both aortic and coronary VSM indicates the classic genomic action of T3 in these cells [44]. The effect of thyroid hormones on SMC also is also evident from studies of cardiac function. The direct effect of iodothyronines on cardiac myocytes, as well as its effects on peripheral vasculature, have also been demonstrated; in fact, the high systemic vascular resistance observed in patients with hypothyroidism is rapidly reversed with thyroid hormone treatment [45]. Moreover, studies using vascular smooth muscle cells isolated from rat aorta and cultured on a deformable matrix have demonstrated that exposure to T3 is able to relax these cells [44].
The demonstration of the presence of TRs in SMCs, together with the numerous observations of ED and/or ejaculatory disorders in thyroid conditions, support the hypothesis of a direct effect of thyroid hormones on corpus cavernosum SMCs. The presence of TRs in these cells provides further evidence for this theory. In light of our results, the need for physicians to investigate the sexual function in men with thyroid disorders is increasingly important.
Acknowledgments
This paper is partially supported by a PRIN grant and by an unrestricted Pfizer Italia grant. Zdenek Kostrouch was supported by the Grant 0021620806 from the Ministry of Education, Youth and Sports of the Czech Republic. We are in debt with Dr Ms. Marie-Hélène Hayles and Rosaria Caruso for English. Authors thank Mrs. Marie Kolářová (Charles University, Prague, Czech Republic) for kind help with immunohistochemistry.
Conflict of Interest
None.
Statement of Authorship
Category 1
-
Conception and Design
Eleonora Carosa; Andrea Lenzi; Emmanuele A. Jannini
-
Acquisition of Data
Eleonora Carosa; Stefania Di Sante; Simona Rossi; Alessandra Castri; Fabio D'Adamo; Piero Ronchi; Zdenek Kostrouch
-
Analysis and Interpretation of Data
Giovanni Luca Gravina; Susanna Dolci
Category 2
-
Drafting the Article
Eleonora Carosa; Giovanni Luca Gravina; Emmanuele A. Jannini
-
Revising It for Intellectual Content
Andrea Lenzi; Emmanuele A. Jannini
Category 3
-
Final Approval of the Completed Article
Eleonora Carosa; Andrea Lenz; Emmanuele A. Jannini
References
- 1.Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–40. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986;324:641–6. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 3.Lazar MA, Hodin RA, Darling DS, Chin WW. Identification of a rat c-erbA alpha-related protein which binds deoxyribonucleic acid but does not bind thyroid hormone. Mol Endocrinol. 1988;2:893–901. doi: 10.1210/mend-2-10-893. [DOI] [PubMed] [Google Scholar]
- 4.Mitsuhashi T, Tennyson G, Nikodem V. Nucleotide sequence of novel cDNAs generated by alternative splicing of a rat thyroid hormone receptor gene transcript. Nucleic Acids Res. 1988;16:5697. doi: 10.1093/nar/16.12.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989;337:659–61. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- 6.Katz D, Reginato MJ, Lazar MA. Functional regulation of thyroid hormone receptor variant TR alpha 2 by phosphorylation. Mol Cell Biol. 1995;15:2341–8. doi: 10.1128/mcb.15.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraichard A, Chassande O, Plateroti M, Roux JP, Trouillas J, Dehay C, Legrand C, Gauthier K, Kedinger M, Malaval L, Rousset B, Samarut J. The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. Embo J. 1997;16:4412–20. doi: 10.1093/emboj/16.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. Embo J. 1998;17:455–61. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jannini EA, Ulisse S, D'Armiento M. Thyroid hormone and male gonadal function. Endocr Rev. 1995;16:443–59. doi: 10.1210/edrv-16-4-443. [DOI] [PubMed] [Google Scholar]
- 10.O'Shea PJ, Williams GR. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol. 2002;175:553–70. doi: 10.1677/joe.0.1750553. [DOI] [PubMed] [Google Scholar]
