Abstract
Zygomycosis is increasingly recognized in immunocompromised hosts. We investigated whether platelets become activated after contact with Zygomycetes and adhere to conidial and hyphal structures using immunofluorescence. The platelets’ influence on fungal viability was evaluated by assessing hyphal elongation and hyphal damage. Platelets became activated and strongly adhered to conidia and hyphae of Zygomycetes. Platelets induced time dependent damage to hyphae and significantly reduced (P < .05) hyphal elongation. We found that platelets possess antifungal capacities against Zygomycetes based on granule dependent mechanisms and significantly reduce fungal growth and spread, both of which are of major importance in evolving invasive disease.
Aspergillus species remain the most common cause of invasive fungal disease [1]. Zygomycetes have recently attracted attention as the second most common opportunistic mold causing invasive disease among patients with hematological malignancies and recipients of transplants [1, 2]. Zygomycosis has a particularly poor prognosis, with mortality rates for disseminated disease that can exceed 90% [2]. Rhizopus, Rhizomucor, Mucor, and Absidia species are most commonly involved [2].
Platelets are the smallest cells of human blood and, with numbers ranging from 150,000 to 300,000 platelets/mm3, are second only to red cells in abundance [3]. They contain 3 distinct cytoplasmatic granule types: dense (δ) granules store mediators that include serotonin and adenosine nucleotide diphosphate; alpha (α) granules are involved in adhesion and coagulation and contain an arsenal of platelet microbicidal proteins (PMPs); and lysosomal (λ) granules contain lysosomal enzymes [4]. Several PMPs have been characterized and have been identified from supernatants of thrombin-stimulated rabbit and human platelets [4].
Platelets are essential components in hemostasis [3, 4], but they also play a role in antimicrobial host defense and exhibit features characteristic of classic cell-mediated immune effector cells [3, 4]. Platelets impair the viability of Staphylococcus aureus and several other bacteria [4] and are capable of binding, aggregating, and internalizing viral particles, which enhance the clearance of pathogens from the bloodstream [4]. However, only few data are available on the role of platelets in defending against fungi [5, 6].
Zygomycetes typically invade blood vessels (angioinvasion), causing local thrombosis with consequent distal hemorrhagic infarction [2]. This and the known antifungal capacities of platelets against Aspergillus species led us to examine the potential antifungal role of platelets in host defense against Zygomycetes.
Materials and methods
Four clinical isolates each of Rhizomucor species, Absidia species, Mucor species, and Rhizopus species were grown on sabouraud dextrose agar (Merck) and incubated at 37°C for 4 days. The conidial suspension was harvested by flooding each colony with 2 mL of Roswell Park Memorial Institute (RPMI) 1640 medium, and the number of conidia was calculated using a hemocytometer and adjusted to a concentration of 1 × 105 colony-forming units/mL, if not otherwise indicated. The viability and inoculum sizes of the various strains were checked by using quantitative colony counts. For hyphal growth, conidia were incubated for 18 h at 37°C; >90% formed hyphae under these conditions.
Fresh platelet concentrates were provided from the local Department of Immunology and Blood Transfusion (Innsbruck Medical University, Innsbruck, Austria). Platelets were collected from regular healthy blood donors and prepared by thrombocytapheresis with Amicus cell separator (Baxter). Platelets were stored for a maximum of 2 days at 22°C and platelet aggregation analyses were used for quality control. The platelet counts were 6 × 108 cells/mL. For selected experiments, the microfilament inhibitor cD (Sigma-Aldrich) was added to platelets at a final concentration of 10 μM.
Platelet activation by Zygomycetes was detected by a fluorescent labelling of CD 42b, an antigen present on the plasma membranes of both resting and activated platelets, and of CD 62P, which is present only on activated platelets. Platelets were mixed with Zygomycetes conidia or hyphae in an effector:target (E:T) ratio of 100:1 and incubated for 30 min at 37°C. Fluorescein isothicyanate–conjugated mouse anti-human CD 42b (Becton Dickinson) and R-Phycoerythrin-conjugated CD 62P (Becton Dickinson) were added using 20 μL/106 cells for 30 min at 37°C, and samples were visualized by fluorescent microscopy. Each sample was assessed in duplicate, and the procedure was repeated 3 times.
Zygomycetes conidia-platelet adherence was determined by a modification of the spectrophotometric method of Herzberg et al [7]. In short, equal volumes (100 μL) of Zygomycetes conidia and platelets appropriate to achieve E:T ratios of 100:1 were mixed, incubated for 30 min at 37°C, and centrifuged at 55 g for 5 min at 4°C. After centrifugation, 100-μL supernatant aliquots were sampled from wells and diluted into 1.9 mL of phosphate buffered saline (Sigma-Aldrich); the OD700 of each supernatant sample was determined spectrophotometrically, and the percentage of Zygomycetes-platelet adherence was calculated by using the following formula: 1—OD reaction supernatant/0.5 × (OD conidia supernatant + OD platelet supernatant) × 100 [8].
