Abstract
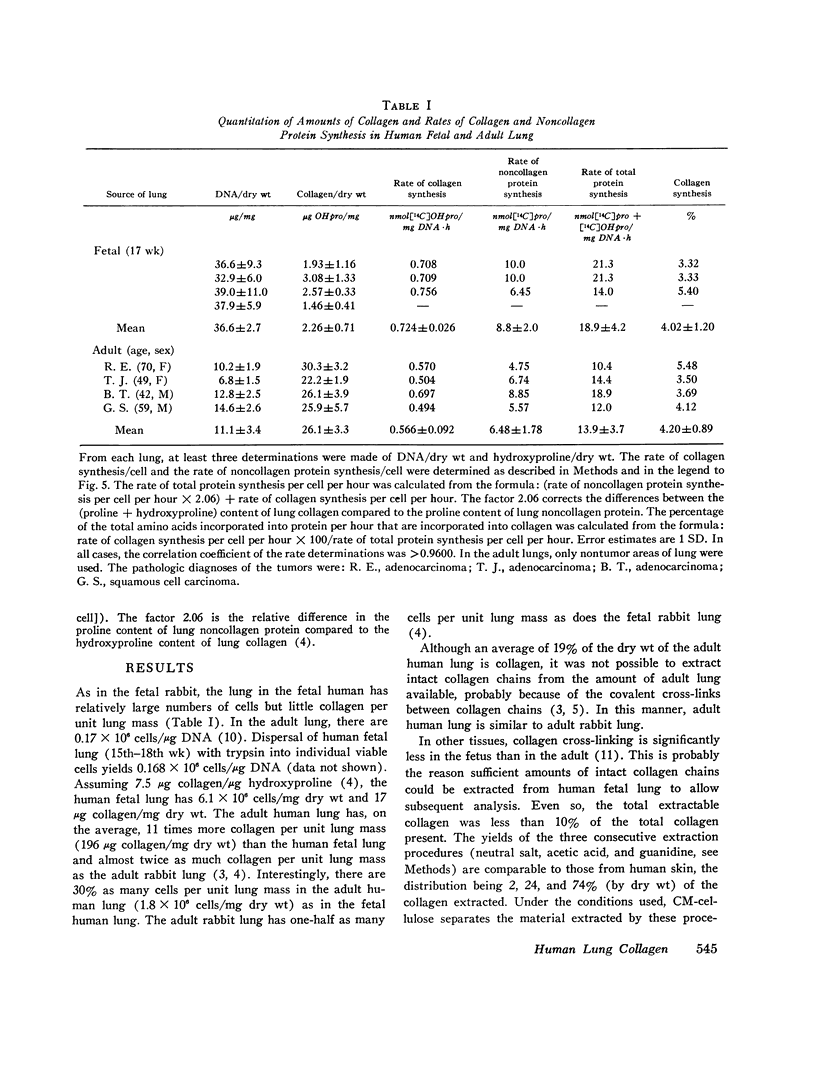
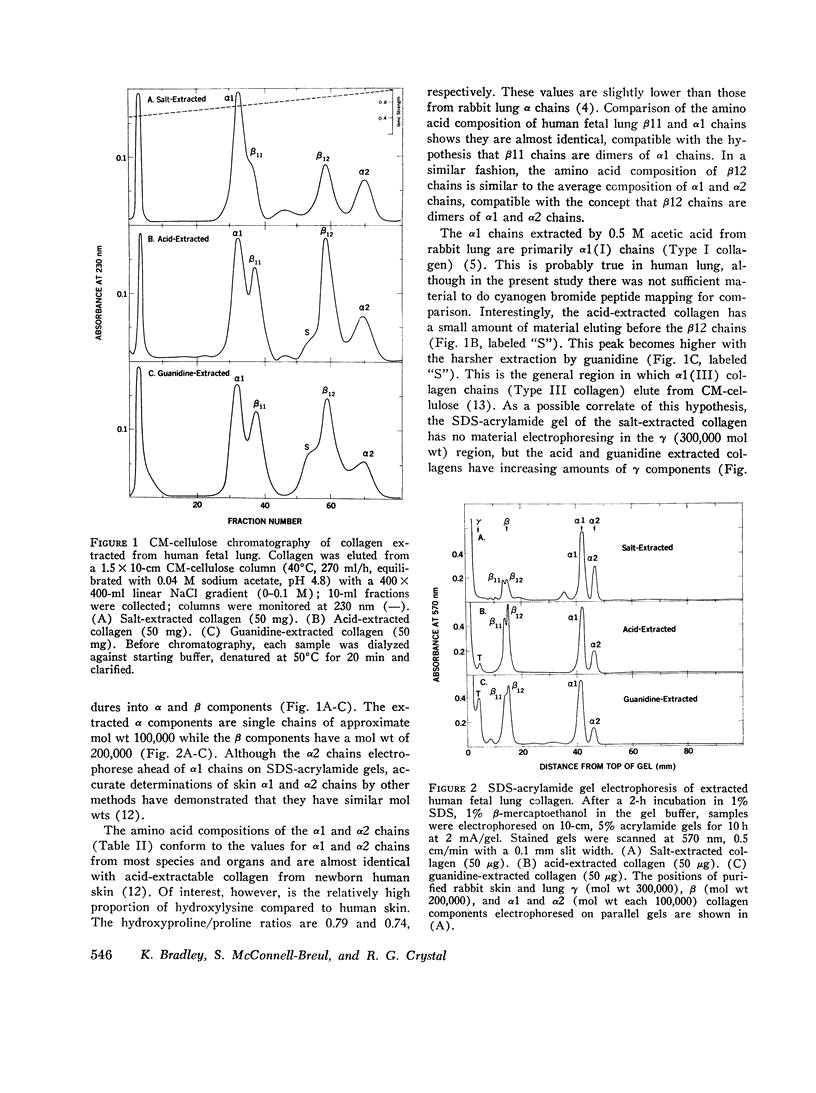
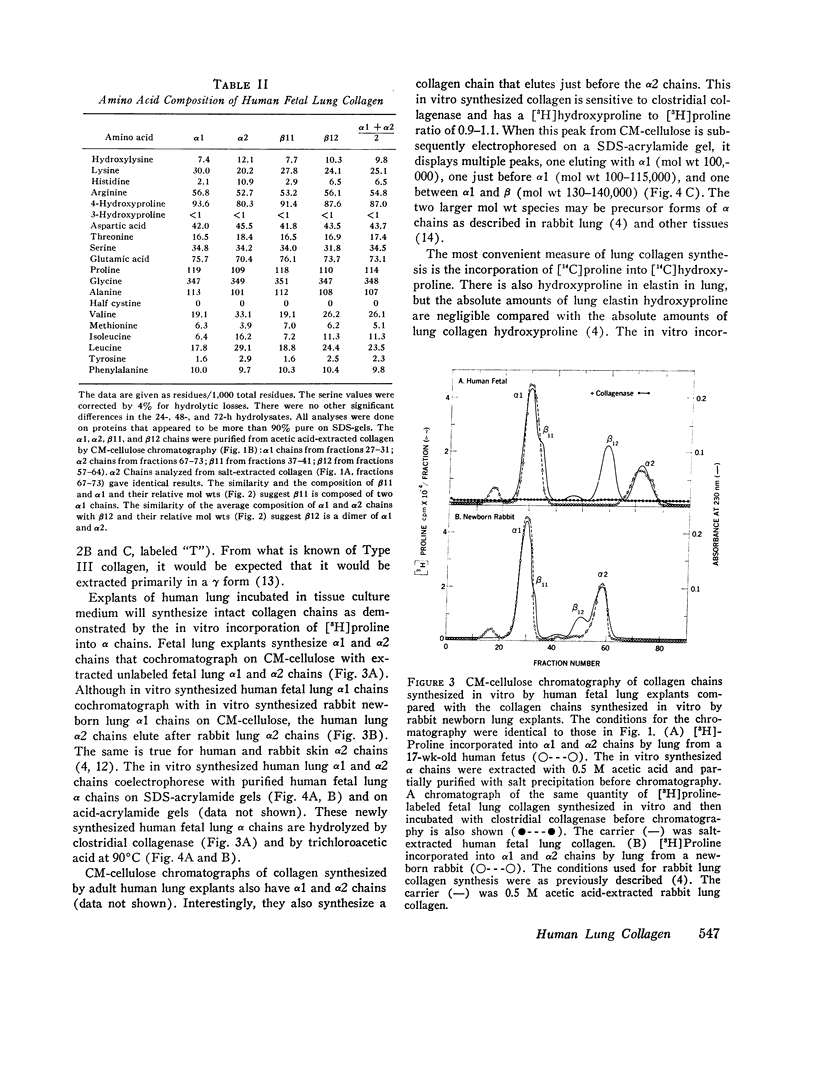
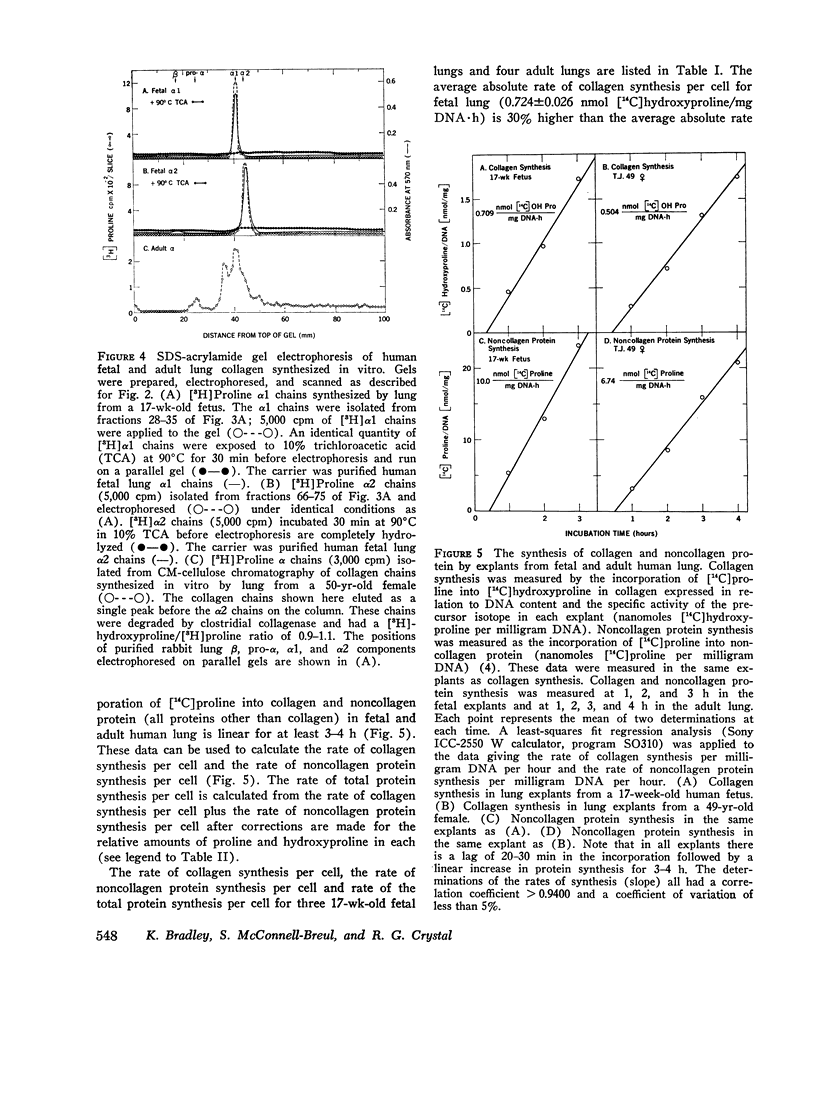
The presence of collagen in lung is fundamental in normal lung structure and function. Methods have been developed to examine human fetal and adult lung collagen with respect to its composition and synthesis. The second trimester fetal lung has a large number of cells per unit lung mass (36.6 plus or minus 2.7 mug DNA/mg dry wt) and relatively small amounts of collagen (17.0 plus or minus 5.3 mug collagen/mg dry wt). The number of cells per unit lung mass in the adult lung (11.1 plus or minus 3.4 mug DNA/mg dry wt) is 30% of the number of cells in the fetal lung, but the adult has 11 times more collagen (196 plus or minus 25 mug collagen/mg dry wt). The composition of fetal lung collagen can be partially characterized by extraction with salt at neutral pH, acetic acid, or guanidine. The extracted chains, representing 10% of the total lung collagen, chromatograph as alpha1 and alpha2 chains, each with a mol wt of 100,000 and an animo acid composition characteristic for collagen but not specific for lung. Short-term explant cultures of