- 11.Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: Evidence for tissue-specific modulation of receptor function. Embo J. 1996;15:3006–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Abel ED, Boers ME, Pazos-Moura C, Moura E, Kaulbach H, Zakaria M, Lowell B, Radovick S, Liberman MC, Wondisford F. Divergent roles for thyroid hormone receptor beta isoforms in the endocrine axis and auditory system. J Clin Invest. 1999;104:291–300. doi: 10.1172/JCI6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellovade TL, Chan J, Vennstrom B, Forrest D, Pfaff DW. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nat Neurosci. 2000;3:472–5. doi: 10.1038/74846. [DOI] [PubMed] [Google Scholar]
- 14.Veronelli A, Mauri C, Zecchini B, Peca MG, Turri O, Valitutti MT, dall’Asta C, Pontiroli AE. Sexual dysfunction is frequent in premenopausal women with diabetes, obesity, and hypothyroidism, and correlates with markers of increased cardiovascular risk. A preliminary report. J Sex Med. 2009;6:1561–8. doi: 10.1111/j.1743-6109.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- 15.Slag MF, Morley JE, Elson MK, Trence DL, Nelson CJ, Nelson AE, Kinlaw WB, Beyer HS, Nuttall FQ, Shafer RB. Impotence in medical clinic outpatients. JAMA. 1983;249:1736–40. [PubMed] [Google Scholar]
- 16.Corona G, Petrone L, Mannucci E, Jannini EA, Mansani R, Magini A, Giommi R, Forti G, Maggi M. Psycho-biological correlates of rapid ejaculation in patients attending an andrologic unit for sexual dysfunctions. Eur Urol. 2004;46:615–22. doi: 10.1016/j.eururo.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Carani C, Isidori AM, Granata A, Carosa E, Maggi M, Lenzi A, Jannini EA. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab. 2005;90:6472–9. doi: 10.1210/jc.2005-1135. [DOI] [PubMed] [Google Scholar]
- 18.Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P. Erectile dysfunction in patients with hyper- and hypothyroidism: How common and should we treat? J Clin Endocrinol Metab. 2008;93:1815–9. doi: 10.1210/jc.2007-2259. [DOI] [PubMed] [Google Scholar]
- 19.Veronelli A, Masu A, Ranieri R, Rognoni C, Laneri M, Pontiroli AE. Prevalence of erectile dysfunction in thyroid disorders: Comparison with control subjects and with obese and diabetic patients. Int J Impot Res. 2006;18:111–4. doi: 10.1038/sj.ijir.3901364. [DOI] [PubMed] [Google Scholar]
- 20.Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1:228–57. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- 21.Carosa E, Rossi S, Giansante N, Gravina GL, Castri A, Dolci S, Botti F, Morelli A, Di Luigi L, Pepe M, Lenzi A, Jannini EA. The ontogenetic expression pattern of type 5 phosphodiesterase correlates with androgen receptor expression in rat corpora cavernosa. J Sex Med. 2009;6:388–96. doi: 10.1111/j.1743-6109.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 22.Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, Orlando C, Vannelli GB, Aversa A, Natali A, Forti G, Giorgi M, Jannini EA, Ledda F, Maggi M. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–63. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 23.Zandieh-Doulabi B, Dop E, Schneiders M, Schiphorst MP, Mansen A, Vennström B, Dijkstra CD, Bakker O, Wiersinga WM. Zonal expression of the thyroid hormone receptor alpha isoforms in rodent liver. J Endocrinol. 2003;179:379–85. doi: 10.1677/joe.0.1790379. [DOI] [PubMed] [Google Scholar]
- 24.Krall JF, Fittingoff M, Rajfer J. Characterization of cyclic nucleotide and inositol 1,4,5-trisphosphate-sensitive calcium-exchange activity of smooth muscle cells cultured from the human corpora cavernosa. Biol Reprod. 1988;39:913–22. doi: 10.1095/biolreprod39.4.913. [DOI] [PubMed] [Google Scholar]
- 25.Granchi S, Vannelli GB, Vignozzi L, Crescioli C, Ferruzzi P, Mancina R, Vinci MC, Forti G, Filippi S, Luconi M, Ledda F, Maggi M. Expression and regulation of endothelin-1 and its receptors in human penile smooth muscle cells. Mol Hum Reprod. 2002;8:1053–64. doi: 10.1093/molehr/8.12.1053. [DOI] [PubMed] [Google Scholar]
- 26.Murray MB, Zilz ND, McCreary NL, MacDonald MJ, Towle HC. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988;263:12770–7. [PubMed] [Google Scholar]
- 27.Jannini EA, Crescenzi A, Rucci N, Screponi E, Carosa E, de Matteis A, Macchia E, Amati G, D’Armiento M. Ontogenetic pattern of thyroid hormone receptor expression in the human testis. J Clin Endocrinol Metab. 2000;85:3453–7. doi: 10.1210/jcem.85.9.6803. [DOI] [PubMed] [Google Scholar]