For detection of Zygomyctes hyphae-platelet adherence, hyphal structures were coincubated with platelets for 30 min. Adherence was measured by microscopic examination. The number of platelets attached to each of the first 100 hyphae was counted. On the basis of results obtained from various microorganisms, fungal isolates exhibiting ±15% platelet adherence were considered as highly active platelet adherence.
Hyphae plus platelets (E:T ratio of 100:1) or cD treated platelets were incubated for 30 min and 60 min at 37°C. The reactions were stopped by adding 0.5 mL of ice-cold double-distilled water, and the tubes were washed twice to lyse platelets. For measurement of hyphal metabolic activity, 2,3-bis [2-methoxy-4-nitro-5-sulfophenyl] 2 H-tetrazolium-5-carboxynilide sodium salt (XTT) plus 40 μg/mL coenzyme Q (2,3-dimethoxy-5-methyl-1,4-benzoquinone) (Sigma) was used [8]. Absorbance was determined at 450 nm using an enzyme-linked immuno-sorbent assay plate reader (ASYS Hitech), and antifungal activity was calculated as the percentage of hyphal damage equal to (1 — X/C) × 100, where X is the optical density of test wells and C is the optical density of control wells with hyphae only. Each sample was assessed in duplicate, and repeated 3 times.
The morphology of conidia treated with platelets, without platelets, and cD-treated platelets was investigated by assessing hyphal elongation [9]. A total of 100 μL each of platelets and conidia (E:T ratio of 100:1) were inoculated into 96-microwell plates (Greiner) and incubated at 37°C. The morphology of the organisms was determined microscopically after 18 h; platelets were lysed with ice-cold water and a micrometer was used for length measurement. The conidial inhibition rate was calculated from the percentage of conidia which did not germinate. Each sample was assessed in triplicate, measuring 50 conidia per sample.
Conidial suspensions were prepared as described above and coincubated with platelets for 1 h and 5 h at 37°C, and the postplatelet effect was assessed [9]. In brief, fungi were washed twice with sterile water, centrifuged at 4000 g for 2 min, and refilled with RPMI 1640. Quantitative cultures of nondiluted samples and 1:100 and 1:1000 dilutions in aqua were spread on sabouraud dextrose agar, incubated at 37°C, and examined visually for growth after 24 h. Colony count, colony size, and pigmentation release of untreated and platelet-treated isolates were determined.
Each set of experiments was performed in triplicate and repeated at least 3 times for each species. The results were expressed as mean ± standard deviation (SD). Student’s t test for paired samples was used to determine statistical significance. P < .05 was considered to be statistically significant.
Results
In all assays performed, platelets adherence for hyphae (Figure 1A and 1B) was 45% ± 2%, 46% ± 4%, 57% ± 3%, 44% ± 7% and for conidia (Figure 1C and 1D) 50% ± 3%, 49% ± 4%, 51% ± 3%, 50% ± 3% for Rhizomucor species, Mucor species, Absidia species, and Rhizopus species, respectively. No difference between the species was observed.
Figure 1.
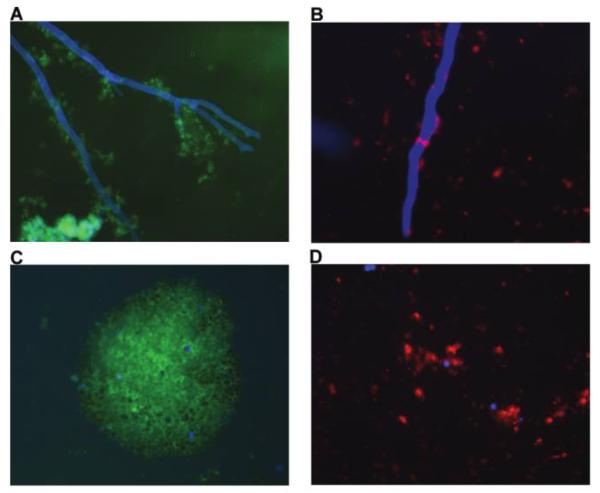
Representative fluorescence photomicrographs of platelet adherence (A, C) and activation (B, D) after contact with either hyphae or conidia of Zygomycetes, which were stained with calcofluor white (blue fluorescence). Platelets adhered to hyphae (A) and conidia (C) of zygomycetes and were visualized by monoclonal anti CD-42b–fluorescein isothicyanate conjugated antibody (green fluorescence). Platelets became activated after contact with either hyphae (B) or conidia (D) of zygomycetes as shown by monoclonal anti-CD62P-PE antibody (red fluorescence), which is presented exclusively on activated platelets.