fetal and adult lung synthesize alpha chains which can be isolated by ion-exchange chromatography. These chains, representing 30-40% of the total collagen synthesized by the explants, coelectrophorese with extracted collagen chains on acrylamide gels: they are destroyed by clostridial collagenase and they have a mol wt of 100,000. Although the composition of the collagen synthesized by these explants can be only partially characterized, the rate of synthesis of both collagen and noncollagen protein can be quantitated. In fetal lung, 4.0 plus or minus 1.2% of the amino acids incorporated into protein per hour are incorporated into collagen. In normal adult lung, this percentage (4.2 plus or minus 0.9%) is remarkably similar. These values are almost identical to the relative rate of collagen synthesis in rabbit lung in the same age range. This technology should be applicable to answer specific questions regarding collagen synthesis and degradation in human lung disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN P., PIEZ K. A. A BIOCHEMICAL STUDY OF HUMAN SKIN COLLAGEN AND THE RELATION BETWEEN INTRA- AND INTERMOLECULAR CROSS-LINKING. J Clin Invest. 1964 Sep;43:1813–1823. doi: 10.1172/JCI105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. The biosynthesis of collagen. Annu Rev Biochem. 1974;43(0):567–603. doi: 10.1146/annurev.bi.43.070174.003031. [DOI] [PubMed] [Google Scholar]
- Bradley K. H., McConnell S. D., Crystal R. G. Lung collagen composition and synthesis. Characterization and changes with age. J Biol Chem. 1974 May 10;249(9):2674–2683. [PubMed] [Google Scholar]
- Bradley K., McConnell-Breul S., Crystal R. G. Lung collagen heterogeneity. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2828–2832. doi: 10.1073/pnas.71.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Click E. M., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha 1 and alpha 2 chains of human skin collagen. Biochemistry. 1970 Nov 24;9(24):4699–4706. doi: 10.1021/bi00826a012. [DOI] [PubMed] [Google Scholar]
- Crystal R. G. Lung collagen: definition, diversity and development. Fed Proc. 1974 Nov;33(11):2248–2255. [PubMed] [Google Scholar]
- Dickie K., Massaro D. Protein transport by lung. Proc Soc Exp Biol Med. 1974 Jan;145(1):154–156. doi: 10.3181/00379727-145-37767. [DOI] [PubMed] [Google Scholar]
- Kaplan P. D., Kuhn C., Pierce J. A. The induction of emphysema with elastase. I. The evolution of the lesion and the influence of serum. J Lab Clin Med. 1973 Sep;82(3):349–356. [PubMed] [Google Scholar]