- 28.Jannini EA, Olivieri M, Francavilla S, Gulino A, Ziparo E, D'Armiento M. Ontogenesis of the nuclear 3,5,3′-triiodothyronine receptor in the rat testis. Endocrinology. 1990;126:2521–6. doi: 10.1210/endo-126-5-2521. [DOI] [PubMed] [Google Scholar]
- 29.Jannini EA, Ulisse S, Piersanti D, Carosa E, Muzi P, Lazar J, D’Armiento M. Early thyroid hormone treatment in rats increases testis size and germ cell number. Endocrinology. 1993;132:2726–8. doi: 10.1210/endo.132.6.8504773. [DOI] [PubMed] [Google Scholar]
- 30.Jannini EA, Dolci S, Ulisse S, Nikodem VM. Developmental regulation of the thyroid hormone receptor alpha 1 mRNA expression in the rat testis. Mol Endocrinol. 1994;8:89–96. doi: 10.1210/mend.8.1.8152433. [DOI] [PubMed] [Google Scholar]
- 31.Jannini EA, Mitsuhashi T, Nikodem VM. Developmental expression of mRNAs from a rat C-erbA genomic locus. Biochem Biophys Res Commun. 1992;184:739–45. doi: 10.1016/0006-291x(92)90652-2. [DOI] [PubMed] [Google Scholar]
- 32.Jannini EA, Carosa E, Rucci N, Screponi E, D'Armiento M. Ontogeny and regulation of variant thyroid hormone receptor isoforms in developing rat testis. J Endocrinol Invest. 1999;22:843–8. doi: 10.1007/BF03343656. [DOI] [PubMed] [Google Scholar]
- 33.Forrest D, Sjoberg M, Vennstrom B. Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. Embo J. 1990;9:1519–28. doi: 10.1002/j.1460-2075.1990.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990;265:10514–21. [PubMed] [Google Scholar]
- 35.Bradley DJ, Towle HC, Young WS., 3rd Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992;12:2288–302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cihan A, Demir O, Demir T, Aslan G, Comlekci A, Esen A. The relationship between premature ejaculation and hyperthyroidism. J Urol. 2009;181:1273–80. doi: 10.1016/j.juro.2008.10.150. [DOI] [PubMed] [Google Scholar]
- 37.Saltó C, Kindblom JM, Johansson C, Wang Z, Gullberg H, Nordström K, Mansén A, Ohlsson C, Thorén P, Forrest D, Vennström B. Ablation of TRalpha2 and a concomitant overexpression of alpha1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol Endocrinol. 2001;15:2115–28. doi: 10.1210/mend.15.12.0750. [DOI] [PubMed] [Google Scholar]
- 38.Giuriato L, Borrione AC, Zanellato AM, Tonello M, Scatena M, Scannapieco G, Pauletto P, Sartore S. Aortic intimal thickening and myosin isoform expression in hyperthyroid rabbits. Arterioscler Thromb. 1991;11:1376–89. doi: 10.1161/01.atv.11.5.1376. [DOI] [PubMed] [Google Scholar]
- 39.Gunasekera RD, Kuriyama H. The influence of thyroid states upon responses of the rat aorta to catecholamines. Br J Pharmacol. 1990;99:541–7. doi: 10.1111/j.1476-5381.1990.tb12965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeniyi KO, Ogunkeye OO, Senok SS, Udoh FV. Influence of the thyroid state on the intrinsic contractile properties of the bladder muscle. Acta Physiol Hung. 1994;82:69–74. [PubMed] [Google Scholar]
- 41.McAllister RM, Grossenburg VD, Delp MD, Laughlin MH. Effects of hyperthyroidism on vascular contractile and relaxation responses. Am J Physiol. 1998:274. doi: 10.1152/ajpendo.1998.274.5.E946. [DOI] [PubMed] [Google Scholar]
- 42.Lofgren M, Fagher K, Woodard G, Arner A. Effects of thyroxine on myosin isoform expression and mechanical properties in guinea-pig smooth muscle. J Physiol. 2002;543:757–66. doi: 10.1113/jphysiol.2002.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuma H, Murakami M, Mori M. Thyroid hormone activation in human vascular smooth muscle cells: Expression of type II iodothyronine deiodinase. Circ Res. 2001;88:313–8. doi: 10.1161/01.res.88.3.313. [DOI] [PubMed] [Google Scholar]
- 44.Ojamaa K, Klemperer JD, Klein I. Acute effects of thyroid hormone on vascular smooth muscle. Thyroid. 1996;6:505–12. doi: 10.1089/thy.1996.6.505. [DOI] [PubMed] [Google Scholar]
- 45.Graettinger JS, Muenster JJ, Checchia CS, Grissom RL, Campbell JA. A correlation of clinical and hemodynamic studies in patients with hypothyroidism. J Clin Invest. 1958;37:502–10. doi: 10.1172/JCI103631. [DOI] [PMC free article] [PubMed] [Google Scholar]