The CD42b surface antigen proved to be constitutively expressed on surfaces of both resting and activated platelets (Figure 1A and 1C). Interaction of platelets either with hyphae or conidia of Zygomycetes clearly induced strong adherence and expression of the CD62P antigen on platelet surfaces (Figure 1B and 1D), an established antigenic marker for platelet activation.
A prolonged incubation time of 30–60 min revealed significantly greater effects, with an increase in hyphal damage for Rhizomucor species, Absidia species, Mucor species, and Rhizopus species. In contrast, cD-treated platelets showed a statistically significant reduced capacity (P < .05) of hyphae to metabolize XTT, compared with untreated platelets (Table 1).
Table 1.
Reduction of Hyphal Elongation and Percentage of Hyphal Damage at 30 min and 60 min of Zygomycetes under Platelet and cD Platelet Treatment
| Hyphal damage, mean % (±SD) |
|||||||
|---|---|---|---|---|---|---|---|
| Hyphal elongation, mean μm (±SD) |
Platelets |
cD platelets |
|||||
| Genus | Control samples | Platelets | cD platelets | 30 min | 60 min | 30 min | 60 min |
| Rhizomucor (n = 1) | 2651 ± 244 | 19.7 ± 7a | 2250 ± 321 | 13.7± 3.0a | 25.3 ± 1.5a | 2.7 ± 1.2 | 5.7 ± 3.5 |
| Mucor (n = 1) | 2780 ± 135 | 19.3 ± 7a | 2304 ± 260 | 16.7 ± 4.0a | 27.0 ± 5.2a | 4.0 ± 2.0 | 7.7 ± 2.5 |
| Absidia (n = 1) | 2699 ± 301 | 20 ± 8a | 2010 ± 560 | 27.0 ± 8.0a | 39.0 ± 2.0a | 3.3 ± 8.0 | 5.3 ± 1.5 |
| Rhizopus (n = 1) | 2554 ± 311 | 19.3 ± 7a | 2400 ± 155 | 16.7 ± 8.0a | 30.7 ± 8.7a | 3.3 ± 2.3 | 4.7 ± 2.1 |
NOTE. SD, standard deviation.
P < .05, for platelets versus control samples and/or cD-treated platelets.
Platelets were found to be strong inhibitors of fungal growth, because conidial germination was significantly decreased under platelet treatment (P < .05): 85%, 91%, 89%, and 85% of conidia did not germinate in Rhizomucor species, Absidia species, Mucor species, and Rhizopus species, respectively, whereas germination in untreated controls samples was 98% (Table 1). The microfilament inhibitor cD neutralized all effects (Table 1).
Visual examination of platelet-pretreated Zygomycetes cultures did not result in different fungal pigmentation, colony count, or colony size after 24 h (data not shown). No difference between platelets-treated and untreated fungi was observed.
Discussion
In this study, we show that platelets adhere to conidia and hyphae of Zygomycetes, undergo activation after exposition to fungi, damage hyphae in a time-dependent manner, and reduce fungal growth, because germination and hyphal elongation were statistically significantly decreased (P < .05). In contrast, cD-pretreated platelets any antifungal effects, which reflects granule-dependent mechanisms.
The interaction between Zygomycetes and platelets resulted in highly activated platelets as CD62P was expressed on their surface. Platelet surface glycoproteins are essential for different platelet functions and play a primary role in the adhesion, ligand-interaction and aggregation [10]. CD62P is stored in α granule membrane and is rapidly transported to the plasma membrane upon activation [10]. This result confirms the observation that platelets interact with various fungi [5, 6].
The exact mechanisms by which platelets interact with microorganisms are as yet little understood. In this study, we showed that platelets adhere to Zygomycetes, the invasive form of the fungus, and tended to spread over hyphal surfaces, because this was also found for aspergilli [5]. Adherence was similar for all Zygomycetes tested, without showing strain-dependent differences, as demonstrated for S. aureus and streptococci [11]. Fibrinogen binds to fungal cell surfaces and has been postulated to play a significant role in platelet attachment to Candida albicans [12]. The nature of hyphal cell wall surface constituents binding these plasma factors remains to be defined.
The changes in the ability to reduce the tetrazolium dye XTT indicated that platelets inflicted significant damage to hyphae of Zygomycetes species. Similar results were found for polymorphomuclear leucocytes [13], which are well known for their hyphal killing of fungi [13]. A study by Simitsopoulou et al [13] found polymorphomuclear leucocytes induced hyphal damage of 3 Zygomycetes, ranging from a mean (±SD) of 15.9% ± 1.7% up to 26.7% ± 6.7%, depending on the species tested. Our data reveal similar or even greater damage caused by human platelets, emphasizing their potent antifungal activity against invading hyphae. The observed damage was time dependent and ranged from 12.5% to 27% after 30 min and was almost twice this level after 60 min.