- LEUCHTENBERGER C., LEUCHTENBERGER R., DAVIS A. M. A microspectrophotometric study of the desoxyribose nucleic acid (DNA) content in cells of normal and malignant human tissues. Am J Pathol. 1954 Jan-Feb;30(1):65–85. [PMC free article] [PubMed] [Google Scholar]
- Maekawa T., Rathinasamy T. K., Altman K. I., Forbes W. F. Changes in collagen with age. I. The extraction of acid soluble collagens from the skin of mice. Exp Gerontol. 1970 Jul;5(2):177–186. doi: 10.1016/0531-5565(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Massaro D., Weiss H., Simon M. R. Protein synthesis and secretion by lung. Am Rev Respir Dis. 1970 Feb;101(2):198–206. doi: 10.1164/arrd.1970.101.2.198. [DOI] [PubMed] [Google Scholar]
- PIERCE J. A., HOCOTT J. B., EBERT R. V. The collagen and elastin content of the lung in emphysema. Ann Intern Med. 1961 Aug;55:210–222. doi: 10.7326/0003-4819-55-2-210. [DOI] [PubMed] [Google Scholar]
- Pierce J. A., Resnick H., Henry P. H. Collagen and elastin metabolism in the lungs, skin, and bones of adult rats. J Lab Clin Med. 1967 Mar;69(3):485–493. [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]