All platelets antifungal effects were abrogated by cD, high-lighting the role of granules in antifungal activity, as was found for Aspergillus species [5]. cD is a microfilament inhibitor that suppresses the formation of contractile microtubules and, therefore, the platelets’ degranulation [5].
The most important finding from this study was the lack of germination and the lack of hyphal elongation of Zygomycetes exposed to platelets. It is widely held that the onset of invasive zygomycosis is dependent on 2 main steps: first, the failure to suppress the germination of spores, and second, the failure to kill growing hyphae [2]. We clearly demonstrated that platelets have the capability of interfering with both steps.
A recent study identified thrombocytopenia as a risk factor for invasive fungal diseases in organ transplant recipients [14]. Thrombocytopenia is often seen in patients with hematological malignancy who experience invasive zygomycoses and is usually associated with the severity and type of underlying disease or its treatment [14]. However, severe thrombocytopenia has also been reported in cases of zygomycoses associated with other medical conditions [15], which suggests that the interaction of platelets with hyphae is involved in vascular damage, angioinvasion and infarction.
In conclusion, our investigations support a strong interaction between platelets and Zygomycetes. Platelets employ granule dependent mechanisms against the invading fungi. Because platelets exhibit antifungal activity and function as immune cells, a better understanding of platelet-dependent host defenses may help in evaluating risk factors and inform therapeutic strategies.
Acknowledgments
We thank J. Peter Donnelly, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
Financial support: Austrian Science Foundation (grant FWF-P17484-B05)
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Richardson M, Lass-Flörl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14(Suppl 4):5–24. doi: 10.1111/j.1469-0691.2008.01978.x. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez CE, Rinaldi MG, Sugar AM. Zygomycosis. Infect Dis Clin North Am. 2002;16:895–914. doi: 10.1016/s0891-5520(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 3.Kehrel BE, Jurk K. Platelets at the interface between hemostasis and innate immunity. Transfus Med Hemother. 2004;31:379–86. [Google Scholar]
- 4.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–57. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 5.Perkhofer S, Kehrel BE, Dierich MP, et al. Human platelets attenuate Aspergillus species via granule-dependent mechanisms. J Infect Dis. 2008;198:1243–6. doi: 10.1086/591458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christin L, Wysong D, Meshulam T, et al. Human platelets damage Aspergillus fumigatus hyphae and may supplement killing neutrophils. Infect Immun. 1998;66:1181–9. doi: 10.1128/iai.66.3.1181-1189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herzberg M, Brintzenhofer K, Clawson C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983;39:1457–69. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antachopoulos C, Meletiadis J, Roilides E, Sein T, Walsh TJ. Rapid susceptibility testing of medically important Zygomycetes by XTT assay. J Clin Microbiol. 2006;44:553–60. doi: 10.1128/JCM.44.2.553-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lass-Flörl C, Dierich MP, Fuchs D, Semenitz E, Jenewein I, Ledochowski M. Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J Antimicrob Chemother. 2001;48:775–9. doi: 10.1093/jac/48.6.775. [DOI] [PubMed] [Google Scholar]
- 10.Chung I, Choudhury A, Lip GY. Platelet activation in acute, decompansated congestive heart failure. Thromb Res. 2007;120:709–13. doi: 10.1016/j.thromres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Hermann A, Lai Q, Albrecht R, Mosher D, Proctor R. Adhesion of Staphylococcus aureus to surface-bound platelets: role of fibrinogen/fibrin and platelet integrins. J Infect Dis. 1993;167:312–22. doi: 10.1093/infdis/167.2.312. [DOI] [PubMed] [Google Scholar]
- 12.Senet JM. Candida adherence phenomena, from commensalism to pathogenicity. Int Microbiol. 1998;1:117–22. [PubMed] [Google Scholar]
- 13.Simitsopoulou M, Roilides E, Maloukou A, Gil-Lamaignere C, Walsh TJ. Interaction of amphotericin B lipid formulations and triazoles with human polymorphomuclear leucocytes for antifungal activity against Zygomycetes. Mycoses. 2008;51:147–54. doi: 10.1111/j.1439-0507.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang FY, Singh N, Gayowski T, et al. Thrombocytopenia in liver transplant recipients: predictors, impact on fungal infections, and role of endogenous thrombopoetin. Transplantation. 2000;69:70–5. doi: 10.1097/00007890-200001150-00014. [DOI] [PubMed] [Google Scholar]
- 15.Bloxham CA, Carr S, Ryan DW, Kesteven PJ, Bexton RS, Griffiths ID, Richards J. Disseminated zygomycosis and systemic lupus erythematosus. Intensive Care Med. 1990;16:201–7. doi: 10.1007/BF01724803. [DOI] [PubMed] [Google Scholar]


